A Relaxed Horse—A Relaxed Client? An Experimental Investigation of the Effects of Therapy Horses’ Stress on Clients’ Stress, Mood, and Anxiety
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Recruitment and Sample Characteristics
2.2. Design
2.3. Materials and Measures
2.3.1. Positive and Negative Affect Schedule (PANAS)
2.3.2. State-Trait Anxiety Inventory (STAI-T and STAI-S)
2.3.3. Beck Depression Inventory II (BDI-II)
2.3.4. Brief Symptom Inventory (BSI)
2.3.5. Pet Attitude Scale (PAS)
2.3.6. Lexington Attachment to Pets Scale (LAPS)
2.3.7. Interpersonal Reactivity Index (Saarbrücker Persönlichkeitsfragebogen, SPF)
2.3.8. Physiological Measurements
Heart Rate and Heart Rate Variability (Horses)
Heart Rate and Heart Rate Variability (Humans)
Salivary Cortisol (Horses and Humans)
2.3.9. Horses
Selection of Horses and Matching of Horses
2.4. Procedure
2.4.1. Telephone Screening
2.4.2. Online Questionnaire
2.4.3. Experimental Session
Experimental Location
Arrival at the Ranch and Baseline Measurement
Standardized Interaction with the First Horse
First Waiting Period
Standardized Interaction with the Second Horse
Second Waiting Period
Questionnaire on Preference for the Horses and Debriefing
2.4.4. Experimental Manipulations: Stress vs. Relaxation Induction
Preparation of the Horses
Baseline Measurement
Stress Induction
Relaxation Induction
2.5. Data Analyses
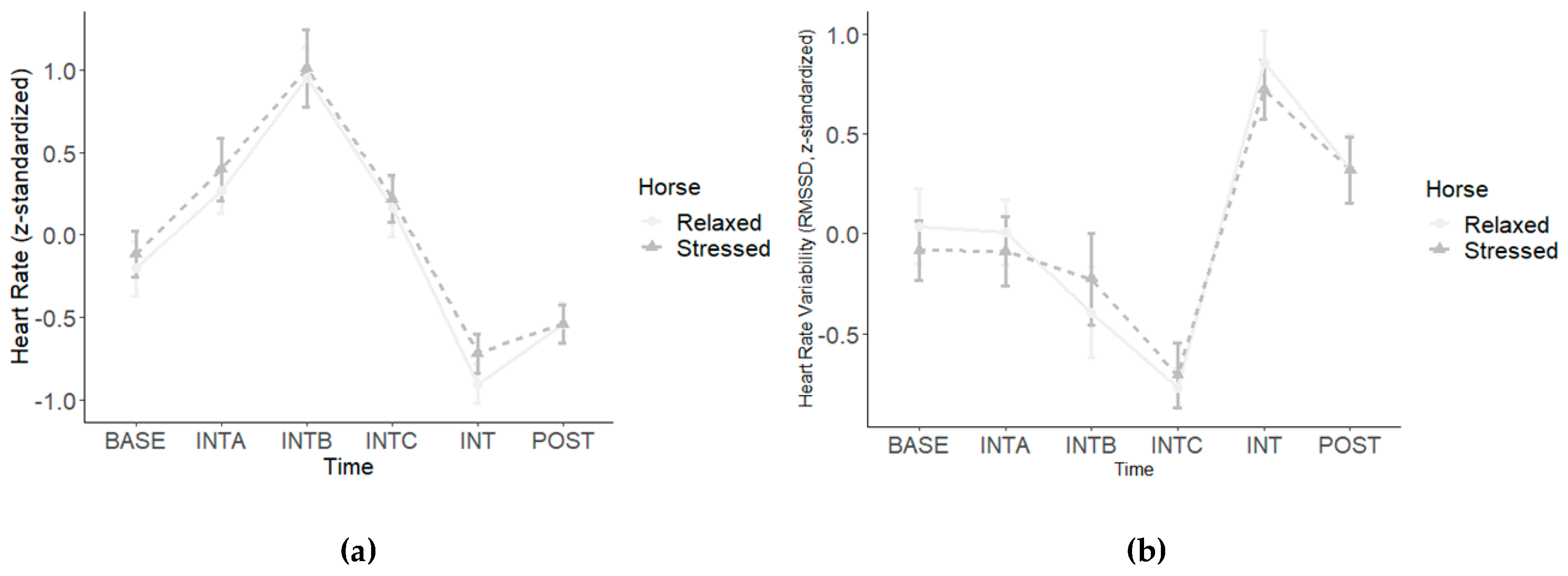
3. Results
3.1. Manipulation Check
3.2. State Anxiety, Negative and Positive Affect, and Human Salivary Cortisol
3.3. Human ECG Data
3.4. Questionnaire on Preference for the Horses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, W.; Alm, K.; Benjamin, J.; Thomas, L.; Anderson, D.; Pohl, L.; Kane, M. Optimal terminology for services in the United States that incorporate horses to benefit people: A consensus document. J. Altern. Complement. Med. 2021, 27, 88–95. [Google Scholar] [CrossRef]
- Stern, C.; Chur-Hansen, A. An umbrella review of the evidence for equine-assisted interventions. Aust. J. Psychol. 2019, 71, 361–374. [Google Scholar] [CrossRef]
- McKinney, C.; Mueller, M.K.; Frank, N. Effects of therapeutic riding on measures of stress in horses. J. Equine Vet. Sci. 2015, 35, 922–928. [Google Scholar] [CrossRef]
- Malinowski, K.; Yee, C.; Tevlin, J.M.; Birks, E.K.; Durando, M.M.; Pournajafi-Nazarloo, H.; Cavaiola, A.A.; McKeever, K.H. The effects of equine assisted therapy on plasma cortisol and oxytocin concentrations and heart rate variability in horses and measures of symptoms of post-traumatic stress disorder in veterans. J. Equine Vet. Sci. 2018, 64, 17–26. [Google Scholar] [CrossRef]
- Contalbrigo, L.; Borgi, M.; De Santis, M.; Collacchi, B.; Tuozzi, A.; Toson, M.; Redaelli, V.; Odore, R.; Vercelli, C.; Stefani, A.; et al. Equine-assisted services (EASs) for children with autism spectrum disorders (ASD): Behavioural and physiological indices of stress in domestic horses (Equus caballus) during riding sessions. Animals 2021, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, A.; Fazio, E.; Cravana, C.; Medica, P. Equine-assisted services: An overview of current scientific contributions on efficacy and outcomes on humans and horses. J. Vet. Behav. 2023, 59, 15–24. [Google Scholar] [CrossRef]
