Seroprevalence of Anti-SARS-CoV-2 Antibodies in Cats during Five Waves of COVID-19 Epidemic in Thailand and Correlation with Human Outbreaks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
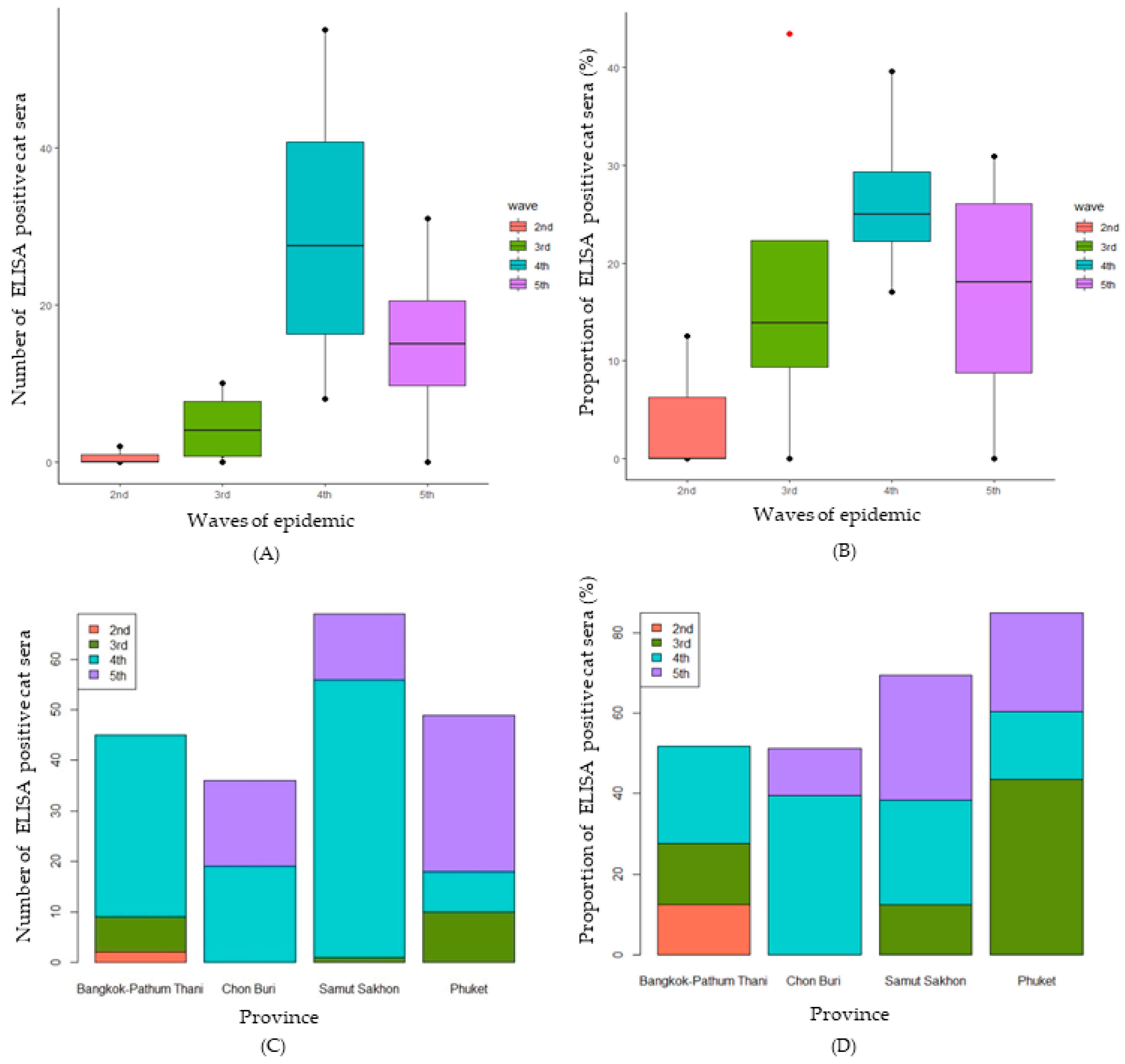
3.1. Prevalence of SARS-CoV-2 Antibody in Cats by Modified SARS-CoV-2 S1 RBD ELISA
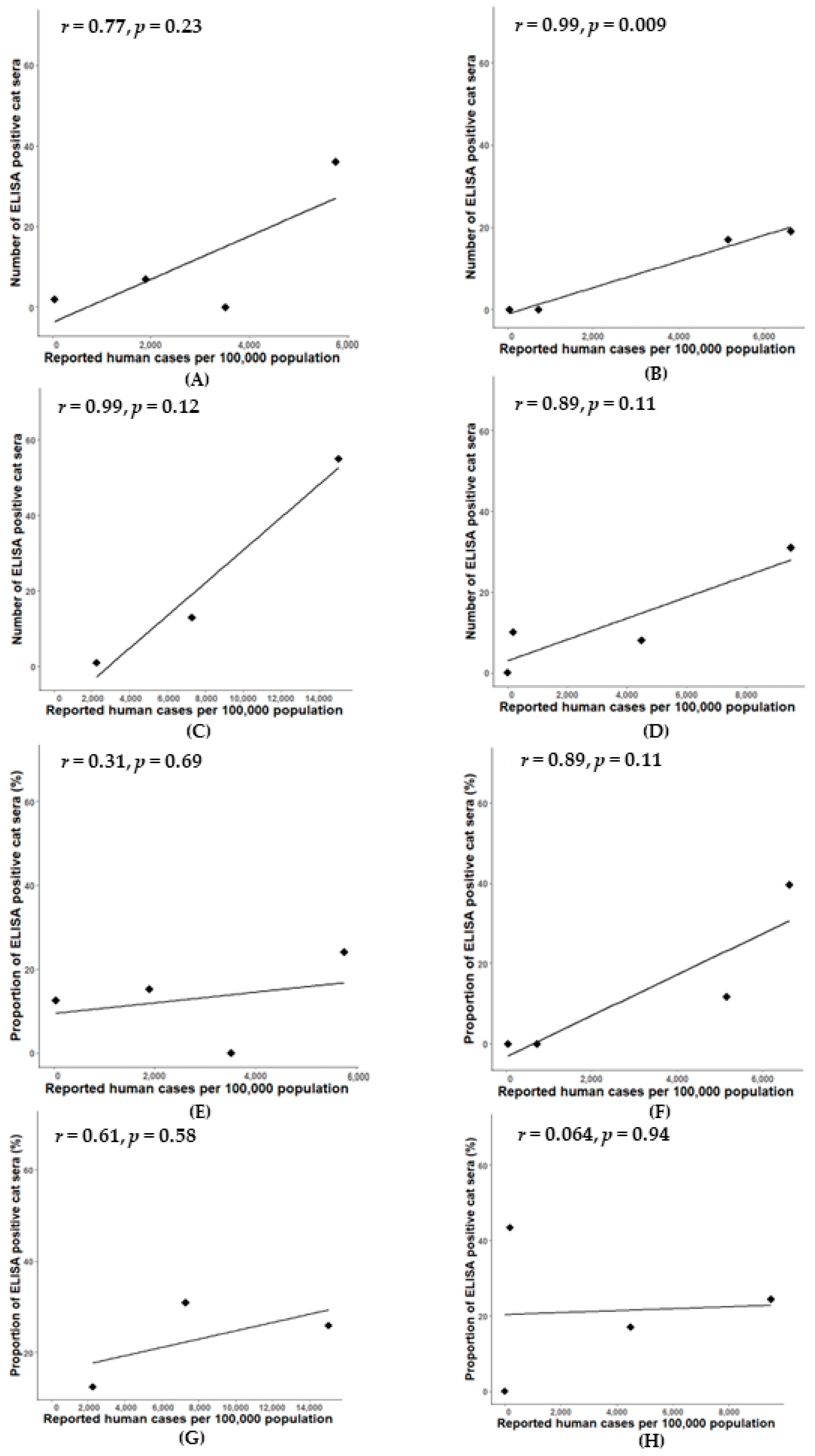
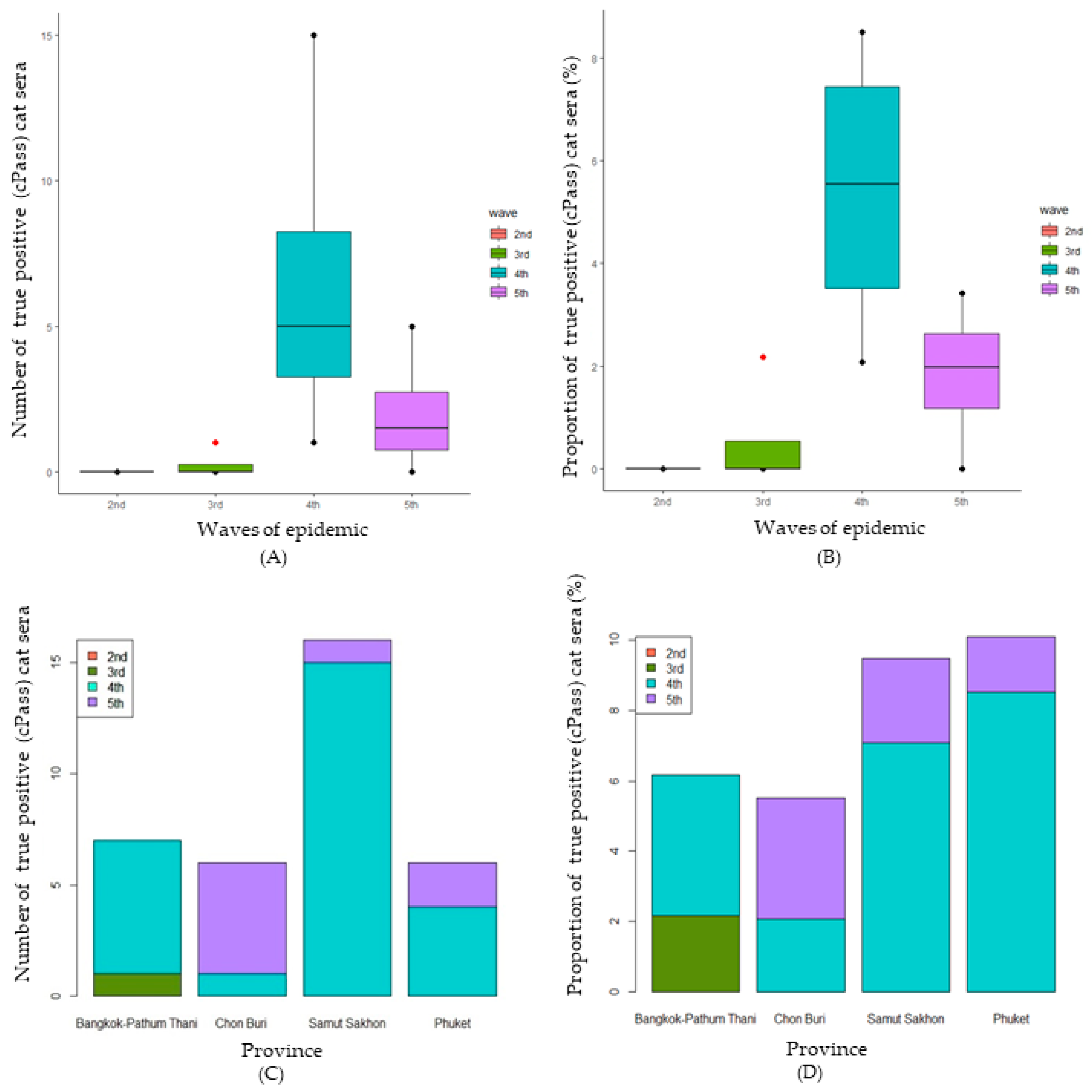
3.2. Confirmation of SARS-CoV-2 Infection in Cats Using cPassTM Neutralization Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puenpa, J.; Rattanakomol, P.; Saengdao, N.; Chansaenroj, J.; Yorsaeng, R.; Suwannakarn, K.; Thanasitthichai, S.; Vongpunsawad, S.; Poovorawan, Y. Molecular characterisation and tracking of severe acute respiratory syndrome coronavirus 2 in Thailand, 2020–2022. Arch. Virol. 2023, 168, 26. [Google Scholar] [CrossRef]
- Triukose, S.; Nitinawarat, S.; Satian, P.; Somboonsavatdee, A.; Chotikarn, P.; Thammasanya, T.; Wanlapakorn, N.; Sudhinaraset, N.; Boonyamalik, P.; Kakhong, B.; et al. Effects of public health interventions on the epidemiological spread during the first wave of the COVID-19 outbreak in Thailand. PLoS ONE 2021, 16, e0246274. [Google Scholar] [CrossRef]
