First Reported Circulation of Equine Influenza H3N8 Florida Clade 1 Virus in Horses in Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Virological Tests
2.3. Genetic Analysis
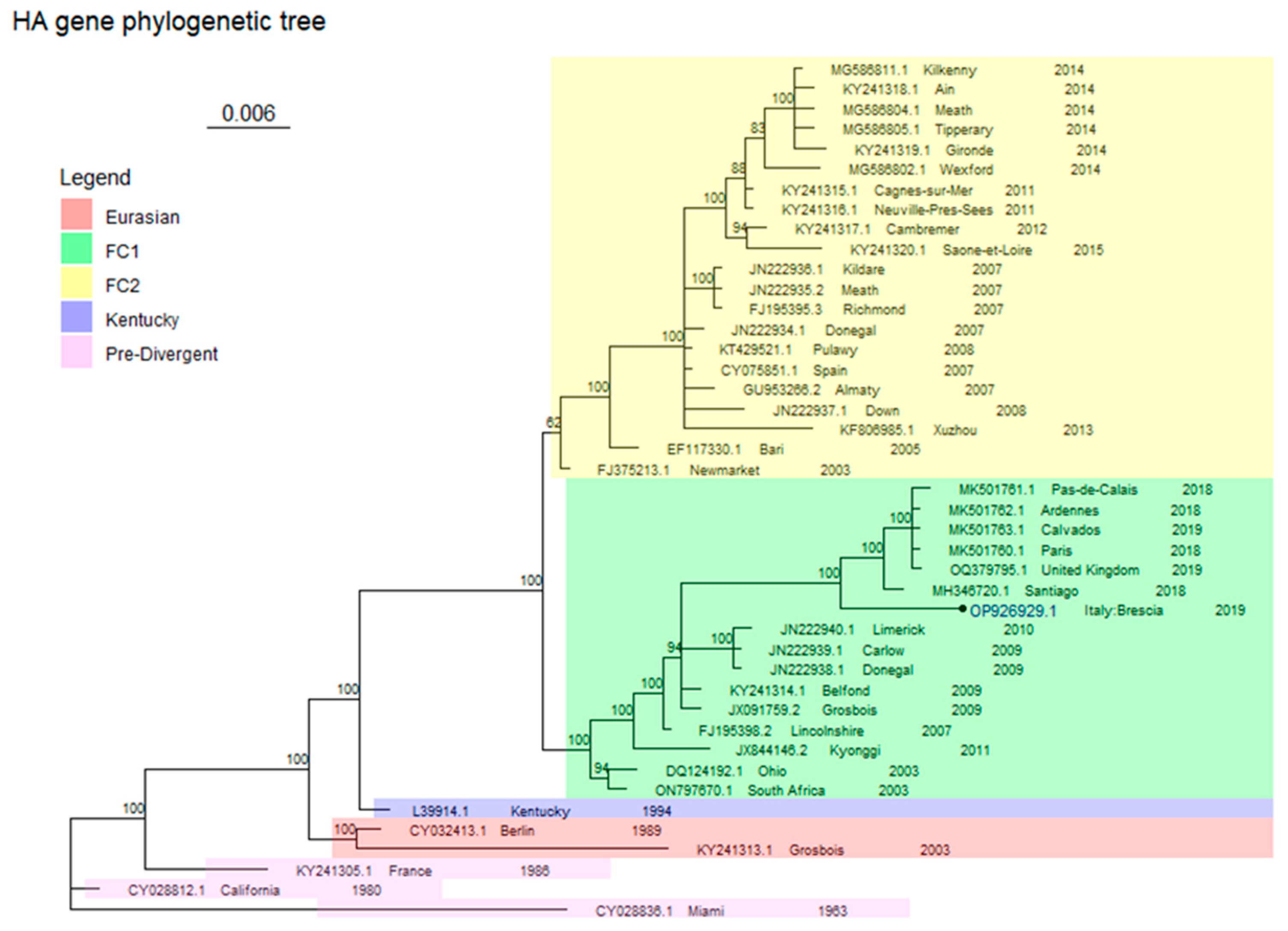
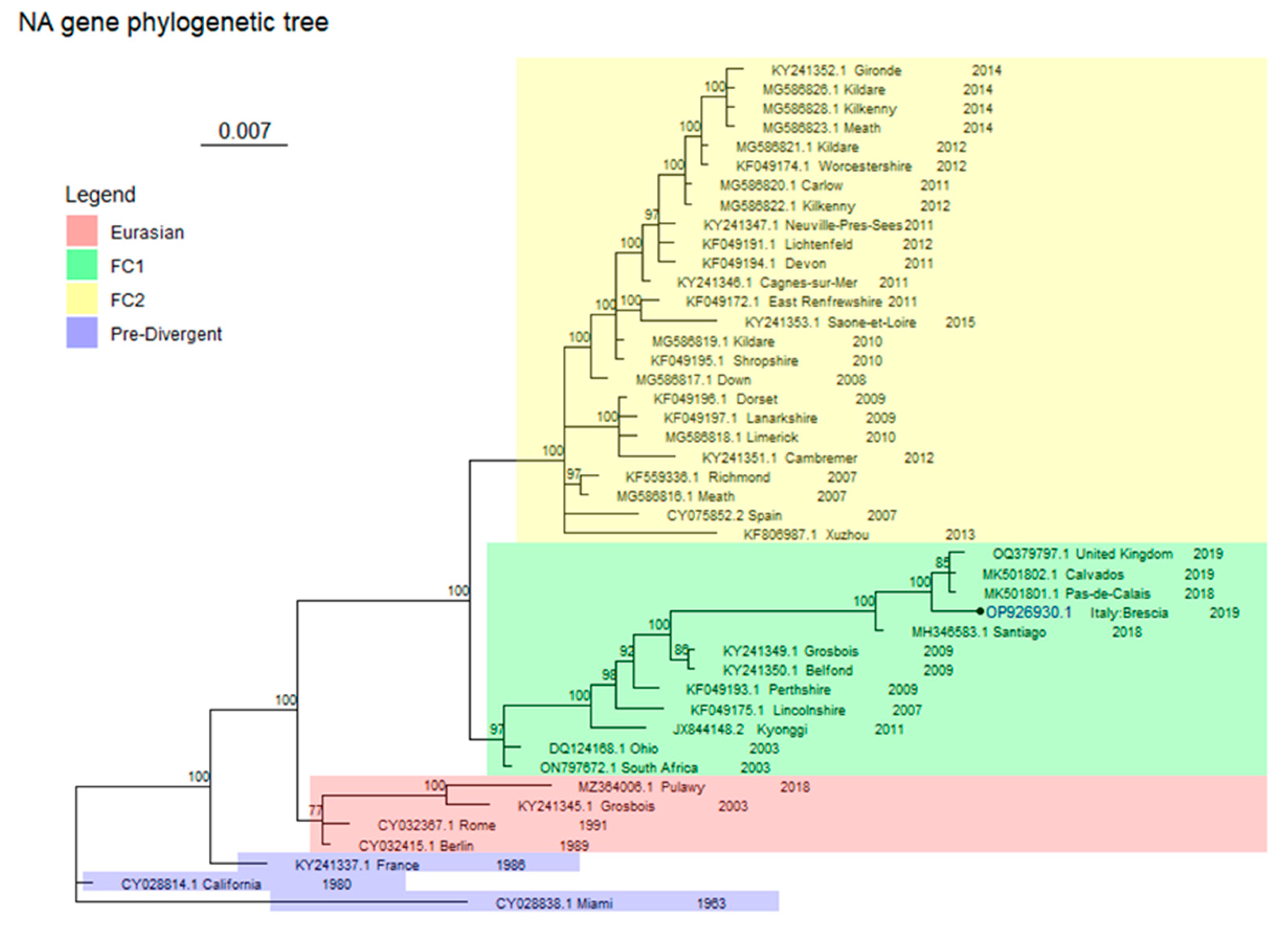
2.4. Phylogenetic Analysis
2.5. Serological Tests
3. Results
Virological Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambers, T.M. Equine Influenza. Cold Spring Harb. Perspect. Med. 2022, 12, a038331. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Ohta, M.; Yamanaka, T.; Kambayashi, Y.; Bannai, H.; Tsujimura, K.; Yamayoshi, S.; Kawaoka, Y.; Cullinane, A. Antigenic differences between equine influenza virus vaccine strains and Florida sublineage clade 1 strains isolated in Europe in 2019. Vet. J. 2021, 272, 105674. [Google Scholar] [CrossRef] [PubMed]
- Oladunni, F.S.; Oseni, S.O.; Martinez-Sobrido, L.; Chambers, T.M. Equine influenza virus and vaccines. Viruses 2021, 13, 1657. [Google Scholar] [CrossRef] [PubMed]
- Alves Beuttemmüller, E.; Woodward, A.; Rash, A.; Dos Santos Ferraz, L.E.; Fernandes Alfieri, A.; Alfieri, A.A.; Elton, D. Characterisation of the epidemic strain of H3N8 equine influenza virus responsible for outbreaks in South America in 2012. Virol. J. 2016, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Olguin-Perglione, C.; Barrandeguy, M.E. An overview of equine influenza in south america. Viruses 2021, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Rash, A. Diagnosis of equine influenza. Vet. Rec. 2017, 181, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Virmani, N.; Singh, B.K.; Gulati, B.R.; Kumar, S. Equine influenza outbreak in India. Vet. Rec. 2008, 163, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Pitel, P.H.; Pronost, S.; Legrand, L.; Fougerolle, S.; Jourdan, M.; Marcillaud-Pitel, C. Florida clade 1 equine influenza virus in France. Vet. Rec. 2019, 184, 101. [Google Scholar] [CrossRef]
