Evaluation of Muscle Long Non-Coding RNA Profile during Rearing and Finishing Phase of Bulls Subjected to Different Prenatal Nutritional Strategies
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Ethics Statement
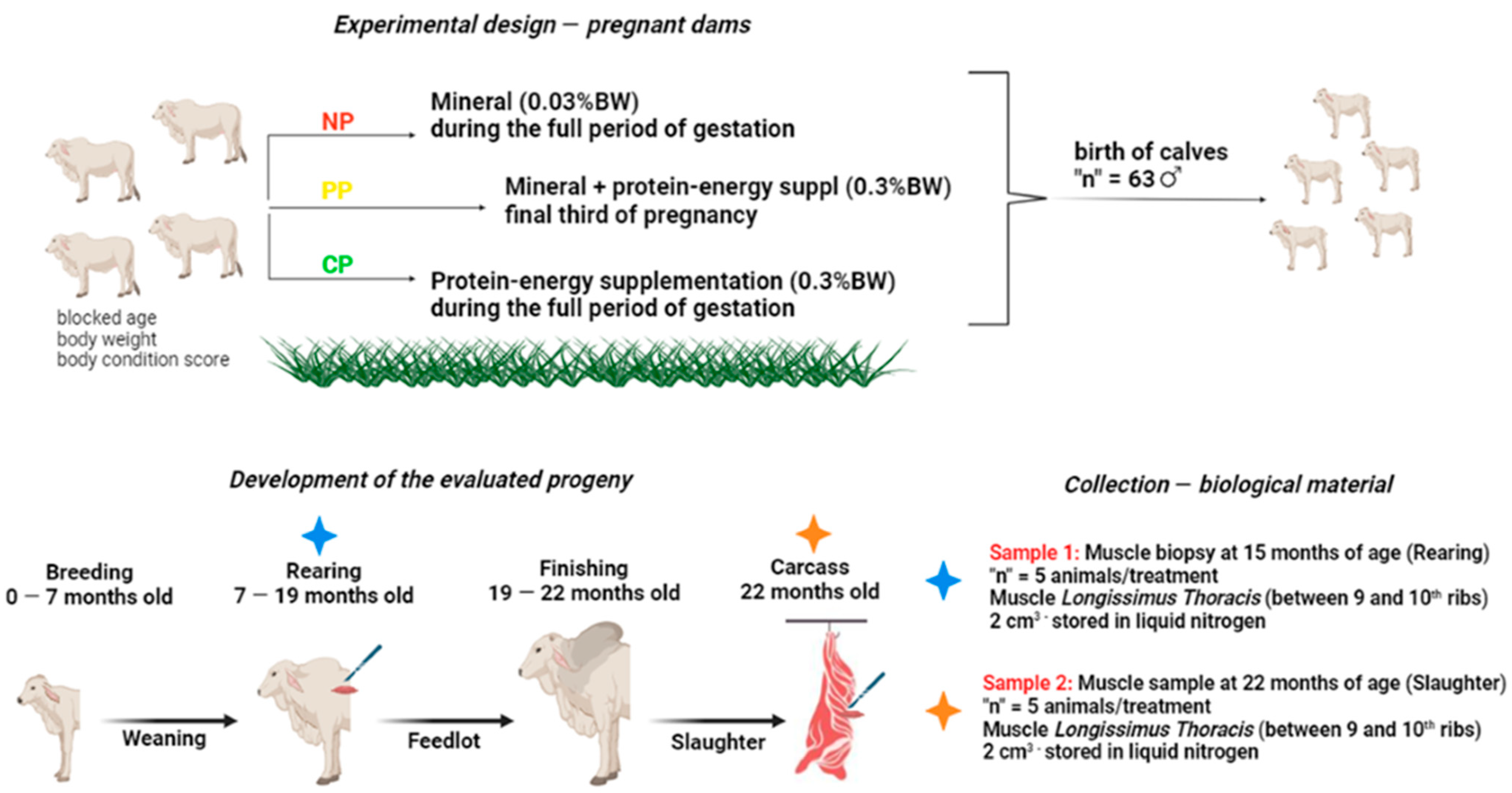
2.2. Experimental Design
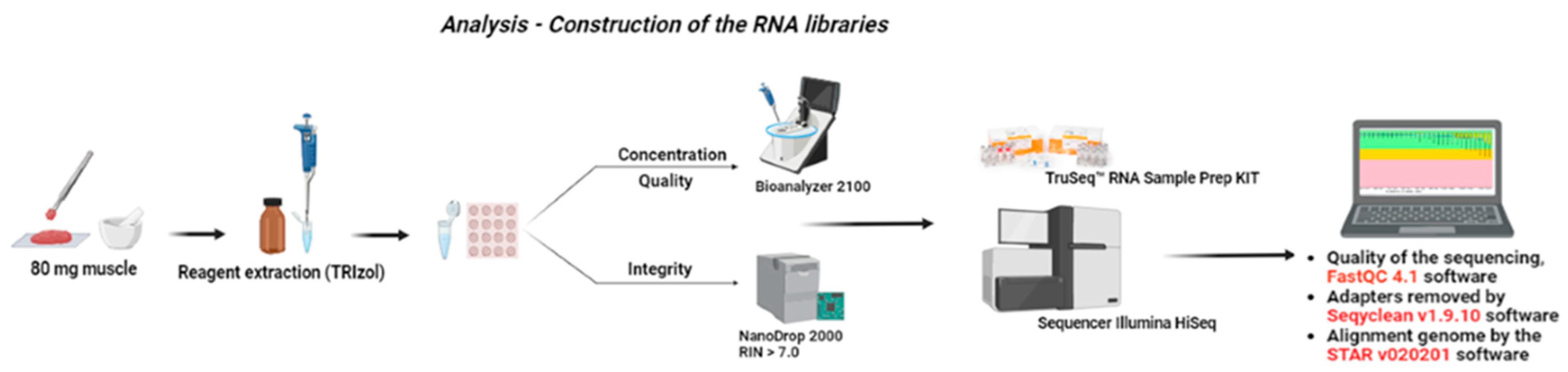
2.3. Sample Collection and RNA Extraction and Sequencing
2.4. Expression of Genes Related to Epigenetic Mechanisms
2.5. lncRNA Differential Expression
2.6. Regulatory Potential and Co-Expression Networks
3. Results
3.1. Differential Expression of Epigenetic Mechanism’s Genes
3.2. Identification of New lncRNA
3.3. Differentially Expressed lncRNA
3.4. lncRNA with Regulatory Potential
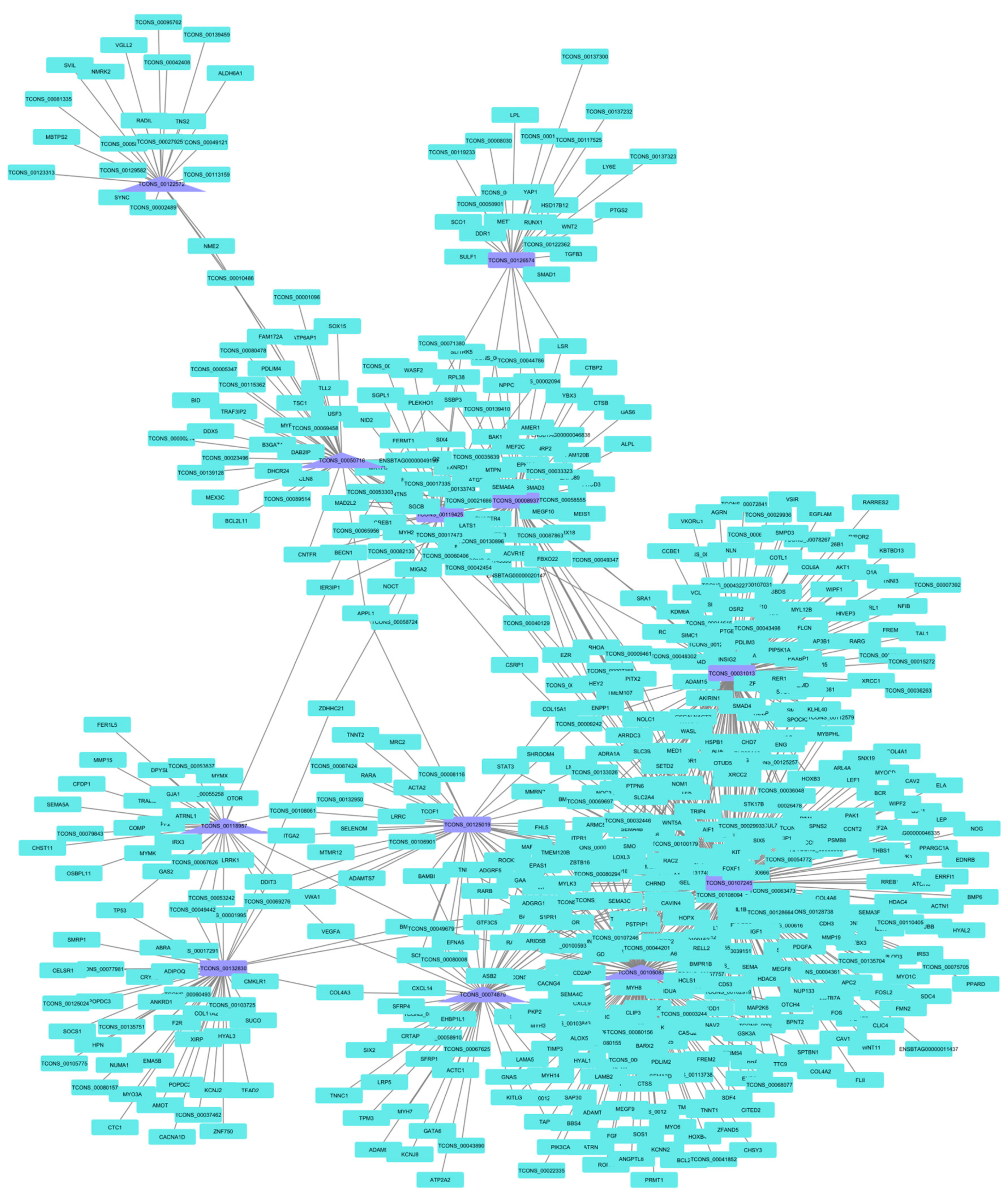
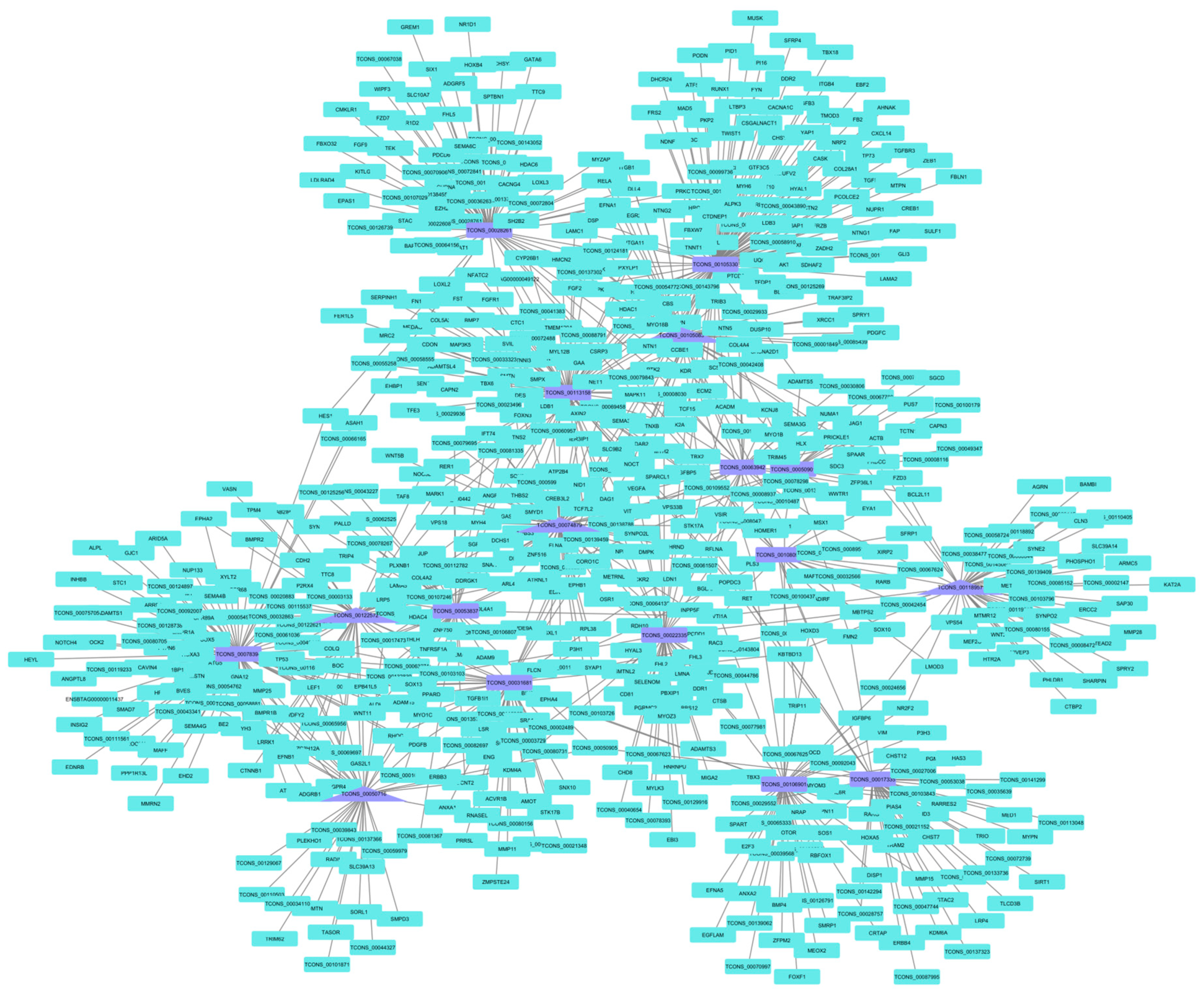
3.5. lncRNA Co-Expression Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noya, A.; Ripoll, G.; Casasús, I.; Sanz, A. Long-Term Effects of Early Maternal Undernutrition on the Growth, Physiological Profiles, Carcass and Meat Quality of Male Beef Offspring. Res. Vet. Sci. 2022, 142, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, M.A. Impact of Maternal Malnutrition during the Periconceptional Period on Mammalian Preimplantation Embryo Development. Domest. Anim. Endocrinol. 2015, 51, 27–45. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-Invited Review: Intrauterine Growth Retardation: Implications for the Animal Sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. Intrauterine Programming of Adult Disease. Mol. Med. Today 1995, 1, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Eisemann, J.H.; Currie, W.B. Hormonal Effects on Partitioning of Nutrients for Tissue Growth: Role of Growth Hormone and Prolactin. Fed. Proc. 1982, 41, 2538–2544. [Google Scholar] [PubMed]
- Close, W.H.; Pettigrew, J.E. Mathematical Models of Sow Reproduction. J. Reprod. Fertil. Suppl. 1990, 13, 83–88. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Means, W.J.; Hess, B.W.; Nathanielsz, P.W.; Du, M. Maternal Nutrient Restriction Affects Properties of Skeletal Muscle in Offspring. J. Physiol. 2006, 575, 241–250. [Google Scholar] [CrossRef]
- Glore, S.R.; Layman, D.K. Cellular Growth of Skeletal Muscle in Weanling Rats during Dietary Restrictions. Growth 1983, 47, 403–410. [Google Scholar]
- Greenwood, P.L.; Hunt, A.S.; Hermanson, J.W.; Bell, A.W. Effects of Birth Weight and Postnatal Nutrition on Neonatal Sheep: II. Skeletal Muscle Growth and Development. J. Anim. Sci. 2000, 78, 50–61. [Google Scholar] [CrossRef]