- Sila, T.; Sunghan, J.; Laochareonsuk, W.; Surasombatpattana, S.; Kongkamol, C.; Ingviya, T.; Siripaitoon, P.; Kositpantawong, N.; Kanchanasuwan, S.; Hortiwakul, T.; et al. Suspected Cat-to-Human Transmission of SARS-CoV-2, Thailand, July–September 2021. Emerg. Infect. Dis. J. 2022, 28, 1485. [Google Scholar] [CrossRef]
- Allendorf, V.; Denzin, N.; Conraths, F.J.; Boden, L.A.; Elvinger, F.; Magouras, I.; Stegeman, A.; Wood, J.L.N.; Urueña, A.C.; Grace, K.E.F.; et al. Does having a cat in your house increase your risk of catching COVID-19? One Health 2022, 14, 100381. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Trujillo, J.D.; Carossino, M.; Meekins, D.A.; Morozov, I.; Madden, D.W.; Indran, S.V.; Bold, D.; Balaraman, V.; Kwon, T.; et al. SARS-CoV-2 infection, disease and transmission in domestic cats. Emerg. Microbes Infect. 2020, 9, 2322–2332. [Google Scholar] [CrossRef]
- Doliff, R.; Martens, P. Cats and SARS-CoV-2: A Scoping Review. Animals 2022, 12, 1413. [Google Scholar] [CrossRef]
- Animals and COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html (accessed on 10 January 2024).
- GUIDELINE for SARS-Coronovirus (CoV)-2 and Cats. Available online: https://www.abcdcatsvets.org/guideline-for-sars-coronavirus-cov-2-and-cats/ (accessed on 1 February 2024).
