Dietary 25 Hydroxyvitamin D3 Improved Serum Concentration Level and Alkaline Phosphatase Activity during Lactation but Had Meager Impact on Post-Farrowing Reproductive Performance in Sows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Dietary Treatments
2.2. Feed Quality Control Analysis
2.3. Environment, Housing, and Management
2.4. Records and Measures
2.5. Blood Sample Collection and Biochemical Analysis
2.6. Alkaline Phosphatase Assay
2.7. Analysis of Serum 25(OH)D3 Concentration
2.8. Statistical Analysis
3. Results
3.1. Post-Farrowing Reproductive Performance
3.2. ALP
3.3. 25(OH)D3
4. Discussion
4.1. Post-Farrowing Reproductive Performance
4.2. ALP
4.3. 25(OH)D3
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foxcroft, G.; Patterson, J. Optimizing breeding management in a competitive world: Gilt and sow aspects. In Proceedings of the AASV 41st Annual Meeting, Omaha, NE, USA, 6–9 March 2010; pp. 6–9. [Google Scholar]
- Koketsu, Y.; Tani, S.; Iida, R. Factors for improving reproductive performance of sows and herd productivity in commercial breeding herds. Porc. Health Manag. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; Witschi, A.-K.; Wenk, C.; Martens, H. Triennial Growth Symposium—Effects of dietary 25-hydroxycholecalciferol and cholecalciferol on blood vitamin D and mineral status, bone turnover, milk composition, and reproductive performance of sows. J. Anim. Sci. 2014, 92, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, M.; Anderson, P.H.; Findlay, D.M.; Morris, H.A.; Atkins, G.J. The metabolism of 25-(OH) vitamin D3 by osteoclasts and their precursors regulates the differentiation of osteoclasts. J. Steroid Biochem. Mol. Biol. 2010, 121, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Wuryastuti, H.; Stowe, H.D.; Miller, E.R. The influence of gestational dietary calcium on serum 1, 25-dihydroxycholecalciferol in sows and their pigs. J. Anim. Sci. 1991, 69, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. Trennial Growth Symposium—Establishment of the 2012 vitamin D requirements in swine with focus on dietary forms and levels of vitamin D. J. Anim. Sci. 2014, 92, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C.; Halekoh, U.; Larsen, T.; Jensen, S.K. Reproductive performance and bone status markers of gilts and lactating sows supplemented with two different forms of vitamin D. J. Anim. Sci. 2010, 88, 202–213. [Google Scholar] [CrossRef]