- Zhu, M.-J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Effect of Maternal Nutrient Restriction in Sheep on the Development of Fetal Skeletal Muscle. Biol. Reprod. 2004, 71, 1968–1973. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. Developmental Programming of Fetal Growth and Development. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 229–247. [Google Scholar] [CrossRef]
- Bollati, V.; Baccarelli, A. Environmental Epigenetics. Heredity 2010, 105, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Gabbianelli, R. Primers on Nutrigenetics and Nutri(Epi)Genomics: Origins and Development of Precision Nutrition. Biochimie 2019, 160, 156–171. [Google Scholar] [CrossRef]
- Şanlı, E.; Kabaran, S. Maternal Obesity, Maternal Overnutrition and Fetal Programming: Effects of Epigenetic Mechanisms on the Development of Metabolic Disorders. Curr. Genom. 2019, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Thayer, Z.M.; Rutherford, J.; Kuzawa, C.W. The Maternal Nutritional Buffering Model: An Evolutionary Framework for Pregnancy Nutritional Intervention. Evol. Med. Public Health 2020, 2020, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kuzawa, C.W. Fetal Origins of Developmental Plasticity: Are Fetal Cues Reliable Predictors of Future Nutritional Environments? Am. J. Hum. Biol. 2005, 17, 5–21. [Google Scholar] [CrossRef]
- Goyal, D.; Limesand, S.W.; Goyal, R.; Limesand, S.; Thornburg, K.; Harding, J. Epigenetic Responses and the Developmental Origins of Health and Disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Meissner, A.; Lander, E.S. The Mammalian Epigenome. Cell 2007, 128, 669–681. [Google Scholar] [CrossRef]
- Deniz, E.; Erman, B. Long Noncoding RNA (lincRNA), a New Paradigm in Gene Expression Control. Funct. Integr. Genom. 2017, 17, 135–143. [Google Scholar] [CrossRef]
- Guttman, M.; Amit, I.; Garber, M.; French, C.; Lin, M.F.; Feldser, D.; Huarte, M.; Zuk, O.; Carey, B.W.; Cassady, J.P.; et al. Chromatin Signature Reveals over a Thousand Highly Conserved Large Non-Coding RNAs in Mammals. Nature 2009, 458, 223–227. [Google Scholar] [CrossRef]
- Mercer, T.R.; Mattick, J.S. Structure and Function of Long Noncoding RNAs in Epigenetic Regulation. Nat. Struct. Mol. Biol. 2013, 20, 300–307. [Google Scholar] [CrossRef]
- Paradis, F.; Wood, K.M.; Swanson, K.C.; Miller, S.P.; McBride, B.W.; Fitzsimmons, C. Maternal Nutrient Restriction in Mid-to-Late Gestation Influences Fetal mRNA Expression in Muscle Tissues in Beef Cattle. BMC Genom. 2017, 18, 632. [Google Scholar] [CrossRef]