- Piewbang, C.; Poonsin, P.; Lohavicharn, P.; Wardhani Sabrina, W.; Dankaona, W.; Puenpa, J.; Poovorawan, Y.; Techangamsuwan, S. SARS-CoV-2 Transmission from Human to Pet and Suspected Transmission from Pet to Human, Thailand. J. Clin. Microbiol. 2022, 60, e01058-22. [Google Scholar] [CrossRef]
- Dileepan, M.; Di, D.; Huang, Q.; Ahmed, S.; Heinrich, D.; Ly, H.; Liang, Y. Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence 2021, 12, 1597–1609. [Google Scholar] [CrossRef]
- Jairak, W.; Charoenkul, K.; Chamsai, E.; Udom, K.; Chaiyawong, S.; Bunpapong, N.; Boonyapisitsopa, S.; Tantilertcharoen, R.; Techakriengkrai, N.; Surachetpong, S.; et al. First cases of SARS-CoV-2 infection in dogs and cats in Thailand. Transbound. Emerg. Dis. 2022, 69, e979–e991. [Google Scholar] [CrossRef]
- Panzera, Y.; Mirazo, S.; Baz, M.; Techera, C.; Grecco, S.; Cancela, F.; Fuques, E.; Condon, E.; Calleros, L.; Camilo, N.; et al. Detection and genome characterisation of SARS-CoV-2 P.6 lineage in dogs and cats living with Uruguayan COVID-19 patients. Mem. Inst. Oswaldo Cruz 2022, 117, e220177. [Google Scholar]
- Antia, R.; Halloran, M.E. Transition to endemicity: Understanding COVID-19. Immunity 2021, 54, 2172–2176. [Google Scholar] [CrossRef]
- Kost, G.J. Diagnostic Strategies for Endemic Coronavirus Disease 2019 (COVID-19): Rapid Antigen Tests, Repeated Testing, and Prevalence Boundaries. Arch. Pathol. Lab. Med. 2021, 146, 16–25. [Google Scholar] [CrossRef]