- Hovey, M.R.; Davis, A.; Chen, S.; Godwin, P.; Porr, C.S. Evaluating stress in riding horses: Part one—Behavior assessment and serum cortisol. J. Equine Vet. Sci. 2021, 96, 103297. [Google Scholar] [CrossRef]
- Potier, J.F.; Louzier, V. Evaluation of stress markers in horses during hippotherapy sessions in comparison to being ridden by beginners. Anim. Welf. 2023, 32, e10. [Google Scholar] [CrossRef]
- Arrazola, A.; Merkies, K. Effect of human attachment style on horse behaviour and physiology during equine-assisted activities—A pilot study. Animals 2020, 10, 1156. [Google Scholar] [CrossRef]
- Mendonça, T.; Bienboire-Frosini, C.; Menuge, F.; Leclercq, J.; Lafont-Lecuelle, C.; Arroub, S.; Pageat, P. The impact of equine-assisted therapy on equine behavioral and physiological responses. Animals 2019, 9, 409. [Google Scholar] [CrossRef]
- Kaiser, L.; Heleski, C.; Siegford, J.; Smith, K. Stress-related behaviors among horses used in a therapeutic riding program. J. Am. Vet. Med. Assoc. 2006, 288, 39–45. [Google Scholar] [CrossRef]
- Condon, V.M.; McGreevy, P.D.; McLean, A.N.; Williams, J.M.; Randle, H. Associations between commonly used apparatus and conflict behaviors reported in the ridden horse in Australia. J. Vet. Behav. 2022, 49, 1–14. [Google Scholar] [CrossRef]
- Cook, W.R. Bit-induced pain: A cause of fear, flight fight and facial neuralgia in the horse. Pferdeheilkunde 2003, 19, 75–82. [Google Scholar] [CrossRef]
- Cook, W.R.; Kibler, M. Behavioural assesment of pain in 66 horses, with and without a bit. Equine Vet. Educ. 2019, 31, 551–560. [Google Scholar] [CrossRef]
- Stehouwer, A. Performance and Stress Levels of Horses in a L1 Dressage Competition Compared for a Cross-under Bitless Bridle and a Bridle Containing a Snaffle Bit. Bachelor Thesis, University of Applied Sciences, Wageningen, The Netherlands, 2014. [Google Scholar]
- Zinsstag, J.; Schelling, E.; Crump, L.; Whittaker, M.; Tanner, M.; Stephen, C. (Eds.) One Health: The Theory and Practice of Integrated Health Approaches, 2nd ed.; CABI Digital Library: Wallingford, Oxfordshire, UK, 2021. [Google Scholar] [CrossRef]
- Chalmers, D.; Dell, C.A. Applying one health to the study of animal-assisted interventions. Ecohealth 2015, 12, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Hediger, K.; Meisser, A.; Zinsstag, J. A one health research framework for animal-assisted interventions. Int. J. Environ. Res. Public Health 2019, 16, 640. [Google Scholar] [CrossRef] [PubMed]
- Menna, L.F.; Santaniello, A.; Todisco, M.; Amato, A.; Borrelli, L.; Scandurra, C.; Fioretti, A. The human–animal relationship as the focus of animal-assisted interventions: A one health approach. Int. J. Environ. Res. Public Health 2019, 16, 3660. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.; Gross, J.J. Digital emotion contagion. Trends Cogn. Sci. 2020, 24, 316–328. [Google Scholar] [CrossRef]
- Herrando, C.; Constantinides, E. Emotional contagion: A brief overview and future directions. Front. Psychol. 2021, 12, 712606. [Google Scholar] [CrossRef]