- Schalch Junior, F.J.; Polizel, G.H.G.; Cançado, F.A.C.Q.; Fernandes, A.C.; Mortari, I.; Pires, P.R.L.; Fukumasu, H.; Santana, M.H.D.A.; Saran Netto, A. Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves. Metabolites 2022, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Polizel, G.H.G.; de Francisco Strefezzi, R.; Cracco, R.C.; Fernandes, A.C.; Zuca, C.B.; Castellar, H.H.; Baldin, G.C.; de Almeida Santana, M.H. Effects of Different Maternal Nutrition Approaches on Weight Gain and on Adipose and Muscle Tissue Development of Young Bulls in the Rearing Phase. Trop. Anim. Health Prod. 2021, 53, 536. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Beline, M.; Polizel, G.H.G.; Cracco, R.C.; Dias, E.F.F.; Furlan, É.; Silva, S.D.L.; Santana, M.H.D.A. Fetal Programming and Its Effects on Meat Quality of Nellore Bulls. Vet. Sci. 2023, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Zhbannikov, I.Y.; Hunter, S.S.; Foster, J.A.; Settles, M.L. Seqyclean: A Pipeline for High-Throughput Sequence Data Preprocessing. In Proceedings of the 8th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics (ACM-BCB 2017), Boston, MA, USA, 20–23 August 2017; Volume 17, pp. 407–416. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Law, C.W.; Smyth, G.K.; Ritchie, M.E.; Alhamdoosh, M.; Su, S.; Dong, X.; Tian, L. RNA-Seq Analysis Is Easy as 1-2-3 with Limma, Glimma and edgeR. F1000Research 2018, 5, 1408. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential Analysis of Gene Regulation at Transcript Resolution with RNA-Seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A Fast and Accurate Coding Potential Calculator Based on Sequence Intrinsic Features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhang, L.; Guo, J.; Niu, Y.; Wu, Y.; Li, H.; Zhao, L.; Li, X.; Teng, X.; Sun, X.; et al. NONCODEV5: A Comprehensive Annotation Database for Long Non-Coding RNAs. Nucleic Acids Res. 2018, 46, D308–D314. [Google Scholar] [CrossRef]
- Alexandre, P.A.; Reverter, A.; Berezin, R.B.; Porto-Neto, L.R.; Ribeiro, G.; Santana, M.H.A.; Ferraz, J.B.S.; Fukumasu, H. Exploring the Regulatory Potential of Long Non-Coding RNA in Feed Efficiency of Indicine Cattle. Genes 2020, 11, 997. [Google Scholar] [CrossRef]
- Blake, J.A.; Christie, K.R.; Dolan, M.E.; Drabkin, H.J.; Hill, D.P.; Ni, L.; Sitnikov, D.; Burgess, S.; Buza, T.; Gresham, C.; et al. Gene Ontology Consortium: Going Forward. Nucleic Acids Res. 2015, 43, D1049–D1056. [Google Scholar] [CrossRef]
- Reverter, A.; Hudson, N.J.; Nagaraj, S.H.; Pérez-Enciso, M.; Dalrymple, B.P. Regulatory Impact Factors: Unraveling the Transcriptional Regulation of Complex Traits from Expression Data. Bioinformatics 2010, 26, 896–904. [Google Scholar] [CrossRef]
- Reverter, A.; Chan, E.K.F. Combining Partial Correlation and an Information Theory Approach to the Reversed Engineering of Gene Co-Expression Networks. Bioinform. Orig. Pap. 2008, 24, 2491–2497. [Google Scholar] [CrossRef]