- Sidiq, Z.; Hanif, M.; Dwivedi, K.K.; Chopra, K.K. Benefits and limitations of serological assays in COVID-19 infection. Indian J. Tuberc. 2020, 67, S163–S166. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 2020, CD013652. [Google Scholar] [CrossRef]
- Udom, K.; Jairak, W.; Chamsai, E.; Charoenkul, K.; Boonyapisitsopa, S.; Bunpapong, N.; Techakriengkrai, N.; Amonsin, A. Serological survey of antibodies against SARS-CoV-2 in dogs and cats, Thailand. Transbound. Emerg. Dis. 2022, 69, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Schuurman, N.; Li, W.; Wang, C.; Smit, L.A.M.; Broens, E.; Wagenaar, J.; van Kuppeveld, F.J.M.; Bosch, B.-J.; Egberink, H. Serologic Screening of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Cats and Dogs during First Coronavirus Disease Wave, the Netherlands. Emerg. Infect. Dis. J. 2021, 27, 1362. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.E.; Vizard, A.L. Determination of the optimal cutoff value for a serological assay: An example using the Johne’s Absorbed EIA. J. Clin. Microbiol. 1993, 31, 1256–1261. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- COVID-19 Infected Situation Reports. Available online: https://covid19.ddc.moph.go.th (accessed on 10 January 2024).