- Schachter, S.; Singer, J. Cognitive, social, and physiological determinants of emotional state. Psychol. Rev. 1962, 69, 379–399. [Google Scholar] [CrossRef]
- Hatfield, E.; Cacioppo, J.T.; Rapson, R.L. Emotional Contagion; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar] [CrossRef]
- Hatfield, E.; Cacioppo, J.T.; Rapson, R.L. Primitive emotional contagion. In Review of Personality and Social Psychology: Emotions and Social Behavior; Clark, M.S., Ed.; Sage: Newbury Park, CA, USA, 1992; pp. 151–177. [Google Scholar]
- Baba, C.; Kawai, M.; Takimoto-Inose, A. Are horses (Equus caballus) sensitive to human emotional cues? Animals 2019, 9, 630. [Google Scholar] [CrossRef]
- Trösch, M.; Cuzol, F.; Parias, C.; Calandreau, L.; Nowak, R.; Lansade, L. Horses categorize human emotions cross-modally based on facial expression and non-verbal vocalizations. Animals 2019, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Trösch, M.; Pellon, S.; Cuzol, F.; Parias, C.; Nowak, R.; Calandreau, L.; Lansade, L. Horses feel emotions when they watch positive and negative horse–human interactions in a video and transpose what they saw to real life. Anim. Cogn. 2020, 23, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Keeling, L.J.; Jonare, L.; Lanneborn, L. Investigating horse–human interactions: The effect of a nervous human. Vet. J. 2009, 181, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Greenall, J.S.; Cornu, L.; Maigrot, A.L.; De La Torre, M.P.; Briefer, E.F. Age, empathy, familiarity, domestication and call features enhance human perception of animal emotion expressions. R. Soc. Open Sci. 2022, 9, 221138. [Google Scholar] [CrossRef] [PubMed]
- Merkies, K.; Crouchman, E.; Belliveau, H. Human ability to determine affective states in domestic horse whinnies. Anthrozoös 2022, 35, 483–494. [Google Scholar] [CrossRef]
- Bell, C.; Rogers, S.; Taylor, J.; Busby, D. Improving the recognition of equine affective states. Animals 2019, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Gronqvist, G.; Rogers, C.; Gee, E.; Martinez, A.; Bolwell, C. Veterinary and equine science students’ interpretation of horse behaviour. Animals 2017, 7, 63. [Google Scholar] [CrossRef]
- Guinnefollau, L.; Gee, E.K.; Bolwell, C.F.; Norman, E.J.; Rogers, C.W. Benefits of animal exposure on veterinary students’ understanding of equine behaviour and self-assessed equine handling skills. Animals 2019, 9, 620. [Google Scholar] [CrossRef]
- Braun, M.N.; Müller-Klein, A.; Sopp, M.R.; Michael, T.; Link-Dorner, U.; Lass-Hennemann, J. The human ability to interpret horses’ expressive behavior: The role of emotion recognition ability and previous experience with horses. Appl. Anim. Behav. Sci. 2024, 271, 106171. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Rietmann, T.R.; Stuart, A.E.A.; Bernasconi, P.; Stauffacher, M.; Auer, J.A.; Weishaupt, M.A. Assessment of mental stress in warmblood horses: Heart rate variability in comparison to heart rate and selected behavioural parameters. Appl. Anim. Behav. Sci. 2004, 88, 121–136. [Google Scholar] [CrossRef]
- Schmidt, A.; Hödl, S.; Möstl, E.; Aurich, J.; Müller, J.; Aurich, C. Cortisol release, heart rate, and heart rate variability in transport-naive horses during repeated road transport. Domest. Anim. Endocrinol. 2010, 39, 205–213. [Google Scholar] [CrossRef]