- Watson-Haigh, N.S.; Kadarmideen, H.N.; Reverter, A. PCIT: An R Package for Weighted Gene Co-Expression Networks Based on Partial Correlation and Information Theory Approaches. Bioinformatics 2010, 26, 411–413. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.; Li, Y.; Song, T.; Wu, Y.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Pei, D.; et al. NONCODEV6: An Updated Database Dedicated to Long Non-Coding RNA Annotation in Both Animals and Plants. Nucleic Acids Res. 2021, 49, D165–D171. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.A.; Raja, J.S.; Hoffman, M.L.; Zinn, S.A.; Govoni, K.E. Poor Maternal Nutrition Inhibits Muscle Development in Ovine Offspring. J. Anim. Sci. Biotechnol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.C.; Du, M.; Nascimento, K.B.; Galvão, M.C.; Meneses, J.A.M.; Schultz, E.B.; Gionbelli, M.P.; Duarte, M.D.S. Skeletal Muscle Development in Postnatal Beef Cattle Resulting from Maternal Protein Restriction during Mid-Gestation. Animals 2021, 11, 860. [Google Scholar] [CrossRef] [PubMed]
- Maresca, S.; Valiente, S.L.; Rodriguez, A.M.; Pavan, E.; Quintans, G.; Long, N.M. Late-Gestation Protein Restriction Negatively Impacts Muscle Growth and Glucose Regulation in Steer Progeny. Domest. Anim. Endocrinol. 2019, 69, 13–18. [Google Scholar] [CrossRef]
- Mulliniks, J.T.; Sawyer, J.E.; Harrelson, F.W.; Mathis, C.P.; Cox, S.H.; Löest, C.A.; Petersen, M.K.; Mulliniks, J.T.; Sawyer, J.E.; Harrelson, F.W.; et al. Effect of Late Gestation Bodyweight Change and Condition Score on Progeny Feedlot Performance. Anim. Prod. Sci. 2015, 56, 1998–2003. [Google Scholar] [CrossRef]
- Mohrhauser, D.A.; Taylor, A.R.; Underwood, K.R.; Pritchard, R.H.; Wertz-Lutz, A.E.; Blair, A.D. The Influence of Maternal Energy Status during Midgestation on Beef Offspring Carcass Characteristics and Meat Quality. J. Anim. Sci. 2015, 93, 786–793. [Google Scholar] [CrossRef]
- Piaggio, L.; Quintans, G.; San Julián, R.; Ferreira, G.; Ithurralde, J.; Fierro, S.; Pereira, A.S.C.; Baldi, F.; Banchero, G.E. Growth, Meat and Feed Efficiency Traits of Lambs Born to Ewes Submitted to Energy Restriction during Mid-Gestation. Animal 2018, 12, 256–264. [Google Scholar] [CrossRef]
- Acton, K.; Mandell, I.B.; Huber, L.-A.; Steele, M.A.; Wood, K.M. PSIX-5 Fetal Programming in an Industry Applied Setting–Effects of Feeding Methionine during Late Gestation on Progeny Performance, Feed Efficiency, and Carcass Quality for Feedlot Steers. J. Anim. Sci. 2020, 98, 411–412. [Google Scholar] [CrossRef]
- Acton, K.; Mandell, I.B.; Huber, L.-A.; Steele, M.A.; Wood, K.M. PSIX-4 Fetal Programming–Maternal Plane of Nutrition Effects on Progeny Performance, Feed Efficiency, and Carcass Quality for Feedlot Steers. J. Anim. Sci. 2020, 98, 411. [Google Scholar] [CrossRef]