- Kannekens-Jager, M.M.; de Rooij, M.M.T.; de Groot, Y.; Biesbroeck, E.; de Jong, M.K.; Pijnacker, T.; Smit, L.A.M.; Schuurman, N.; Broekhuizen-Stins, M.J.; Zhao, S.; et al. SARS-CoV-2 infection in dogs and cats is associated with contact to COVID-19-positive household members. Transbound. Emerg. Dis. 2022, 69, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Michelitsch, A.; Schön, J.; Hoffmann, D.; Beer, M.; Wernike, K. The Second Wave of SARS-CoV-2 Circulation—Antibody Detection in the Domestic Cat Population in Germany. Viruses 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Suphanchaimat, R.; Teekasap, P.; Nittayasoot, N.; Phaiyarom, M.; Cetthakrikul, N. Forecasted Trends of the New COVID-19 Epidemic Due to the Omicron Variant in Thailand, 2022. Vaccines 2022, 10, 1024. [Google Scholar] [CrossRef]
- Sánchez-Morales, L.; Sánchez-Vizcaíno, J.M.; Pérez-Sancho, M.; Domínguez, L.; Barroso-Arévalo, S. The Omicron (B.1.1.529) SARS-CoV-2 variant of concern also affects companion animals. Front. Vet. Sci. 2022, 9, 940710. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Saied, A.A.; Tiwari, R.; Dhama, K. SARS-CoV-2 infection in domestic and feral cats: Current evidence and implications. Vet. Q. 2021, 41, 228–231. [Google Scholar] [CrossRef]
- What You Should Know about COVID-19 and Pets. Available online: https://www.cdc.gov/coronavirus/2019-ncov/downloads/covid-19-pets-prevention.pdf (accessed on 10 January 2024).
- Choi, J.Y.; Smith, D.M. SARS-CoV-2 Variants of Concern. Yonsei Med. J. 2021, 62, 961–968. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Torjesen, I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ 2021, 375, n2943. [Google Scholar] [CrossRef]
- Klein, C.; Michelitsch, A.; Allendorf, V.; Conraths, F.J.; Beer, M.; Denzin, N.; Wernike, K. Dogs and Cats Are Less Susceptible to the Omicron Variant of Concern of SARS-CoV-2: A Field Study in Germany, 2021/2022. Transbound. Emerg. Dis. 2023, 2023, 1868732. [Google Scholar] [CrossRef]
- Saied, A.A.; Metwally, A.A. SARS-CoV-2 variants of concerns in animals: An unmonitored rising health threat. Virus Dis. 2022, 33, 466–476. [Google Scholar] [CrossRef]
- Halaji, M.; Heiat, M.; Faraji, N.; Ranjbar, R. Epidemiology of COVID-19: An updated review. J. Res. Med. Sci. 2021, 26, 82. [Google Scholar]
- Kerner, G.; Quintana-Murci, L. The genetic and evolutionary determinants of COVID-19 susceptibility. Eur. J. Hum. Genet. 2022, 30, 915–921. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Falfán-Valencia, R. Genetics Insight for COVID-19 Susceptibility and Severity: A Review. Front. Immunol. 2021, 12, 622176. [Google Scholar] [CrossRef]
- Wongcha-um, P. Thailand Expands Lockdown Areas as COVID-19 Cases Surge. Available online: https://www.reuters.com/world/asia-pacific/thailand-expands-lockdown-areas-covid-19-cases-surge-2021-07-18/ (accessed on 10 January 2024).