- Whitlock, F.; Murcia, P.R.; Newton, J.R. A Review on Equine Influenza from a Human Influenza Perspective. Viruses 2022, 14, 1312. [Google Scholar] [CrossRef]
- Walker-Panse, L.; Rash, A.; Huckstep, J.; Payne, S.; Blake, S.; Whitlock, F.; Elton, D.; Newton, R.; Bryant, N.A. Equine influenza virus surveillance in the United Kingdom from 2019 to 2021. Equine Vet. J. 2021, 78–79. [Google Scholar] [CrossRef]
- Damiani, A.M.; Scicluna, M.T.; Ciabatti, I.; Cardeti, G.; Sala, M.; Vulcano, G.; Cordioli, P.; Martella, V.; Amaddeo, D.; Autorino, G.L. Genetic characterization of equine influenza viruses isolated in Italy between 1999 and 2005. Virus Res. 2008, 131, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Rash, A.S.; Russell, C.A.; Ross, J.; Cooke, A.; Bowman, S.; MacRae, S.; Lewis, N.S.; Paillot, R.; Zanoni, R.; et al. Antigenic and genetic variations in European and North American equine influenza virus strains (H3N8) isolated from 2006 to 2007. Vet. Microbiol. 2009, 138, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Legrand, L.J.; Pitel, P.H.Y.; Cullinane, A.A.; Fortier, G.D.; Pronost, S.L. Genetic evolution of equine influenza strains isolated in France from 2005 to 2010. Equine Vet. J. 2015, 47, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Autorino, G.L.; Frontoso, R.; Cersini, A.; Manna, G.; Damiani, A.M.; Scicluna, M.T. Case Report of Equine Influenza in Italy, in 2014. J. Equine Vet. Sci. 2015, 35, 779–783. [Google Scholar] [CrossRef]
- Elton, D.; Cullinane, A. Equine influenza: Antigenic drift and implications for vaccines. Equine Vet. J. 2013, 45, 768–769. [Google Scholar] [CrossRef] [PubMed]
