Assessment of Full-Fat Tenebrio molitor as Feed Ingredient for Solea senegalensis: Effects on Growth Performance and Lipid Profile
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source and Composition of TM Meal
2.2. Experimental Diets
2.3. Fish and Experimental Design
2.4. Fish Sampling
2.5. Growth Performance and Nutrient Utilization
2.6. Biochemical Analysis
2.6.1. Proximate Composition of Diets and Tissues
2.6.2. Total Lipids, Lipid Classes and FA Analyses
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Proximate Composition
3.2. Lipid Classes and Fatty Acid Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Nijdam, D.; Rood, T.; Westhoek, H. The price of protein: Review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Policy 2012, 37, 760–770. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Bakke-McKellep, A.M.; Penn, M.H.; Salas, P.M.; Refstie, S.; Sperstad, S.; Landsverk, T.; Ringø, E.; Krogdahl, Å. Effects of dietary soyabean meal, inulin and oxytetracycline on intestinal microbiota and epithelial cell stress, apoptosis and proliferation in the teleost Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2007, 97, 699–713. [Google Scholar] [CrossRef] [PubMed]
- UrÁN, P.A.; Schrama, J.W.; Rombout, J.H.W.M.; Obach, A.; Jensen, L.; Koppe, W.; Verreth, J.A.J. Soybean meal-induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquac. Nutr. 2008, 14, 324–330. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Sogari, G.; Bellezza Oddon, S.; Gasco, L.; van Huis, A.; Spranghers, T.; Mancini, S. Review: Recent advances in insect-based feeds: From animal farming to the acceptance of consumers and stakeholders. Animal 2023, 17, 100904. [Google Scholar] [CrossRef]
- Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects—Future Prospects for Food and Feed Security; FAO Forestry Paper 171; FAO: Rome, Italy, 2013. [Google Scholar]
- Quang Tran, H.; Van Doan, H.; Stejskal, V. Environmental consequences of using insect meal as an ingredient in aquafeeds: A systematic view. Rev. Aquac. 2022, 14, 237–251. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Gasco, L.; Finke, M.; Huis, A. Can diets containing insects promote animal health? J. Insects Food Feed 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Hua, K. A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture 2021, 530, 735732. [Google Scholar] [CrossRef]
- Marco, M.; Martinez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Alfiko, Y.; Xie, D.; Astuti, R.T.; Wong, J.; Wang, L. Insects as a feed ingredient for fish culture: Status and trends. Aquac. Fish. 2022, 7, 166–178. [Google Scholar] [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Mastoraki, M.; Mollá Ferrándiz, P.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture 2020, 528, 735511. [Google Scholar] [CrossRef]
- Piccolo, G.; Iaconisi, V.; Marono, S.; Gasco, L.; Loponte, R.; Nizza, S.; Bovera, F.; Parisi, G. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata). Anim. Feed Sci. Technol. 2017, 226, 12–20. [Google Scholar] [CrossRef]