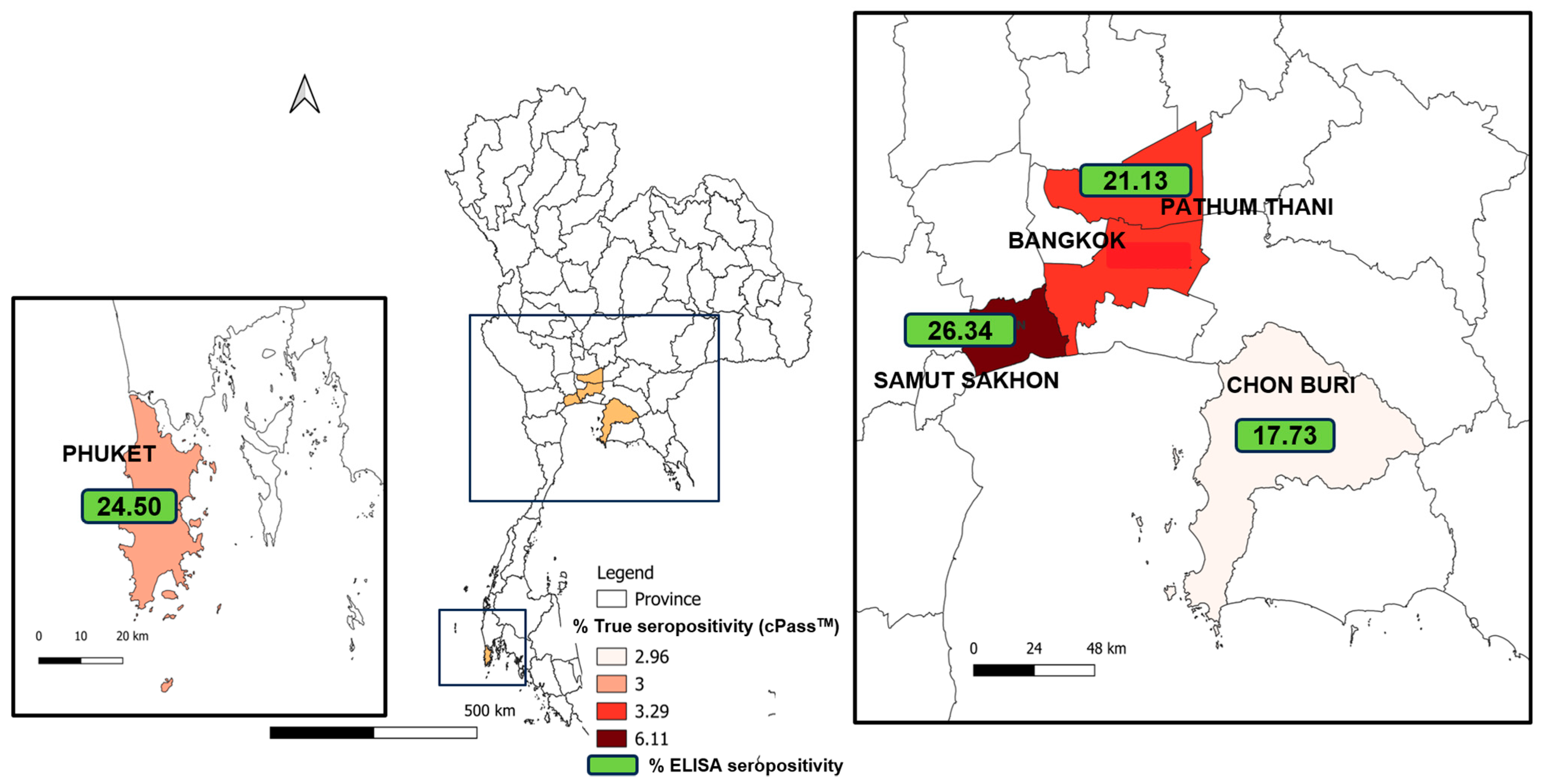
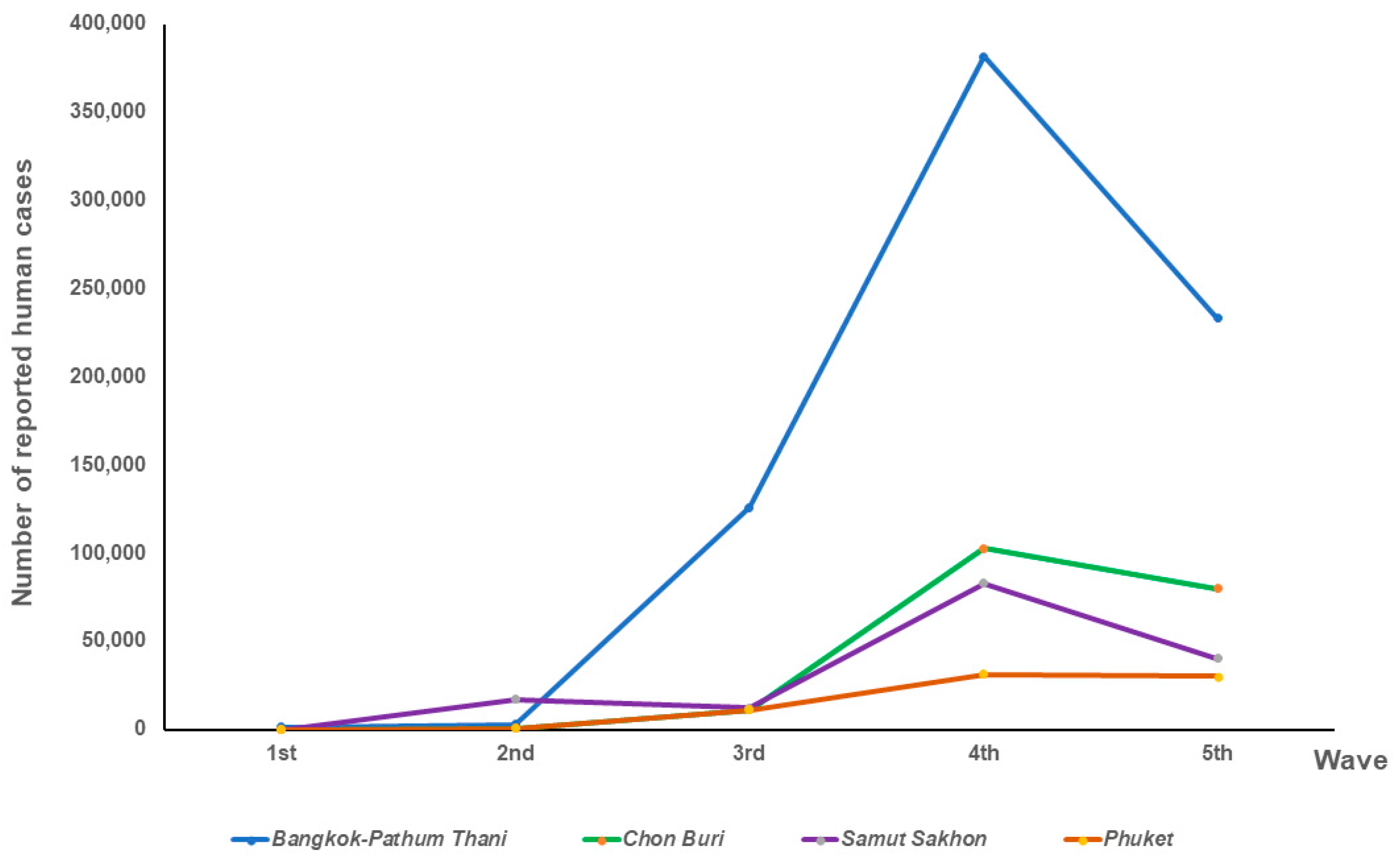

| Location | Epidemic Wave | No. of Cat Serum Samples | No. of Positive ELISA | Percentage of Positive Samples by ELISA | No. of Positive cPass Tests | Percentage of Positive Samples by cPass Test | No. of Reported Human Cases | Human Cases/ 100,000 Population |
|---|---|---|---|---|---|---|---|---|
| Bangkok–Pathum Thani | 1st | 0 | 0 | 0 | 0 | 0 | 1336 | 19.99 |
| 2nd | 16 | 2 | 12.50 | 0 | 0 | 2943 | 44.38 | |
| 3rd | 46 | 7 | 15.22 | 1 | 2.17 | 125,788 | 1896.80 | |
| 4th | 150 | 36 | 24.00 | 6 | 4.00 | 381,766 | 5756.77 | |
| 5th | 1 | 0 | 0 | 0 | 0 | 233,372 | 3519.09 | |
| Total | 213 | 45 | 21.13 | 7 | 3.29 | 745,205 | 11,237.18 | |
| Chon Buri | 1st | 0 | 0 | 0 | 0 | 0 | 71 | 45.92 |
| 2nd | 5 | 0 | 0 | 0 | 0 | 662 | 42.61 | |
| 3rd | 4 | 0 | 0 | 0 | 0 | 11,254 | 724.40 | |
| 4th | 48 | 19 | 39.58 | 1 | 2.08 | 102,794 | 6616.67 | |
| 5th | 146 | 17 | 11.64 | 5 | 3.42 | 80,052 | 5152.81 | |
| Total | 203 | 36 | 17.73 | 6 | 2.96 | 194,833 | 12,541.06 | |