- OIE Expert Surveillance Panel on Equine Influenza Vaccine Composition—Videoconference, 16 April 2020. Bull. Off. 2020, 2020, 1–5. [CrossRef]
- Spackman, E.; Senne, D.A.; Myers, T.J.; Bulaga, L.L.; Garber, L.P.; Perdue, M.L.; Lohman, K.; Daum, L.T.; Suarez, D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 2002, 40, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Heine, H.G.; Foord, A.J.; Wang, J.; Valdeter, S.; Walker, S.; Morrissy, C.; Wong, F.Y.K.; Meehan, B. Detection of highly pathogenic zoonotic influenza virus H5N6 by reverse-transcriptase quantitative polymerase chain reaction. Virol. J. 2015, 12, 10–13. [Google Scholar] [CrossRef]
- Laconi, A.; Fortin, A.; Bedendo, G.; Shibata, A.; Sakoda, Y.; Awuni, J.A.; Go-Maro, E.; Arafa, A.; Maken Ali, A.S.; Terregino, C.; et al. Detection of avian influenza virus: A comparative study of the in silico and in vitro performances of current RT-qPCR assays. Sci. Rep. 2020, 10, 8441. [Google Scholar] [CrossRef]
- OIE. Equine Influenza (Infection with Equine Influenza Virus); Elsevier: Amsterdam, The Netherlands, 2019; Chapter 3.6.7.
- Woodward, A.L.; Rash, A.S.; Blinman, D.; Bowman, S.; Chambers, T.M.; Daly, J.M.; Damiani, A.; Joseph, S.; Lewis, N.; McCauley, J.W.; et al. Development of a surveillance scheme for equine influenza in the UK and characterisation of viruses isolated in Europe, Dubai and the USA from 2010–2012. Vet. Microbiol. 2014, 169, 113–127. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Larget, B.; Alfaro, M.E. Bayesian phylogenetic model selection using reversible jump Markov chain Monte Carlo. Mol. Biol. Evol. 2004, 21, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Volume 1, Available online: https://www.r-project.org (accessed on 1 September 2023).