- Basto, A.; Valente, L.M.P.; Conde-Sieira, M.; Soengas, J.L. Central regulation of food intake is not affected by inclusion of defatted Tenebrio molitor larvae meal in diets for European sea bass (Dicentrarchus labrax). Aquaculture 2021, 544, 737088. [Google Scholar] [CrossRef]
- Fabrikov, D.; Barroso, F.G.; Sánchez-Muros, M.J.; Hidalgo, M.C.; Cardenete, G.; Tomás-Almenar, C.; Melenchón, F.; Guil-Guerrero, J.L. Effect of feeding with insect meal diet on the fatty acid compositions of sea bream (Sparus aurata), tench (Tinca tinca) and rainbow trout (Oncorhynchus mykiss) fillets. Aquaculture 2021, 545, 737170. [Google Scholar] [CrossRef]
- Villalta, M.; Estévez, A.; Bransden, M.P.; Bell, J.G. The effect of graded concentrations of dietary DHA on growth, survival and tissue fatty acid profile of Senegal sole (Solea senegalensis) larvae during the Artemia feeding period. Aquaculture 2005, 249, 353–365. [Google Scholar] [CrossRef]
- Borges, P.; Oliveira, B.; Casal, S.; Dias, J.; Conceição, L.; Valente, L.M.P. Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. Br. J. Nutr. 2009, 102, 1007–1014. [Google Scholar] [CrossRef]
- Carballo, C.; Berbel, C.; Guerrero-Cózar, I.; Jiménez-Fernández, E.; Cousin, X.; Bégout, M.L.; Manchado, M. Evaluation of different tags on survival, growth and stress response in the flatfish Senegalese sole. Aquaculture 2018, 494, 10–18. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; Official Methods: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Olsen, R.E.; Henderson, R.J. The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J. Exp. Mar. Biol. Ecol. 1989, 129, 189–197. [Google Scholar] [CrossRef]
- Christie, W.W.; Han, X. Lipid Analysis: Isolation, Separation, Identification, and Structural Analysis of Lipids; Amer Oil Chemists Society: Bridgwater, UK, 2003. [Google Scholar]
- Hachero-Cruzado, I.; Rodriguez-Rua, A.; Roman-Padilla, J.; Ponce, M.; Fernandez-Diaz, C.; Manchado, M. Characterization of the genomic responses in early Senegalese sole larvae fed diets with different dietary triacylglycerol and total lipids levels. Comp. Biochem. Physiol. Part D Genom. Proteom. 2014, 12, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Rodiles, A.; Herrera, M.; Hachero-Cruzado, I.; Ruiz-Jarabo, I.; Mancera, J.M.; Cordero, M.L.; Lall, S.P.; Alarcón, F. Tissue composition, blood biochemistry and histology of digestive organs in Senegalese sole (Solea senegalensis) juveniles fed diets containing different plant protein ingredients. Aquac. Nutr. 2014, 21, 767–779. [Google Scholar] [CrossRef]
- Sokal, R.; Rohlf, F. Biometry: The Principles and Practice of Statistics in Biological Research; W. H. Freeman: New York, NY, USA, 2013. [Google Scholar]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Gasco, L.; Biasato, I.; Enes, P.; Gai, F. Chapter 16 - Potential and challenges for the use of insects as feed for aquaculture. In Mass Production of Beneficial Organisms, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 465–492. [Google Scholar]