- von Lewinski, M.; Biau, S.; Erber, R.; Ille, N.; Aurich, J.; Faure, J.M.; Möstl, E.; Aurich, C. Cortisol release, heart rate and heart rate variability in the horse and its rider: Different responses to training and performance. Vet. J. 2013, 197, 229–232. [Google Scholar] [CrossRef]
- Peeters, M.; Closson, C.; Beckers, J.F.; Vandenheede, M. Rider and horse salivary cortisol levels during competition and impact on performance. J. Equine Vet. Sci. 2013, 33, 155–160. [Google Scholar] [CrossRef]
- Sikorska, U.; Maśko, M.; Ciesielska, A.; Zdrojkowski, Ł.; Domino, M. Role of Cortisol in Horse’s Welfare and Health. Agriculture 2023, 13, 2219. [Google Scholar] [CrossRef]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Lass-Hennemann, J.; Peyk, P.; Streb, M.; Holz, E.; Michael, T. Presence of a dog reduces subjective but not physiological stress responses to an analog trauma. Front. Psychol. 2014, 5, 1010. [Google Scholar] [CrossRef]
- Lass-Hennemann, J.; Schäfer, S.K.; Römer, S.; Holz, E.; Streb, M.; Michael, T. Therapy dogs as a crisis intervention after traumatic events? An experimental study. Front. Psychol. 2018, 9, 1627. [Google Scholar] [CrossRef]
- Matijczak, A.; Yates, M.S.; Ruiz, M.C.; Santos, L.R.; Kazdin, A.E.; Raila, H. The influence of interactions with pet dogs on psychological distress. Emotion 2023, 24, 384–396. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Krohne, H.W.; Egloff, B.; Kohlmann, C.W.; Tausch, A. Untersuchung mit einer deutschen Form der Positive and Negative Affect Schedule (PANAS). Diagnostica 1996, 42, 139–156. [Google Scholar] [CrossRef]
- Laux, L.; Glanzmann, P.; Schaffner, P.; Spielberger, C.D. Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisungen; Beltz Test GmbH: Weinheim, Germany, 1981. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E.; Vagg, P.R.; Jacobs, G.A. State-Trait Anxiety Inventory (STAI) for Adults-Manual; Mind Garden Inc.: Menlo Park, CA, USA, 1970. [Google Scholar]
- Hautzinger, M.; Keller, F.; Kühner, C. Beck Depressions-Inventar (BDI-II); Harcourt Test Services: Frankfurt am Main, Germany, 2006. [Google Scholar]
- Franke, G.H. Brief Symptom Inventory (BSI) von LR Derogatis:(Kurzform der SCL-90-R); Beltz Test: Weinheim, Germany, 2000. [Google Scholar]
- Römpke, A.K. Adaptation of the Pet Attitude Scale to German. Soc. Anim. 2019, 27, 11–24. [Google Scholar] [CrossRef]
- Templer, D.; Arikawa, H.; Canfield, M.; Munsell, K.; Tangan, K. Modification of the pet attitude scale. Soc. Anim. 2004, 12, 137–142. [Google Scholar] [CrossRef]
- Templer, D.I.; Salter, C.A.; Dickey, S.; Baldwin, R.; Veleber, D.M. The construction of a pet attitude scale. Psychol. Rec. 1981, 31, 343–348. [Google Scholar] [CrossRef]
- Templer, D.I.; Arikawa, H. The pet attitude scale. In The Psychology of the Human-Animal Bond: A Resource for Clinicians and Researchers; Springer: New York, NY, USA, 2011; pp. 335–359. [Google Scholar] [CrossRef]
- Johnson, T.P.; Garrity, T.F.; Stallones, L. Psychometric evaluation of the Lexington attachment to pets scale (LAPS). Anthrozoös 1992, 5, 160–175. [Google Scholar] [CrossRef]