- Yu, G. Using ggtree to Visualize Data on Tree-Like Structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Cullinane, A.; Gahan, J.; Walsh, C.; Nemoto, M.; Entenfellner, J.; Olguin-Perglione, C.; Garvey, M.; Fu, T.Q.H.; Venner, M.; Yamanaka, T.; et al. Evaluation of current equine influenza vaccination protocols prior to shipment, guided by OIE standards. Vaccines 2020, 8, 107. [Google Scholar] [CrossRef]
- Fougerolle, S.; Fortier, C.; Legrand, L.; Jourdan, M.; Marcillaud-Pitel, C.; Pronost, S.; Paillot, R. Success and limitation of equine influenza vaccination: The first incursion in a decade of a florida clade 1 equine influenza virus that shakes protection despite high vaccine coverage. Vaccines 2019, 7, 174. [Google Scholar] [CrossRef] [PubMed]
- Fougerolle, S.; Legrand, L.; Lecouturier, F.; Sailleau, C.; Paillot, R.; Hans, A.; Pronost, S. Genetic evolution of equine influenza virus strains (H3N8) isolated in France from 1967 to 2015 and the implications of several potential pathogenic factors. Virology 2017, 505, 210–217. [Google Scholar] [CrossRef]
- Both, G.W.; Sleigh, M.J.; Cox, N.J.; Kendal, A.P. Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: Multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J. Virol. 1983, 48, 52–60. [Google Scholar] [CrossRef]
- Whitlock, F.; Grewar, J.; Newton, R. An epidemiological overview of the equine influenza epidemic in Great Britain during 2019. Equine Vet. J. 2022, 55, 153–164. [Google Scholar] [CrossRef]
- OIE. Expert surveillance panel on equine influenza vaccine composition. Woah 2022, 2022, 1–3. [Google Scholar]
- Chambers, T.M. A brief introduction to equine influenza and equine influenza viruses. Methods Mol. Biol. 2020, 2123, 355–360. [Google Scholar] [CrossRef]
- European Commission Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (“Animal Health Law”). Off. J. Eur. Union 2016, 84, 1–208.