- Long, N.M.; Prado-Cooper, M.J.; Krehbiel, C.R.; DeSilva, U.; Wettemann, R.P. Effects of Nutrient Restriction of Bovine Dams during Early Gestation on Postnatal Growth, Carcass and Organ Characteristics, and Gene Expression in Adipose Tissue and Muscle. J. Anim. Sci. 2010, 88, 3251–3261. [Google Scholar] [CrossRef]
- Wilson, T.B.; Faulkner, D.B.; Shike, D.W. Influence of Prepartum Dietary Energy on Beef Cow Performance and Calf Growth and Carcass Characteristics. Livest. Sci. 2016, 184, 21–27. [Google Scholar] [CrossRef]
- Block, J.J.; Blair, A.D.; Funston, R.N.; Webb, M.J.; Underwood, K.R.; Gonda, M.G.; Harty, A.A.; Salverson, R.R.; Olson, K.C. Influence of Maternal Protein Restriction in Primiparous Heifers during Mid- and/or Late Gestation on Progeny Feedlot Performance and Carcass Characteristics. Animals 2022, 12, 588. [Google Scholar] [CrossRef]
- Oattes, J.L.; Shao, T.; Henley, P.A.; Shike, D.W. Fetal Programming Effects of Early Weaning on Subsequent Parity Calf Performance. Transl. Anim. Sci. 2021, 5, txab049. [Google Scholar] [CrossRef]
- Stalker, L.A.; Adams, D.C.; Klopfenstein, T.J.; Feuz, D.M.; Funston, R.N. Effects of Pre- and Postpartum Nutrition on Reproduction in Spring Calving Cows and Calf Feedlot Performance. J. Anim. Sci. 2006, 84, 2582–2589. [Google Scholar] [CrossRef]
- Cracco, R.C.; Ruy, I.M.; Polizel, G.H.G.; Fernandes, A.C.; Furlan, É.; Baldin, G.C.; Santos, G.E.C.; Santana, M.H.D.A. Evaluation of Maternal Nutrition Effects in the Lifelong Performance of Male Beef Cattle Offspring. Vet. Sci. 2023, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental Epigenomics and Disease Susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.P.; Al-Hasan, Y. Impact of Oxidative Stress in Fetal Programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Lan, X.; Cretney, E.C.; Kropp, J.; Khateeb, K.; Berg, M.A.; Peñagaricano, F.; Magness, R.; Radunz, A.E.; Khatib, H. Maternal Diet during Pregnancy Induces Gene Expression and DNA Methylation Changes in Fetal Tissues in Sheep. Front. Genet. 2013, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.S.; Paulino, P.V.R.; Nascimento, C.S.; Botelho, M.E.; Martins, T.S.; Filho, S.C.V.; Guimarães, S.E.F.; Serão, N.V.L.; Dodson, M.V.; Du, M.; et al. Maternal Overnutrition Enhances mRNA Expression of Adipogenic Markers and Collagen Deposition in Skeletal Muscle of Beef Cattle Fetuses. J. Anim. Sci. 2014, 92, 3846–3854. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, M.-J.; Dodson, M.V.; Du, M. Developmental Programming of Fetal Skeletal Muscle and Adipose Tissue Development. J. Genom. 2013, 1, 29. [Google Scholar] [CrossRef]
- Costa, T.C.; Gionbelli, M.P.; Duarte, M.D.S. Fetal Programming in Ruminant Animals: Understanding the Skeletal Muscle Development to Improve Meat Quality. Anim. Front. 2021, 11, 66–73. [Google Scholar] [CrossRef]