- Coffey, J.; Hines, E.; Starkey, J.; Starkey, C.; Chung, T. Feeding 25-hydroxycholecalciferol improves gilt reproductive performance and fetal vitamin D status. J. Anim. Sci. 2012, 90, 3783–3788. [Google Scholar] [CrossRef] [PubMed]
- Flohr, J.R.; Tokach, M.D.; Dritz, S.S.; Derouchey, J.M.; Goodband, R.D.; Nelssen, J.L.; Henry, S.C.; Tokach, L.M.; Potter, M.L.; Goff, J.P.; et al. Effects of supplemental vitamin D3 on serum 25-hydroxycholecalciferol and growth of preweaning and nursery pigs. J. Anim. Sci. 2014, 92, 152–163. [Google Scholar] [CrossRef]
- Shaheen, S.; Noor, S.S.; Barakzai, Q. Serum alkaline phosphatase screening for vitamin D deficiency states. J. Coll. Physicians Surg. Pak. 2012, 22, 424–427. [Google Scholar]
- Khailova, L.; Robison, J.; Jaggers, J.; Ing, R.; Lawson, S.; Treece, A.; Soranno, D.; Osorio Lujan, S.; Davidson, J.A. Tissue alkaline phosphatase activity and expression in an experimental infant swine model of cardiopulmonary bypass with deep hypothermic circulatory arrest. J. Inflamm. 2020, 17, 27. [Google Scholar] [CrossRef]
- Krawitt, E.L.; Korson, R. Effect of vitamin D on brush border alkaline phosphatase in the rat small intestine. Enzyme 1972, 13, 278–285. [Google Scholar] [CrossRef]
- Kiran, B.; Prema, A.; Thilagavathi, R.; Rani, R.J. Serum 25-Hydroxy vitamin D, calcium, phosphorus and alkaline phosphatase levels in healthy adults above the age of 20 living in Potheri Village of Kancheepuram District, Tamilnadu. J. Appl. Pharm. Sci. 2014, 4, 030–034. [Google Scholar] [CrossRef][Green Version]
- McCarthy, R.A.; McCallion, N.; Molloy, E.J. Relationship Between Vitamin D and Alkaline Phosphatase Levels in Very Low Birth Weight (VLBW) Infants. Arch. Dis. Child. 2008, 93, ps315. [Google Scholar]
- Kamao, M.; Tatematsu, S.; Hatakeyama, S.; Sakaki, T.; Sawada, N.; Inouye, K.; Ozono, K.; Kubodera, N.; Reddy, G.S.; Okano, T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J. Biol. Chem. 2004, 279, 15897–15907. [Google Scholar] [CrossRef] [PubMed]
- Molnar, F.; Sigueiro, R.; Sato, Y.; Araujo, C.; Schuster, I.; Antony, P.; Peluso, J.; Muller, C.; Mourino, A.; Moras, D.; et al. 1alpha,25(OH)2-3-epi-vitamin D3, a natural physiological metabolite of vitamin D3: Its synthesis, biological activity and crystal structure with its receptor. PLoS ONE 2011, 6, e18124. [Google Scholar] [CrossRef] [PubMed]