- Decreto del Presidente della Republica 8 febbraio 1954, n. 320. Regolamento di polizia veterinaria 1954, 320. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=1954-06-24&atto.codiceRedazionale=054U0320&elenco30giorni=false (accessed on 1 September 2023).
| Farm ID | No. Horses with cl. Signs | No. of Positive Horses | No. of Stabled Horses | Morbidity Rate (%) |
|---|---|---|---|---|
| 1 | 12 | 12 | 21 | 57.14% |
| 2 | 1 | 1 | 2 | 50.00% |
| 3 | 1 | 1 | 8 | 12.50% |
| 4 | 7 | 5 | 52 | 9.62% |
| 5 | 1 | 1 | ND | ND |
| Region | Province | Farm ID | Horse ID | Age (Years) | Breed | Sex |
|---|---|---|---|---|---|---|
| Molise | Campobasso | 1 | 1 | 8 | TB | M |
| 2 | 7 | AA | M | |||
| 3 | 4 | TB | M | |||
| 4 | 10 | TB | M | |||
| 5 | 9 | TB | M | |||
| 6 | 11 | AA | M | |||
| 7 | 6 | ND | F | |||
| 8 | ND | ND | ND | |||
| 9 | 5 | TB | M | |||
| 10 | 8 | TB | M | |||
| 11 | 11 | TB | M | |||
| 12 | ND | ND | ND | |||
| 2 | 13 | 9 | ND | M | ||
| 3 | 14 | 4 | TB | M | ||
| Lombardia | Brescia | 4 | 15 | 9 | H | M |
| 16 | 7 | ND | F | |||
| 17 | 3 | ND | M | |||
| 18 | 8 | ND | M | |||
| 19 | 10 | ND | ND | |||
| Bergamo | 5 | 20 | 8 | ND | M |
| Origin of Samples | Serological Results | Virological Results | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haemagglutination Inhibition * | Single Radial Haemolysis ** | Nasal Swab | Allantoic Fluid | |||||||||||||
| Region | Province | Farm ID | Horse ID | H7N7 | H3N8 Am FC1 | H3N8 Eu | H7N7 | H3N8 Am FC1 | H3N8 Eu | HA | RRT PCR | HA | RRT PCR | |||
| Area | p° | Area | p° | Area | p° | |||||||||||
| Molise | Campobasso | 1 | 1 | <8 | 128 | 128 | 0 | 0 | 141 | + | 124 | + | - | + | - | |
| 2 | <8 | 64 | 128 | 0 | 0 | 149 | + | 164 | ++ | - | + | - | ||||
| 3 | <8 | 512 | 1024 | 0 | 0 | 304 | ++ | 272 | ++ | - | + | - | ||||
| 4 | 128 | 128 | 64 | 121 | + | 233 | ++ | 186 | ++ | - | + | - | ||||
| 5 | <8 | 32 | <8 | 0 | 0 | 170 | ++ | 40 | - | - | + | - | ||||
| 6 | <8 | 16 | <8 | 0 | 0 | 72 | - | 0 | 0 | - | + | - | ||||
| 7 | <8 | 128 | 8 | 0 | 0 | 115 | + | 0 | 0 | - | + | - | ||||
| 8 | 64 | 8 | 8 | 126 | + | 130 | + | 123 | + | - | + | - | ||||
| 9 | <8 | 256 | 256 | 0 | 0 | 263 | ++ | 263 | ++ | - | + | - | ||||
| 10 | 128 | 16 | 64 | 127 | + | 163 | ++ | 164 | ++ | - | + | - | ||||
| 11 | <8 | 64 | 32 | 0 | 0 | 204 | ++ | 184 | ++ | - | + | - | ||||
| 12 | <8 | 64 | <8 | 0 | 0 | 99 | + | 0 | 0 | - | + | - | ||||
| 2 | 13 | <8 | 16 | <8 | 0 | 0 | 84 | - | 0 | 0 | - | + | - | |||
| 3 | 14 | <8 | 128 | 128 | 0 | 0 | 223 | ++ | 179 | ++ | - | + | - | |||
| Lombardia | Brescia | 4 | 15 | <8 | <8 | <8 | 0 | 0 | 0 | 0 | 0 | 0 | - | + | + (32) $ | + |