- Sánchez, P.; Ambrosio, P.P.; Flos, R. Stocking density and sex influence individual growth of Senegalese sole (Solea senegalensis). Aquaculture 2010, 300, 93–101. [Google Scholar] [CrossRef]
- Salas-Leiton, E.; Anguís, V.; Rodríguez-Rúa, A.; Cañavate, J.P. Stocking homogeneous size groups does not improve growth performance of Senegalese sole (Solea senegalensis, Kaup 1858) juveniles: Individual growth related to fish size. Aquac. Eng. 2010, 43, 108–113. [Google Scholar] [CrossRef]
- Fabrikov, D.; Sánchez-Muros, M.J.; Barroso, F.G.; Tomás-Almenar, C.; Melenchón, F.; Hidalgo, M.C.; Morales, A.E.; Rodriguez-Rodriguez, M.; Montes-Lopez, J. Comparative study of growth performance and amino acid catabolism in Oncorhynchus mykiss, Tinca tinca and Sparus aurata and the catabolic changes in response to insect meal inclusion in the diet. Aquaculture 2020, 529, 735731. [Google Scholar] [CrossRef]
- Bruni, L.; Pastorelli, R.; Viti, C.; Gasco, L.; Parisi, G. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 2018, 487, 56–63. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- Henry, M.A.; Gai, F.; Enes, P.; Peréz-Jiménez, A.; Gasco, L. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 83, 308–313. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Yu, Y.-P. Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus×O. aureus. Aquaculture 1999, 179, 439–446. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Khosravi, S.; Yoon, K.-Y.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Lee, S.-M. Mealworm, Tenebrio molitor, as a feed ingredient for juvenile olive flounder, Paralichthys olivaceus. Aquac. Rep. 2021, 20, 100747. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio Molitor Meal in Rainbow Trout (Oncorhynchus Mykiss) Diets: Effects on Animal Performance, Nutrient Digestibility and Chemical Composition of Fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 2018, 496, 79–87. [Google Scholar]
- Jeong, S.-M.; Khosravi, S.; Mauliasari, I.R.; Lee, S.-M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for rainbow trout (Oncorhynchus mykiss) fry. Fish. Aquat. Sci. 2020, 23, 12. [Google Scholar] [CrossRef]
- Zacour, A.C.; Silva, M.E.; Cecon, P.R.; Bambirra, E.A.; Vieira, E.C. Effect of dietary chitin on cholesterol absorption and metabolism in rats. J. Nutr. Sci. Vitaminol. 1992, 38, 609–613. [Google Scholar] [CrossRef]
- Hirano, S. Chitin Biotechnology Applications. In Biotechnology Annual Review; El-Gewely, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 2, pp. 237–258. [Google Scholar]
- Kılınç, A.; Teke, M.; Önal, S.; Telefoncu, A. Immobilization of Pancreatic Lipase on Chitin and Chitosan. Prep. Biochem. Biotechnol. 2006, 36, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.Ø.; Penn, M.; Øverland, M.; Shearer, K.D.; Krogdahl, Å.; Mydland, L.T.; Storebakken, T. High inclusion of partially deshelled and whole krill meals in diets for Atlantic salmon (Salmo salar). Aquaculture 2010, 310, 164–172. [Google Scholar] [CrossRef]