- Batista, E.O.S.; Cardoso, B.O.; Oliveira, M.L.; Cuadros, F.D.C.; Mello, B.P.; Sponchiado, M.; Monteiro, B.M.; Pugliesi, G.; Binelli, M. Supplemental Progesterone Induces Temporal Changes in Luteal Development and Endometrial Transcription in Beef Cattle. Domest. Anim. Endocrinol. 2019, 68, 126–134. [Google Scholar] [CrossRef]
- Du, M.; Wang, B.; Fu, X.; Yang, Q.; Zhu, M.J. Fetal Programming in Meat Production. Meat Sci. 2015, 109, 40–47. [Google Scholar] [CrossRef]
- al Aboud, N.; Tupper, C.; Jialal, I. Genetics, Epigenetic Mechanism; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Glendining, K.A.; Jasoni, C.L. Maternal High Fat Diet-Induced Obesity Modifies Histone Binding and Expression of Oxtr in Offspring Hippocampus in a Sex-Specific Manner. Int. J. Mol. Sci. 2019, 20, 329. [Google Scholar] [CrossRef]
- Yang, K.; Cai, W.; Xu, J.L.; Shi, W. Maternal High-Fat Diet Programs Wnt Genes through Histone Modification in the Liver of Neonatal Rats. J. Mol. Endocrinol. 2012, 49, 107–114. [Google Scholar] [CrossRef]
- Blin, G.; Liand, M.; Mauduit, C.; Chehade, H.; Benahmed, M.; Simeoni, U.; Siddeek, B. Maternal Exposure to High-Fat Diet Induces Long-Term Derepressive Chromatin Marks in the Heart. Nutrients 2020, 12, 181. [Google Scholar] [CrossRef]
- Bekdash, R.A. Early Life Nutrition and Mental Health: The Role of DNA Methylation. Nutrients 2021, 13, 3111. [Google Scholar] [CrossRef]
- Lecorguillé, M.; Teo, S.; Phillips, C.M. Maternal Dietary Quality and Dietary Inflammation Associations with Offspring Growth, Placental Development, and DNA Methylation. Nutrients 2021, 13, 3130. [Google Scholar] [CrossRef]
- Keleher, M.R.; Zaidi, R.; Shah, S.; Oakley, M.E.; Pavlatos, C.; El Idrissi, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. Maternal High-Fat Diet Associated with Altered Gene Expression, DNA Methylation, and Obesity Risk in Mouse Offspring. PLoS ONE 2018, 13, e0192606. [Google Scholar] [CrossRef]
- Liew, L.C.; Singh, M.B.; Bhalla, P.L. An RNA-Seq Transcriptome Analysis of Histone Modifiers and RNA Silencing Genes in Soybean during Floral Initiation Process. PLoS ONE 2013, 8, e77502. [Google Scholar] [CrossRef]
- Scott, E.Y.; Mansour, T.; Bellone, R.R.; Brown, C.T.; Mienaltowski, M.J.; Penedo, M.C.; Ross, P.J.; Valberg, S.J.; Murray, J.D.; Finno, C.J. Identification of Long Non-Coding RNA in the Horse Transcriptome. BMC Genom. 2017, 18, 511. [Google Scholar] [CrossRef]
- Ilott, N.E.; Ponting, C.P. Predicting Long Non-Coding RNAs Using RNA Sequencing. Methods 2013, 63, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; He, X.; Hoadley, K.A.; Parker, J.S.; Hayes, D.N.; Perou, C.M. Comparison of RNA-Seq by Poly (A) Capture, Ribosomal RNA Depletion, and DNA Microarray for Expression Profiling. BMC Genom. 2014, 15, 419. [Google Scholar] [CrossRef]