| 16 | <8 | 32 | 16 | 0 | 0 | 140 | + | 114 | + | - | + | - | ||||
| 17 | <8 | <8 | <8 | 0 | 0 | 0 | 0 | 0 | 0 | - | + | + (32) $ | + | |||
| 18 | <8 | <8 | <8 | 0 | 0 | 94 | + | 118 | + | - | + | - | ||||
| 19 | <8 | <8 | 8 | 0 | 0 | 82 | - | 119 | + | - | + | - | ||||
| Bergamo | 5 | 20 | <8 | <8 | <8 | 0 | 0 | 90 | + | 85 | + | - | + | - | ||
| Lineage | ||||
|---|---|---|---|---|
| Protection Level | SRH Area (mm2) | H7N7 | H3N8 Am FC1 | H3N8 Eu |
| Negative | 0 | 17 | 2 | 6 |
| Not protected | 0 < mm2 < 85 | 0 | 3 | 1 |
| Clinically protected | 85 ≤ mm2 ≤ 150 | 3 | 8 | 6 |
| Protected against infection | >150 | 0 | 7 | 7 |
| Brescia/19038317_3/2019 HA (OP926929.1) | Brescia/19038317_3/2019 NA (OP926930.1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Location | A.N. | Query Cov. | E Value | % Identity | A.N. | Query Cov. | E Value | % Identity |
| Santiago | MH346720.1 | 100% | 0 | 98.49 | MH346583.1 | 100% | 0 | 98.99 |
| Paris | MK501760.1 | 100% | 0 | 98.39 | / | / | / | / |
| U.K. | OQ379795.1 | 100% | 0 | 98.39 | OQ379797.1 | 100% | 0 | 99.29 |
| Pas-de-Calais | MK501761.1 | 100% | 0 | 98.28 | MK501801.1 | 100% | 0 | 99.39 |
| Ardennes | MK501762.1 | 100% | 0 | 98.28 | / | / | / | / |
| Calvados | MK501763.1 | 100% | 0 | 98.28 | MK501763.1 | 100% | 0 | 99.39 |
| Amino Acid Position | 236 | 255 | 265 | 273 | 274 | 280 | 285 | 289 * | 302 | 322 | 323 | 325 | 337 | 385 | 400 | 463 | 467 | 477 | 487 | 492 | 502 | 516 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USI49340.1 | South africa | V | I | V | L | K | V | V | I | S | I | R | N | P | A | R | K | Q | G | D | G | Y | K |
| ABA39846.1 | Ohio | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| WCL41102.1 | UK | I | . | . | . | . | . | . | . | . | . | . | . | . | T | . | R | L | . | N | . | . | . |
| AWW20564.1 | Santiago | I | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | L | . | N | . | . | . |
| QBA97753.1 | Calvados | I | . | . | . | . | . | . | . | . | . | . | . | . | T | . | R | L | . | N | . | . | . |
| QBA97752.1 | Ardennes | I | . | . | . | . | . | . | . | . | . | . | . | . | T | . | R | L | . | N | . | . | . |
| QBA97751.1 | Pas-de-calais | I | . | . | . | . | . | . | . | . | . | . | . | . | T | . | R | L | . | N | . | . | . |
| QBA97750.1 | Paris | I | . | . | . | . | . | . | . | . | . | . | . | . | T | . | R | L | . | N | . | . | . |
| WAA38452.1 | Brescia | I | . | . | . | . | . | A | T | P | . | . | . | . | . | . | R | L | . | N | . | . | . |
| ABY81492.1 | Miami | . | V | I | M | R | I | A | T | P | V | K | S | Q | . | K | . | . | N | . | E | D | R |