- Lensmeyer, G.; Poquette, M.; Wiebe, D.; Binkley, N. The C-3 Epimer of 25-Hydroxyvitamin D3 Is Present in Adult Serum. J. Clin. Endocrinol. Metab. 2012, 97, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.F.; Trankle, W.G.; Norman, A.W.; Okamura, W.H. 14-Epi Stereoisomers of 25-Hydroxy-and 1. alpha., 25-Dihydroxyvitamin D3: Synthesis, Isomerization to Previtamins, and Biological Studies. J. Med. Chem. 1994, 37, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Liu, W.; Huang, L.; Cheng, L.; Xu, Z. Efficient biotransformation of vitamin D 3 to 25-hydroxyvitamin D 3 by a newly isolated Bacillus cereus strain. Appl. Microbiol. Biotechnol. 2020, 104, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Guy, E.; Lieve, V.; Natacha, R.; Frank, C.; Pierre De, C.; Maurits, V.; Giuseppe, T.-V.; Dino, M.; Roger, B.; Annemieke, V. Superagonistic Action of 14-epi-Analogs of 1,25-Dihydroxyvitamin D Explained by Vitamin D Receptor-Coactivator Interaction. Mol. Pharmacol. 2005, 67, 1566. [Google Scholar] [CrossRef]
- Latimer, G.W.; International, A.O.A.C. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- ISO:9831; Animal Feeding Stuffs, Animal Products, and Faeces or Urine—Determination of Gross Calorific Value—Bomb Calorimeter Method. International Organization for Standardization: Geneve, Switzerland, 1998. Available online: https://www.iso.org/obp/ui/#iso:std:iso:9831:ed-1:v1:en (accessed on 22 January 2024).
- Cook, R.D.; Weisberg, S. Applied Regression including Computing and Graphics; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Hadley, W. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; p. 260. [Google Scholar] [CrossRef]
- Russell, L. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R package version 1.4.2; An alternative to least squares means, Am Stat. 2019. Available online: https://github.com/rvlenth/emmeans (accessed on 24 October 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Thekkoot, D.M.; Kemp, R.A.; Rothschild, M.F.; Plastow, G.S.; Dekkers, J.C. Estimation of genetic parameters for traits associated with reproduction, lactation, and efficiency in sows. J. Anim. Sci. 2016, 94, 4516–4529. [Google Scholar] [CrossRef]
- Piles, M.; MartÌ, J.; Reixach, J.; S·nchez, J.P. Genetic parameters of sow feed efficiency during lactation and its underlying traits in a Duroc population. Animal 2020, 14, 889–898. [Google Scholar] [CrossRef]