- Paulus, C. Der Saarbrücker Persönlichkeitsfragebogen SPF(IRI) zur Messung von Empathie: Psychometrische Evaluation der deutschen Version des Interpersonal Reactivity Index. 2009. Available online: https://www.psycharchives.org/en/item/2b090feb-4f9e-4ea2-9524-13151172f615 (accessed on 21 December 2023). [CrossRef]
- Davis, M.H. Interpersonal Reactivity Index (IRI); APA PsycTests: Washington, DC, USA, 1980. [Google Scholar] [CrossRef]
- Ille, N.; Erber, R.; Aurich, C.; Aurich, J. Comparison of heart rate and heart rate variability obtained by heart rate monitors and simultaneously recorded electrocardiogram signals in nonexercising horses. J. Vet. Behav. 2014, 9, 341–346. [Google Scholar] [CrossRef]
- Squibb, K.; Griffin, K.; Favier, R.; Ijichi, C. Poker Face: Discrepancies in behaviour and affective states in horses during stressful handling procedures. Appl. Anim. Behav. Sci. 2018, 202, 34–38. [Google Scholar] [CrossRef]
- Blechert, J.; Peyk, P.; Liedlgruber, M.; Wilhelm, F.H. ANSLAB: Integrated multichannel peripheral biosignal processing in psychophysiological science. Behav. Res. Methods 2016, 48, 1528–1545. [Google Scholar] [CrossRef]
- Bourdillon, N.; Schmitt, L.; Yazdani, S.; Vesin, J.M.; Millet, G.P. Minimal window duration for accurate HRV recording in athletes. Front. Neurosci. 2017, 11, 456. [Google Scholar] [CrossRef]
- Munoz, M.L.; van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; de Geus, E.J.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M.; et al. Validity of (ultra-)short recordings for heart rate variability measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef]
- Dressendörfer, R.A.; Kirschbaum, C.; Rohde, W.; Stahl, F.; Strasburger, C.J. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. Mol. Biol. 1992, 43, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Lovallo, W.R.; Al'Absi, M.; Blick, K.; Whitsett, T.L.; Wilson, M.F. Stress-like adrenocorticotropin responses to caffeine in young healthy men. Pharmacol. Biochem. Behav. 1996, 55, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, D.L.; McAuley, E. Cortisol and affective responses to exercise. J. Sports Sci. 1998, 16, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Schrieks, I.C.; Joosten, M.M.; Klöpping-Ketelaars, W.A.A.; Witkamp, R.F.; Hendriks, H.F. Moderate alcohol consumption after a mental stressor attenuates the endocrine stress response. Alcohol 2016, 57, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Smiet, E.; Van Dierendonck, M.C.; Sleutjens, J.; Menheere, P.P.C.A.; Van Breda, E.; De Boer, D.; Back, W.; Wijnberg, I.D.; Van der Kolk, J.H. Effect of different head and neck positions on behaviour, heart rate variability and cortisol levels in lunged Royal Dutch Sport horses. Vet. J. 2014, 202, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.; Esch, L.; Farmer, K.; Marr, I. Basic needs in horses?—A literature review. Animals 2021, 11, 1798. [Google Scholar] [CrossRef]
- Tierärztliche Vereinigung für Tierschutz: Tiere im Sozialen Einsatz. Available online: https://www.tierschutz-tvt.de/alle-merkblaetter-und-stellungnahmen/#c304 (accessed on 22 December 2023).