- Campos, C.; Valente, L.M.P.; Borges, P.; Bizuayehu, T.; Fernandes, J.M.O. Dietary lipid levels have a remarkable impact on the expression of growth-related genes in Senegalese sole (Solea senegalensis Kaup). J. Exp. Biol. 2010, 213, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Basto, A.; Calduch-Giner, J.; Oliveira, B.; Petit, L.; Sá, T.; Maia, M.R.G.; Fonseca, S.C.; Matos, E.; Pérez-Sánchez, J.; Valente, L.M.P. The Use of Defatted Tenebrio molitor Larvae Meal as a Main Protein Source Is Supported in European Sea Bass (Dicentrarchus labrax) by Data on Growth Performance, Lipid Metabolism, and Flesh Quality. Front. Physiol. 2021, 12, 659567. [Google Scholar] [CrossRef] [PubMed]
- Iaconisi, V.; Bonelli, A.; Pupino, R.; Gai, F.; Parisi, G. Mealworm as dietary protein source for rainbow trout: Body and fillet quality traits. Aquaculture 2018, 484, 197–204. [Google Scholar] [CrossRef]
- Brodtkorb, T.; Rosenlund, G.; Lie, Ø. Effects of dietary levels of 20:5n-3 and 22:6n-3 on tissue lipid composition in juvenile Atlantic salmon, Salmo salar, with emphasis on brain and eye. Aquac. Nutr. 1997, 3, 175–187. [Google Scholar] [CrossRef]
- Morais, S.; Castanheira, F.; Martinez-Rubio, L.; Conceição, L.E.C.; Tocher, D.R. Long chain polyunsaturated fatty acid synthesis in a marine vertebrate: Ontogenetic and nutritional regulation of a fatty acyl desaturase with Δ4 activity. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2012, 1821, 660–671. [Google Scholar] [CrossRef]
- Borges, P.; Reis, B.; Fernandes, T.J.R.; Palmas, Â.; Castro-Cunha, M.; Médale, F.; Oliveira, M.B.P.P.; Valente, L.M.P. Senegalese sole juveniles can cope with diets devoid of supplemental fish oil while preserving flesh nutritional value. Aquaculture 2014, 418-419, 116–125. [Google Scholar] [CrossRef]

| Diets | ||||||
|---|---|---|---|---|---|---|
| TM | CTRL | FM5 | FM10 | PP10 | PP15 | |
| Ingredients (% dw) | ||||||
| Fish meal LT94 1 | - | 30.00 | 26.50 | 23.20 | 30.00 | 30.00 |
| Squid meal 2 | - | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| CPSP90 3 | - | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Krill meal 4 | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Wheat gluten 5 | - | 8.70 | 8.70 | 8.70 | 6.80 | 5.90 |
| Soybean protein concentrate 6 | - | 12.00 | 12.00 | 12.00 | 9.90 | 8.90 |
| Pea protein concentrate 7 | - | 9.00 | 9.00 | 9.00 | 7.10 | 6.20 |
| Wheat meal 8 | - | 12.10 | 12.00 | 11.60 | 11.40 | 10.20 |
| Tenebrio molitor meal 9 | - | 0.00 | 5.00 | 10.00 | 10.00 | 15.00 |
| Fish oil 10 | - | 5.40 | 5.40 | 5.40 | 5.00 | 4.00 |
| Soybean oil 11 | - | 3.00 | 1.60 | 0.30 | 0.00 | 0.00 |