- Cabrera, R.A.; Boyd, R.D.; Jungst, S.B.; Wilson, E.R.; Johnston, M.E.; Vignes, J.L.; Odle, J. Impact of lactation length and piglet weaning weight on long-term growth and viability of progeny1,2. J. Anim. Sci. 2010, 88, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Hawe, S.J.; Scollan, N.; Gordon, A.; Magowan, E. Impact of sow lactation feed intake on the growth and suckling behavior of low and average birthweight pigs to 10 weeks of age. Transl. Anim. Sci. 2020, 4, 655–665. [Google Scholar] [CrossRef]
- Kilbride, A.L.; Mendl, M.; Statham, P.; Held, S.; Harris, M.; Marchant-Forde, J.N.; Booth, H.; Green, L.E. Risks associated with preweaning mortality in 855 litters on 39 commercial outdoor pig farms in England. Prev. Vet. Med. 2014, 117, 189–199. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Uddin, M.K.; Hasan, S.; Peltoniemi, O.; Oliviero, C. The effect of piglet vitality, birth order, and blood lactate on the piglet growth performances and preweaning survival. Porc. Health Manag. 2022, 8, 52. [Google Scholar] [CrossRef]
- Di Martino, G.; Stefani, A.L.; Lippi, G.; Gagliazzo, L.; McCormick, W.; Gabai, G.; Bonfanti, L. The degree of acceptability of swine blood values at increasing levels of hemolysis evaluated through visual inspection versus automated quantification. J. Vet. Diagn. 2015, 27, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Wubuli, A.; Gerlinger, C.; Reyer, H.; Oster, M.; Muráni, E.; Trakooljul, N.; Ponsuksili, S.; Wolf, P.; Wimmers, K. Reduced phosphorus intake throughout gestation and lactation of sows is mitigated by transcriptional adaptations in kidney and intestine. BMC Genom. 2020, 21, 626. [Google Scholar] [CrossRef] [PubMed]
- Golder, H.M.; McGrath, J.; Lean, I.J. Effect of 25-hydroxyvitamin D3 during prepartum transition and lactation on production, reproduction, and health of lactating dairy cows. J. Dairy Sci. 2021, 104, 5345–5374. [Google Scholar] [CrossRef]
- Kozai, M.; Yamamoto, H.; Ishiguro, M.; Harada, N.; Masuda, M.; Kagawa, T.; Takei, Y.; Otani, A.; Nakahashi, O.; Ikeda, S.; et al. Thyroid Hormones Decrease Plasma 1α,25-Dihydroxyvitamin D Levels Through Transcriptional Repression of the Renal 25-Hydroxyvitamin D3 1α-Hydroxylase Gene (CYP27B1). Endocrinology 2013, 154, 609–622. [Google Scholar] [CrossRef]