- Hama, H.; Yogo, M.; Matsuyama, Y. Effects of stroking horses on both humans’ and horses’ heart rate responses. Jpn. Psychol. Res. 1996, 38, 66–73. [Google Scholar] [CrossRef]
- Thorbergson, Z.W.; Nielsen, S.G.; Beaulieu, R.J.; Doyle, R.E. Physiological and behavioral responses of horses to wither scratching and patting the neck when under saddle. J. Appl. Anim. Welf. Sci. 2016, 19, 245–259. [Google Scholar] [CrossRef]
- Aurich, J.; Wulf, M.; Ille, N.; Erber, R.; von Lewinski, M.; Palme, R.; Aurich, C. Effects of season, age, sex, and housing on salivary cortisol concentrations in horses. Domest. Anim. Endocrinol. 2015, 52, 11–16. [Google Scholar] [CrossRef]
- Bohák, Z.S.; Szabó, F.; Beckers, J.F.; de Sousa, N.M.; Kutasi, O.; Nagy, K.; Szenci, O. Monitoring the circadian rhythm of serum and salivary cortisol concentrations in the horse. Domest. Anim. Endocrinol. 2013, 45, 38–42. [Google Scholar] [CrossRef]
- O’Brien, R.G.; Kaiser, M.K. MANOVA method for analyzing repeated measures designs: An extensive primer. Psychol. Bull. 1985, 97, 316–333. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 21 December 2023).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 21 December 2023).
- Katayama, M.; Kubo, T.; Yamakawa, T.; Fujiwara, K.; Nomoto, K.; Ikeda, K.; Mogi, K.; Nagasawa, M.; Kikusui, T. Emotional contagion from humans to dogs is facilitated by duration of ownership. Front. Psychol. 2019, 10, 1678. [Google Scholar] [CrossRef] [PubMed]
- Professional Association of Therapeutic Horsemanship (PATH) International. Available online: https://pathintl.org/publications/2022-fact-sheet/ (accessed on 22 January 2024).
- Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and psychophysiological effects of human-animal interactions: The possible role of oxytocin. Front. Psychol. 2012, 3, 234. [Google Scholar] [CrossRef] [PubMed]
- Ein, N.; Li, L.; Vickers, K. The effect of pet therapy on the physiological and subjective stress response: A meta-analysis. Stress Health 2018, 34, 477–489. [Google Scholar] [CrossRef]