| Amino Acid Position | 66 | 67 | 68 | 70 | 72 | 74 | 82 | 123 | 145 | 158 | 159 | 172 | 196 | 199 | 203 | 211 | 248 | 250 | 256 | 258 | 259 | 266 | 269 | 287 | 299 | 304 | 336 | 340 | 341 | 354 | 388 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USI49342.1 | South Africa | T | S | T | K | I | R | N | S | V | K | I | S | N | I | N | I | K | R | D | R | V | S | G | I | I | S | S | N | K | T | R |
| ABA39858.1 | Ohio | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | E | . | . | . | . | . | . | . | . |
| AWW20361.1 | Santiago | I | . | . | . | . | K | . | . | I | . | . | . | . | . | . | . | . | K | N | K | . | . | . | . | . | . | N | . | . | . | . |
| WCL41104.1 | United Kingdom | I | . | . | . | M | K | . | . | I | . | . | . | . | . | . | . | . | K | N | K | . | . | . | . | . | . | N | . | . | . | . |
| QBA98125.1 | Calvados | I | . | . | . | M | K | . | . | I | . | . | . | . | . | . | . | . | K | N | K | . | . | . | . | . | . | N | . | . | . | . |
| QBA98124.1 | Pas-de-calais | I | . | . | . | M | K | . | . | I | . | . | . | . | . | . | . | . | K | N | K | . | . | . | . | . | . | N | . | . | . | . |
| WAA38453.1 | Brescia | I | . | . | . | M | K | . | . | I | . | V | . | . | . | . | . | . | K | N | K | . | . | . | . | . | . | N | . | . | . | . |
| ABY81495.1 | Miami | . | N | I | E | . | E | S | L | . | E | V | A | A | V | H | V | Q | . | . | . | I | N | . | V | V | P | . | S | Q | N | K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, I.; Tofani, S.; Lelli, D.; Vincifori, G.; Rosone, F.; Carvelli, A.; Diaconu, E.L.; La Rocca, D.; Manna, G.; Sabatini, S.; et al. First Reported Circulation of Equine Influenza H3N8 Florida Clade 1 Virus in Horses in Italy. Animals 2024, 14, 598. https://doi.org/10.3390/ani14040598
Ricci I, Tofani S, Lelli D, Vincifori G, Rosone F, Carvelli A, Diaconu EL, La Rocca D, Manna G, Sabatini S, et al. First Reported Circulation of Equine Influenza H3N8 Florida Clade 1 Virus in Horses in Italy. Animals. 2024; 14(4):598. https://doi.org/10.3390/ani14040598
Chicago/Turabian StyleRicci, Ida, Silvia Tofani, Davide Lelli, Giacomo Vincifori, Francesca Rosone, Andrea Carvelli, Elena Lavinia Diaconu, Davide La Rocca, Giuseppe Manna, Samanta Sabatini, and et al. 2024. "First Reported Circulation of Equine Influenza H3N8 Florida Clade 1 Virus in Horses in Italy" Animals 14, no. 4: 598. https://doi.org/10.3390/ani14040598
APA StyleRicci, I., Tofani, S., Lelli, D., Vincifori, G., Rosone, F., Carvelli, A., Diaconu, E. L., La Rocca, D., Manna, G., Sabatini, S., Costantini, D., Conti, R., Pacchiarotti, G., & Scicluna, M. T. (2024). First Reported Circulation of Equine Influenza H3N8 Florida Clade 1 Virus in Horses in Italy. Animals, 14(4), 598. https://doi.org/10.3390/ani14040598