- Thayer, M.T.; Nelssen, J.L.; Langemeier, A.J.; Morton, J.M.; Gonzalez, J.M.; Kruger, S.R.; Ou, Z.; Makowski, A.J.; Bergstrom, J.R. The effects of maternal dietary supplementation of cholecalciferol (vitamin D3) and 25(OH)D3 on sow and progeny performance1. Transl. Anim. Sci. 2019, 3, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Y.; Lv, G.; Zhuo, Y.; Lin, Y.; Feng, B.; Fang, Z.; Che, L.; Li, J.; Xu, S.; et al. Improving maternal vitamin D status promotes prenatal and postnatal skeletal muscle development of pig offspring. Nutrition 2016, 32, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Hong, J.S.; Han, T.H.; Fang, L.H.; Chung, W.L.; Kim, Y.Y. Effects of dietary vitamin levels on physiological responses, blood profiles, and reproductive performance in gestating sows. J. Anim. Sci. Technol. 2019, 61, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Rodney, R.M.; Celi, P.; McGrath, J.J.; Golder, H.M.; Anderson, S.T.; McNeill, D.M.; Fraser, D.R.; Lean, I.J. Metabolic and production responses to calcidiol treatment in mid-lactation dairy cows. Anim. Prod. Sci. 2019, 59, 449–460. [Google Scholar] [CrossRef]
- Grez-Capdeville, M.; Crenshaw, T.D. Estimation of phosphorus requirements of sows based on 24-h urinary phosphorus excretion during gestation and lactation. Br. J. Nutr. 2022, 128, 377–388. [Google Scholar] [CrossRef]

| Treatments | Active Substance/Product | Mass (g)/Metric Ton of Feed | Dose per kg of Diet | Product Conc. IU/Metric Ton |
|---|---|---|---|---|
| T1 a/ | 500,000 | 4 | 2000 IU | 2,000,000 |
| T2 b/ | 69.7 mg | 360 | 25 μg/kg | 2,000,000 |
| T3 b/ | 12.5 g | 4 | 50 μg/kg | 2,000,000 |
| Ingredient, % | Gestating Sow | Lactating Sow |
|---|---|---|
| Broken rice | 10.00 | 35.00 |
| Tapioca meal (70%) | 30.00 | 5.00 |
| Rice barn | 23.29 | 15.00 |
| Wheat barn | 15.00 | 12.00 |
| Soybean oil | 1.99 | 5.70 |
| Soybean meal (45.5%) | 15.78 | 22.44 |
| L-lysine | 0.15 | 0.65 |
| DL-methionine | 0.08 | 0.13 |
| L-threonine | 0.07 | 0.09 |
| Monodicalcium phosphate | 1.08 | 1.41 |
| Calcium carbonate | 1.79 | 1.80 |
| Salt | 0.25 | 0.27 |
| Premix 1 | 0.50 | 0.50 |
| ¶ Optiphose® | 0.01 | 0.01 |
| Calculated nutrient composition, % | ||
| Metabolizable energy, MJ/kg | 12.34 | 13.81 |
| Crude protein | 14.00 | 17.50 |
| Crude fat | 6.22 | 8.79 |
| Crude fiber | 5.97 | 4.71 |
| Calcium | 1.15 | 1.15 |
| Total phosphorus | 0.86 | 0.85 |
| Available phosphorus | 0.41 | 0.45 |
| Sodium | 0.32 | 0.32 |
| Lysine | 0.80 | 1.36 |
| Methionine | 0.27 | 0.38 |