- Weikard, R.; Hadlich, F.; Kuehn, C. Identification of Novel Transcripts and Noncoding RNAs in Bovine Skin by Deep next Generation Sequencing. BMC Genom. 2013, 14, 789. [Google Scholar] [CrossRef]
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal Muscle Is an Endocrine Organ. J. Pharmacol. Sci. 2014, 125, 125–131. [Google Scholar] [CrossRef]
- Orr, B.O.; Fetter, R.D.; Davis, G.W. Retrograde Semaphorin–Plexin Signalling Drives Homeostatic Synaptic Plasticity. Nature 2017, 550, 109–113. [Google Scholar] [CrossRef]
- Ma, X.; Fu, D.; Chu, M.; Ding, X.; Wu, X.; Guo, X.; Kalwar, Q.; Pei, J.; Bao, P.; Liang, C.; et al. Genome-Wide Analysis Reveals Changes in Polled Yak Long Non-Coding RNAs in Skeletal Muscle Development. Front. Genet. 2020, 11, 365. [Google Scholar] [CrossRef]
- Marín-García, J. Molecular Determinants of Cardiac Neovascularization. Post-Genom. Cardiol. 2014, 279–303. [Google Scholar] [CrossRef]
- von Maltzahn, J.; Chang, N.C.; Bentzinger, C.F.; Rudnicki, M.A. Wnt Signaling in Myogenesis. Trends Cell Biol. 2012, 22, 602–609. [Google Scholar] [CrossRef] [PubMed]
| Ingredients/Nutrients | Mineral Supplement | Protein–Energy Supplement |
|---|---|---|
| Corn (%) | 35.00 | 60.00 |
| Soybean meal (%) | - | 30.00 |
| Dicalcium phosphate (%) | 10.00 | - |
| Urea 45% (%) | - | 2.50 |
| Salt (%) | 30.00 | 5.00 |
| Minerthal 160 MD (%) * | 25.00 | 2.50 |
| Total digestible nutrients (%) | 26.76 | 67.55 |
| Crude protein (%) | 2.79 | 24.78 |
| Non-protein nitrogen (%) | - | 7.03 |
| Acid detergent fiber (%) | 1.25 | 4.76 |
| Neutral detergent fiber (%) | 4.29 | 11.24 |
| Fat (%) | 1.26 | 2.61 |
| Calcium (g/kg) | 74.11 | 6.20 |
| Phosphorus (g/kg) | 59.38 | 7.24 |
| Filtering | Connections Performed |
|---|---|
| PP − NP | Connections that appeared only in the PP and not in the NP |
| PP | Relations exclusive to the PP |
| CP − NP | Relations from the CP that did not appear in the NP |
| CP | Relations exclusive to the CP |
| PP + CP − NP | Relations that appeared only in the PP and CP, and not in the NP |
| Period | Contrast | Transcript | Identification | p-Value | Adj. p-Value |
|---|---|---|---|---|---|
| 15 m | NP vs. PP | TCONS_00030990 | 0.0048 | 0.99 | |
| CP vs. PP | TCONS_00038113 | NONBTAT030133.1 | 0.0017 | 0.99 | |
| TCONS_00044746 | NONBTAT029274.1 | 0.0052 | 0.99 | ||
| TCONS_00057377 | NONBTAT031951.1 | 0.0077 | 0.99 | ||
| 22 m | NP vs. CP | TCONS_00092235 | 2.26 × 10−7 | 0.0001 | |
| NP vs. PP | TCONS_00092235 | 0.0004 | 0.19 | ||
| NP vs. CP | TCONS_00052474 | NONBTAT027406.1 | 0.0030 | 0.80 | |
| TCONS_00073566 | 0.0044 | 0.80 | |||