| Samut Sakhon | 1st | 0 | 0 | 0 | 0 | 0 | 14 | 2.54 |
| 2nd | 0 | 0 | 0 | 0 | 0 | 17,109 | 3100.20 | |
| 3rd | 8 | 1 | 12.50 | 0 | 0 | 12,407 | 2248.19 | |
| 4th | 212 | 55 | 25.94 | 15 | 7.08 | 82,921 | 15,025.54 | |
| 5th | 42 | 13 | 30.95 | 1 | 2.38 | 40,194 | 7283.28 | |
| Total | 262 | 69 | 26.34 | 16 | 6.11 | 152,645 | 27,659.74 | |
| Phuket | 1st | 0 | 0 | 0 | 0 | 0 | 209 | 51.72 |
| 2nd | 3 | 0 | 0 | 0 | 0 | 3 | 0.74 | |
| 3rd | 23 | 10 | 43.48 | 0 | 0 | 769 | 189.77 | |
| 4th | 47 | 8 | 17.02 | 4 | 8.51 | 18,164 | 4482.34 | |
| 5th | 127 | 31 | 24.41 | 2 | 1.57 | 38,414 | 9479.44 | |
| Total | 200 | 49 | 24.50 | 6 | 3.00 | 57,559 | 14,203.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongyuan, S.; Thanongsaksrikul, J.; Srimanote, P.; Phongphaew, W.; Eiamcharoen, P.; Thengchaisri, N.; Bosco-Lauth, A.; Decaro, N.; Yodsheewan, R. Seroprevalence of Anti-SARS-CoV-2 Antibodies in Cats during Five Waves of COVID-19 Epidemic in Thailand and Correlation with Human Outbreaks. Animals 2024, 14, 761. https://doi.org/10.3390/ani14050761