| Methionine + Cystine | 0.48 | 0.63 |
| Threonine | 0.53 | 0.68 |
| Tryptophan | 0.17 | 0.21 |
| Diet | Gestating Sow | Lactating Sow | ||||
|---|---|---|---|---|---|---|
| Treatment | T1 ‡ | T2 ‡ | T3 ‡ | T1 ‡ | T2 ‡ | T3 ‡ |
| Gross energy 1, MJ/kg | 18.77 | 19.31 | 19.40 | 18.02 | 17.67 | 17.61 |
| Crude protein, % | 15.30 | 15.37 | 15.09 | 19.49 | 18.79 | 18.93 |
| Ether extract, % | 7.78 | 8.26 | 7.85 | 12.54 | 11.78 | 12.23 |
| Crude fiber, % | 4.32 | 4.67 | 4.31 | 3.91 | 3.46 | 3.98 |
| Crude ash, % | 7.46 | 7.07 | 7.12 | 7.50 | 7.19 | 7.11 |
| Calcium 2, % | 1.29 | 1.22 | 1.18 | 1.39 | 1.35 | 1.27 |
| Phosphorus, % | 0.81 | 0.81 | 0.80 | 0.96 | 0.89 | 0.87 |
| Diet | Level (ng/g) | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| Gestation | <2.00 | 20.20 | 57.30 |
| Lactation | <2.00 | 22.70 | 50.00 |
| Parameter | T1 | T2 | T3 | p-Value |
|---|---|---|---|---|
| Mean ± SEM | ||||
| Gestation length, days | 117.93 ± 0.20 | 118.04 ± 0.20 | 118.41 ± 0.20 | 1/ 0.168 |
| Lactation length, days | 23.74 ± 0.21 b | 24.49 ± 0.22 a | 24.30 ± 0.22 ab | 1/ 0.039 * |
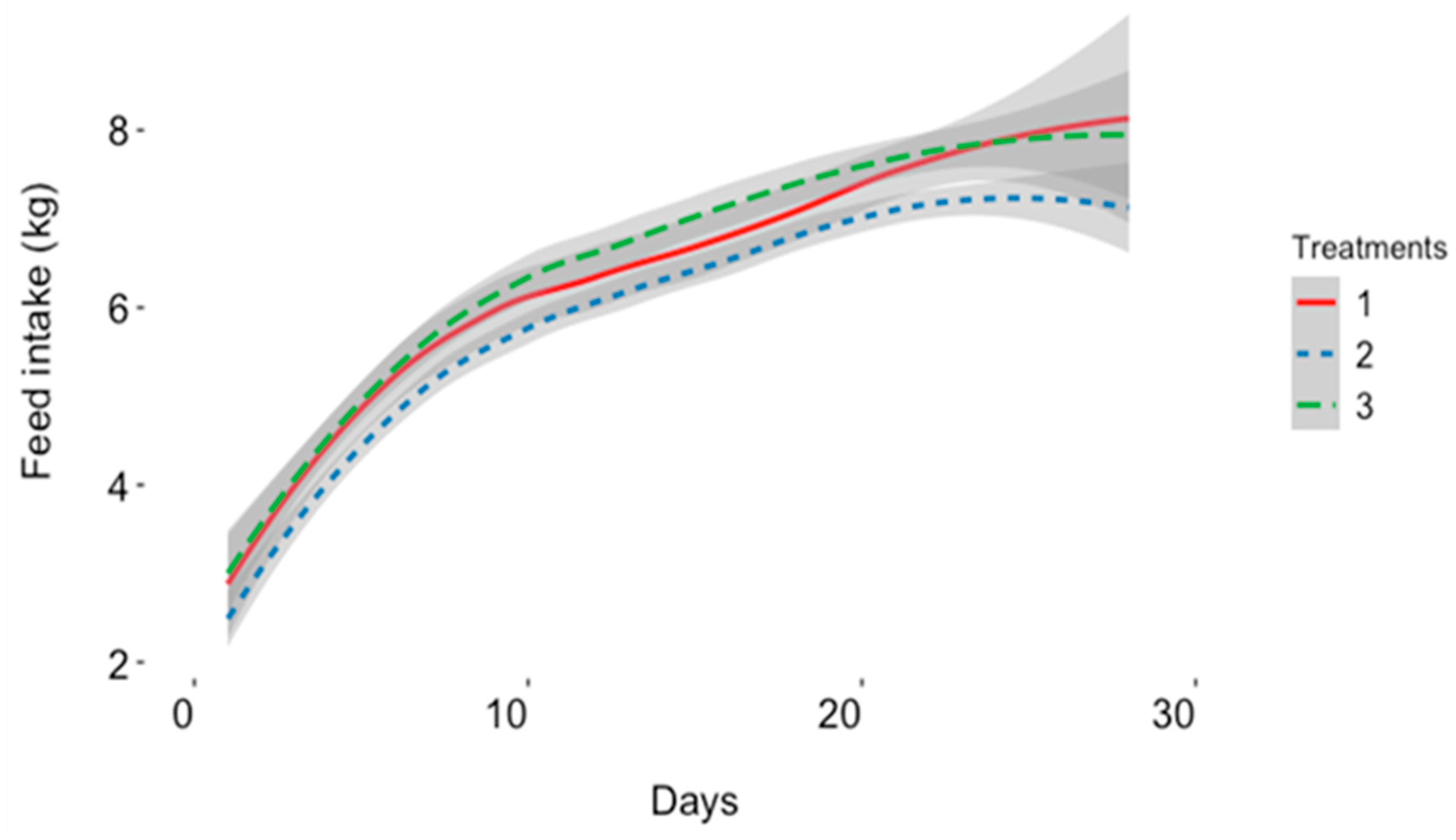
| LFI, kg/day | 6.37 ± 0.15 ab | 6.11 ± 0.14 b | 6.58 ± 0.14 a | 1/ 0.023 * |
| BW before farrowing, kg | 297.60 ± 2.79 | 298.25 ± 2.77 | 299.46 ± 2.85 | 1/ 0.895 |
| BW at weaning, kg | 257.23 ± 3.15 | 252.89 ± 3.26 | 260.59 ± 3.27 | 1/ 0.250 |
| BF before farrowing, kg | 18.35 ± 0.25 | 18.80 ± 0.25 | 18.65 ± 0.25 | 1/ 0.414 |
| BF at weaning, mm | 17.78 ± 0.24 | 17.33 ± 0.25 | 17.65 ± 0.25 | 1/ 0.421 |
| BW loss, % ¶ | 14.40 ± 0.57 | 14.84 ± 0.60 | 13.50 ± 0.60 | 1/ 0.104 |
| BF loss, % ¶ | 10.32 ± 1.28 | 11.99 ± 1.33 | 8.37 ± 1.34 | 1/ 0.156 |
| Total born, head | 15.73 ± 0.38 | 16.45 ± 0.37 | 15.72± 0.38 | 1/ 0.278 |
| Piglets BW, kg | 1.47 ± 0.03 | 1.40 ± 0.03 | 1.41 ± 0.03 | 1/ 0.140 |
| Piglets born alive, head | 14.15 ± 0.36 | 14.94 ± 0.35 | 14.67 ± 0.36 | 1/ 0.286 |
| Stillborn piglets, head | 1.61 ± 0.15 | 1.39 ± 0.15 | 1.13 ± 0.16 | 1/ 0.100 |
| Mummified piglets, head | 0.48 ± 0.09 | 0.66 ± 0.09 | 0.69 ± 0.09 | 1/ 0.228 |
| Pre-wean mortality, head | 0.81 ± 0.13 a | 1.26 ± 0.13 b | 0.84 ± 0.13 ab | 1/ 0.029 * |
| Weaned piglets, head | 11.42 ± 0.13 a | 10.97 ± 0.13 b | 11.39 ± 0.13 a | 1/ 0.029 * |
| Time of farrowing, hour | 4.39 ± 0.28 | 4.15 ± 0.25 | 4.16 ± 0.31 | 1/ 0.086 |
| Oxytocin dose, ml | 0.86 ± 0.13 | 1.05 ± 0.13 | 1.13 ± 0.14 | 1/ 0.294 |