| TCONS_00007180 | 0.0062 | 0.83 | |||
| CP vs. PP | TCONS_00030818 | 0.0085 | 0.99 | ||
| TCONS_00039302 | NONBTAT031112.1 | 0.0037 | 0.99 |
| Treatment | lncRNA | Identification | Connections |
|---|---|---|---|
| PP | TCONS_00107245 | NONBTAT031978.1 | 247 |
| TCONS_00105083 | - | 167 | |
| TCONS_00031013 | NONBTAT028263.1 | 131 | |
| TCONS_00008937 | - | 74 | |
| TCONS_00074879 | NONBTAT028969.1 | 55 | |
| TCONS_00132830 | NONBTAT031353.1 | 44 | |
| TCONS_00050716 | NONBTAT028732.1 | 42 | |
| TCONS_00125019 | - | 41 | |
| TCONS_00119425 | NONBTAT026662.2 | 39 | |
| TCONS_00118957 | NONBTAT021767.2 | 34 | |
| TCONS_00126574 | NONBTAT031687.1 | 28 | |
| TCONS_00122572 | - | 24 | |
| TCONS_00132533 | NONBTAT031349.1 | - | |
| CP | TCONS_00105330 | NONBTAT030235.1 | 108 |
| TCONS_00113158 | NONBTAT030355.1 | 87 | |
| TCONS_00078394 | - | 85 | |
| TCONS_00028261 | NONBTAT026662.2 | 71 | |
| TCONS_00074879 | NONBTAT028969.1 | 68 | |
| TCONS_00118957 | NONBTAT021767.2 | 50 | |
| TCONS_00106901 | NONBTAT019405.2 | 48 | |
| TCONS_00022335 | - | 47 | |
| TCONS_00031681 | - | 46 | |
| TCONS_00105083 | - | 45 | |
| TCONS_00122572 | - | 43 | |
| TCONS_00017335 | - | 42 | |
| TCONS_00053837 | - | 42 | |
| TCONS_00050716 | NONBTAT028732.1 | 42 | |
| TCONS_00063942 | NONBTAT028058.1 | 35 | |
| TCONS_00050901 | NONBTAT028721.1 | 24 | |
| TCONS_00108094 | NONBTAT027378.1 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cracco, R.C.; Alexandre, P.A.; Polizel, G.H.G.; Fernandes, A.C.; de Almeida Santana, M.H. Evaluation of Muscle Long Non-Coding RNA Profile during Rearing and Finishing Phase of Bulls Subjected to Different Prenatal Nutritional Strategies. Animals 2024, 14, 652. https://doi.org/10.3390/ani14040652
Cracco RC, Alexandre PA, Polizel GHG, Fernandes AC, de Almeida Santana MH. Evaluation of Muscle Long Non-Coding RNA Profile during Rearing and Finishing Phase of Bulls Subjected to Different Prenatal Nutritional Strategies. Animals. 2024; 14(4):652. https://doi.org/10.3390/ani14040652
Chicago/Turabian StyleCracco, Roberta Cavalcante, Pamela Almeida Alexandre, Guilherme Henrique Gebim Polizel, Arícia Christofaro Fernandes, and Miguel Henrique de Almeida Santana. 2024. "Evaluation of Muscle Long Non-Coding RNA Profile during Rearing and Finishing Phase of Bulls Subjected to Different Prenatal Nutritional Strategies" Animals 14, no. 4: 652. https://doi.org/10.3390/ani14040652
APA StyleCracco, R. C., Alexandre, P. A., Polizel, G. H. G., Fernandes, A. C., & de Almeida Santana, M. H. (2024). Evaluation of Muscle Long Non-Coding RNA Profile during Rearing and Finishing Phase of Bulls Subjected to Different Prenatal Nutritional Strategies. Animals, 14(4), 652. https://doi.org/10.3390/ani14040652