Thongyuan S, Thanongsaksrikul J, Srimanote P, Phongphaew W, Eiamcharoen P, Thengchaisri N, Bosco-Lauth A, Decaro N, Yodsheewan R. Seroprevalence of Anti-SARS-CoV-2 Antibodies in Cats during Five Waves of COVID-19 Epidemic in Thailand and Correlation with Human Outbreaks. Animals. 2024; 14(5):761. https://doi.org/10.3390/ani14050761
Chicago/Turabian StyleThongyuan, Suporn, Jeeraphong Thanongsaksrikul, Potjanee Srimanote, Wallaya Phongphaew, Piyaporn Eiamcharoen, Naris Thengchaisri, Angela Bosco-Lauth, Nicola Decaro, and Rungrueang Yodsheewan. 2024. "Seroprevalence of Anti-SARS-CoV-2 Antibodies in Cats during Five Waves of COVID-19 Epidemic in Thailand and Correlation with Human Outbreaks" Animals 14, no. 5: 761. https://doi.org/10.3390/ani14050761
APA StyleThongyuan, S., Thanongsaksrikul, J., Srimanote, P., Phongphaew, W., Eiamcharoen, P., Thengchaisri, N., Bosco-Lauth, A., Decaro, N., & Yodsheewan, R. (2024). Seroprevalence of Anti-SARS-CoV-2 Antibodies in Cats during Five Waves of COVID-19 Epidemic in Thailand and Correlation with Human Outbreaks. Animals, 14(5), 761. https://doi.org/10.3390/ani14050761