| Litter fecal score, score | 1.44 ± 0.13 | 1.70± 0.13 | 1.41 ± 0.13 | 2/ 0.399 |
| Treatment Effects | ||||
|---|---|---|---|---|
| Parameters | T1 | T2 | T3 | p-Value |
| Mean ± SEM | ||||
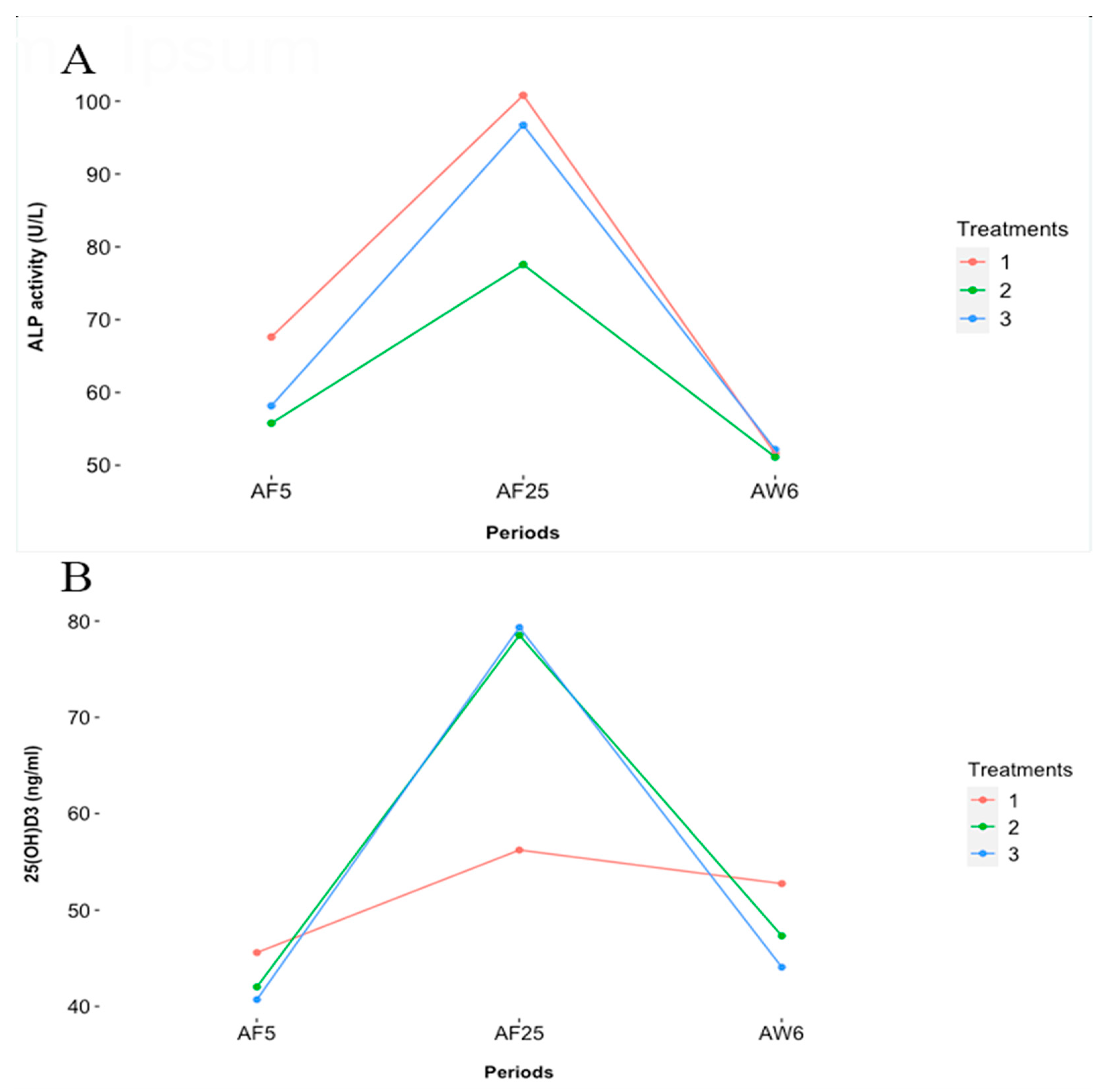
| ALP, U/L ‡ | 73.35 ± 10.02 | 61.49 ± 10.02 | 69.02 ± 10.36 | 1/ 0.820 |
| ALP AF5, U/L | 67.62 ± 6.81 | 55.78 ± 6.81 | 58.17 ± 7.04 | 2/ 0.527 |
| ALP AF25, U/L | 100.82 ± 10.86 | 77.58 ± 10.86 | 96.66 ± 11.24 | 2/ 0.865 |
| ALP AW6, U/L | 51.65 ± 4.70 | 51.15 ± 4.70 | 52.16 ± 4.86 | 2/ 0.805 |
| 25(OH)D3, ng/mL ‡ | 54.67 ± 9.37 | 61.96 ± 9.01 | 58.20 ± 8.98 | 1/ 0.880 |
| 25(OH)D3, AF5, ng/mL | 54.90 ± 7.84 | 49.69 ± 7.09 | 40.23 ± 7.52 | 2/ 0.724 |
| 25(OH)D3, AF25, ng/mL | 55.78 ± 11.83 | 80.86 ± 10.59 | 80.02 ± 10.53 | 2/ 0.219 |
| 25(OH)D3, AW6, ng/mL | 60.62 ± 9.14 | 48.55 ± 8.86 | 52.09 ± 7.97 | 2/ 0.637 |
| Period effects | ||||
| AF5 | AF25 | AW6 | ||
| ALP, U/L | 60.58 ± 6.14 a | 91.58 ± 6.14 b | 51.64 ± 6.14 c | 1/ <0.001 *** |
| 25(OH)D3, ng/mL | 42.59 ± 5.76 a | 72.33 ± 5.43 b | 47.75 ± 5.73 a | 1/ <0.001 *** |
| Treatment × Period | ||||
| ALP, U/L † | — | — | — | 1/ 0.028 * |
| 25(OH)D3, ng/mL † | — | — | — | 1/ 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okafor, P.C.J.; Homwong, N. Dietary 25 Hydroxyvitamin D3 Improved Serum Concentration Level and Alkaline Phosphatase Activity during Lactation but Had Meager Impact on Post-Farrowing Reproductive Performance in Sows. Animals 2024, 14, 419. https://doi.org/10.3390/ani14030419
Okafor PCJ, Homwong N. Dietary 25 Hydroxyvitamin D3 Improved Serum Concentration Level and Alkaline Phosphatase Activity during Lactation but Had Meager Impact on Post-Farrowing Reproductive Performance in Sows. Animals. 2024; 14(3):419. https://doi.org/10.3390/ani14030419
Chicago/Turabian StyleOkafor, Prester C. John, and Nitipong Homwong. 2024. "Dietary 25 Hydroxyvitamin D3 Improved Serum Concentration Level and Alkaline Phosphatase Activity during Lactation but Had Meager Impact on Post-Farrowing Reproductive Performance in Sows" Animals 14, no. 3: 419. https://doi.org/10.3390/ani14030419
APA StyleOkafor, P. C. J., & Homwong, N. (2024). Dietary 25 Hydroxyvitamin D3 Improved Serum Concentration Level and Alkaline Phosphatase Activity during Lactation but Had Meager Impact on Post-Farrowing Reproductive Performance in Sows. Animals, 14(3), 419. https://doi.org/10.3390/ani14030419