- Anderson, M.K.; Friend, T.H.; Evans, J.W.; Bushong, D.M. Behavioral assessment of horses in therapeutic riding programs. Appl. Anim. Behav. Sci. 1999, 63, 11–24. [Google Scholar] [CrossRef]



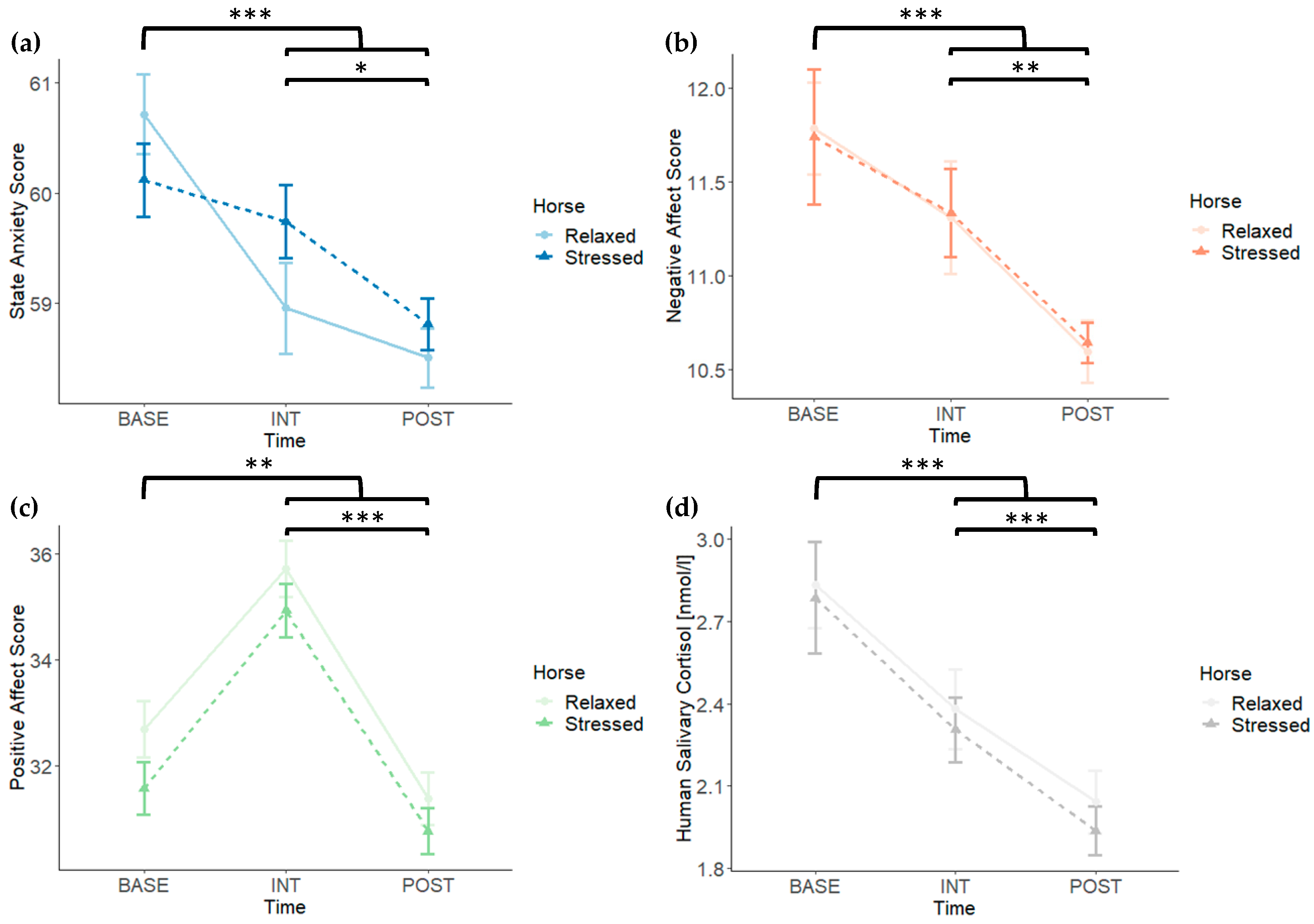
| Time | Horse | Time × Horse | ||||
|---|---|---|---|---|---|---|
| F(2, 40) | p | F(1, 41) | p | F(2, 40) | p | |
| State Anxiety | 34.34 | <0.001 | 0.21 | 0.648 | 1.86 | 0.169 |
| Negative Affect | 19.34 | <0.001 | 0.00 | 0.978 | 0.02 | 0.976 |
| Positive Affect | 33.30 | <0.001 | 3.65 | 0.063 | 0.08 | 0.919 |
| Salivary Cortisol | 17.69 | <0.001 | 0.17 | 0.686 | 0.03 | 0.967 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Klein, A.; Braun, M.N.; Ferreira de Sá, D.S.; Michael, T.; Link-Dorner, U.; Lass-Hennemann, J. A Relaxed Horse—A Relaxed Client? An Experimental Investigation of the Effects of Therapy Horses’ Stress on Clients’ Stress, Mood, and Anxiety. Animals 2024, 14, 604. https://doi.org/10.3390/ani14040604
Müller-Klein A, Braun MN, Ferreira de Sá DS, Michael T, Link-Dorner U, Lass-Hennemann J. A Relaxed Horse—A Relaxed Client? An Experimental Investigation of the Effects of Therapy Horses’ Stress on Clients’ Stress, Mood, and Anxiety. Animals. 2024; 14(4):604. https://doi.org/10.3390/ani14040604
Chicago/Turabian StyleMüller-Klein, Alicia, Moritz Nicolai Braun, Diana S. Ferreira de Sá, Tanja Michael, Ulrike Link-Dorner, and Johanna Lass-Hennemann. 2024. "A Relaxed Horse—A Relaxed Client? An Experimental Investigation of the Effects of Therapy Horses’ Stress on Clients’ Stress, Mood, and Anxiety" Animals 14, no. 4: 604. https://doi.org/10.3390/ani14040604
APA StyleMüller-Klein, A., Braun, M. N., Ferreira de Sá, D. S., Michael, T., Link-Dorner, U., & Lass-Hennemann, J. (2024). A Relaxed Horse—A Relaxed Client? An Experimental Investigation of the Effects of Therapy Horses’ Stress on Clients’ Stress, Mood, and Anxiety. Animals, 14(4), 604. https://doi.org/10.3390/ani14040604