| Soybean lecithin 12 | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Methionine 13 | - | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Lysine 14 | - | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Betaine 15 | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride 16 | - | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Digestive system improver 17 | - | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin and mineral premix 18 | - | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Vitamin C 19 | - | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Guar gum 20 | - | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Proximate composition (% dw) | ||||||
| Moisture | 7.78 | 6.49 | 6.16 | 6.18 | 7.29 | 6.50 |
| Ash | 2.92 | 8.07 | 7.68 | 7.37 | 8.03 | 8.17 |
| Protein | 43.77 | 54.03 | 53.81 | 53.68 | 52.95 | 52.73 |
| Lipid | 35.05 | 15.59 | 15.46 | 16.51 | 16.16 | 17.11 |
| Diets | ||||||
|---|---|---|---|---|---|---|
| TM | CTRL | FM5 | FM10 | PP10 | PP15 | |
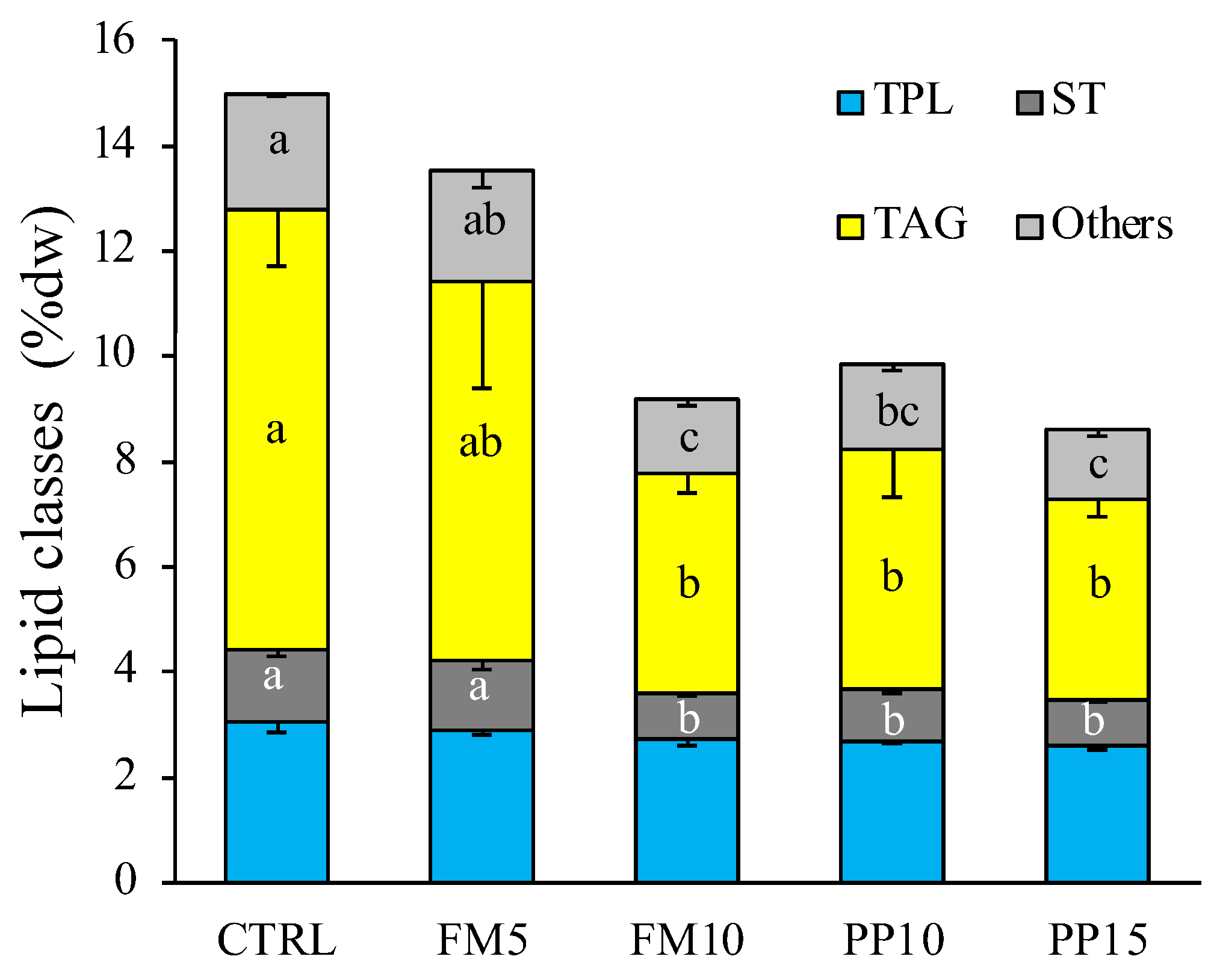
| Lipid classes (%TL) | ||||||
| Lyso-phosphatidylcholine | 0.29 | 0.84 | 1.12 | 1.08 | 1.25 | 1.20 |
| Sphingomyelin | 0.41 | 0.43 | 0.26 | 0.50 | 0.39 | 0.65 |
| Phosphatidylcholine | 1.94 | 4.97 | 5.71 | 5.26 | 5.44 | 5.13 |
| Phosphatidylserine | 0.36 | 1.52 | 2.57 | 2.51 | 2.07 | 1.91 |
| Phosphatidylinositol | 0.38 | 1.93 | 1.35 | 1.62 | 1.49 | 1.83 |
| Phosphatidylethanolamine | 2.54 | 2.11 | 2.90 | 2.51 | 2.19 | 2.57 |
| Diacylglycerol | 3.01 | 3.19 | 3.23 | 3.85 | 3.48 | 3.11 |
| Sterols | 7.36 | 9.65 | 10.94 | 10.20 | 10.37 | 9.70 |
| Free fatty acids | 34.58 | 13.53 | 17.29 | 19.16 | 19.12 | 20.09 |
| Triacylglycerol | 43.45 | 47.08 | 42.77 | 41.90 | 43.24 | 42.59 |
| Sterol esters | 4.10 | 6.22 | 6.19 | 5.19 | 5.65 | 5.61 |
| Fatty acids (% TFA) | ||||||
| 14:0 | 4.18 | 1.69 | 1.92 | 2.16 | 2.31 | 2.63 |
| 16:0, PA | 17.98 | 17.91 | 18.40 | 19.13 | 19.33 | 19.48 |
| 18:0 | 2.55 | 5.19 | 4.94 | 4.81 | 4.73 | 4.45 |
| Total Saturated FA | 24.72 | 26.19 | 26.45 | 27.25 | 27.44 | 27.56 |
| 16:1n-7 | 2.82 | 3.41 | 3.56 | 3.74 | 3.93 | 3.89 |
| 18:1n-9, OA | 51.75 | 17.96 | 22.46 | 26.90 | 26.64 | 30.44 |
| 18:1n-7 | 0.10 | 2.37 | 2.08 | 1.87 | 1.90 | 1.66 |
| 20:1n-9 | 0.10 | 1.52 | 1.39 | 1.29 | 1.37 | 1.18 |
| 22:1n-11 | nd | 0.96 | 0.89 | 0.79 | 0.93 | 0.80 |
| Total Monounsaturated FA | 55.86 | 27.39 | 31.75 | 36.02 | 36.33 | 39.48 |
| 18:2n-6, LA | 18.00 | 19.54 | 16.65 | 13.89 | 12.13 | 12.89 |
| 20:4n-6, ARA | 0.00 | 1.10 | 1.06 | 1.01 | 1.00 | 0.83 |
| 22:5n-6 | nd | 0.79 | 0.76 | 0.73 | 0.74 | 0.59 |
| Total n-6 Polyunsaturated FA | 18.00 | 22.05 | 19.13 | 16.25 | 14.41 | 14.82 |
| 18:3n-3, LNA | 0.29 | 2.42 | 1.85 | 1.30 | 1.13 | 0.99 |
| 18:4n-3 | nd | 0.77 | 0.71 | 0.63 | 0.71 | 0.61 |
| 20:5n-3, EPA | nd | 5.73 | 5.38 | 4.81 | 5.24 | 4.50 |
| 22:5n-3, DPA | nd | 0.91 | 0.99 | 0.94 | 0.98 | 0.82 |
| 22:6n-3, DHA | nd | 13.05 | 12.37 | 11.62 | 12.37 | 10.01 |
| Total n-3 Polyunsaturated FA | 0.29 | 23.61 | 22.00 | 19.94 | 21.12 | 17.49 |
| CTRL | FM5 | FM10 | PP10 | PP15 | p-Value | |
|---|---|---|---|---|---|---|
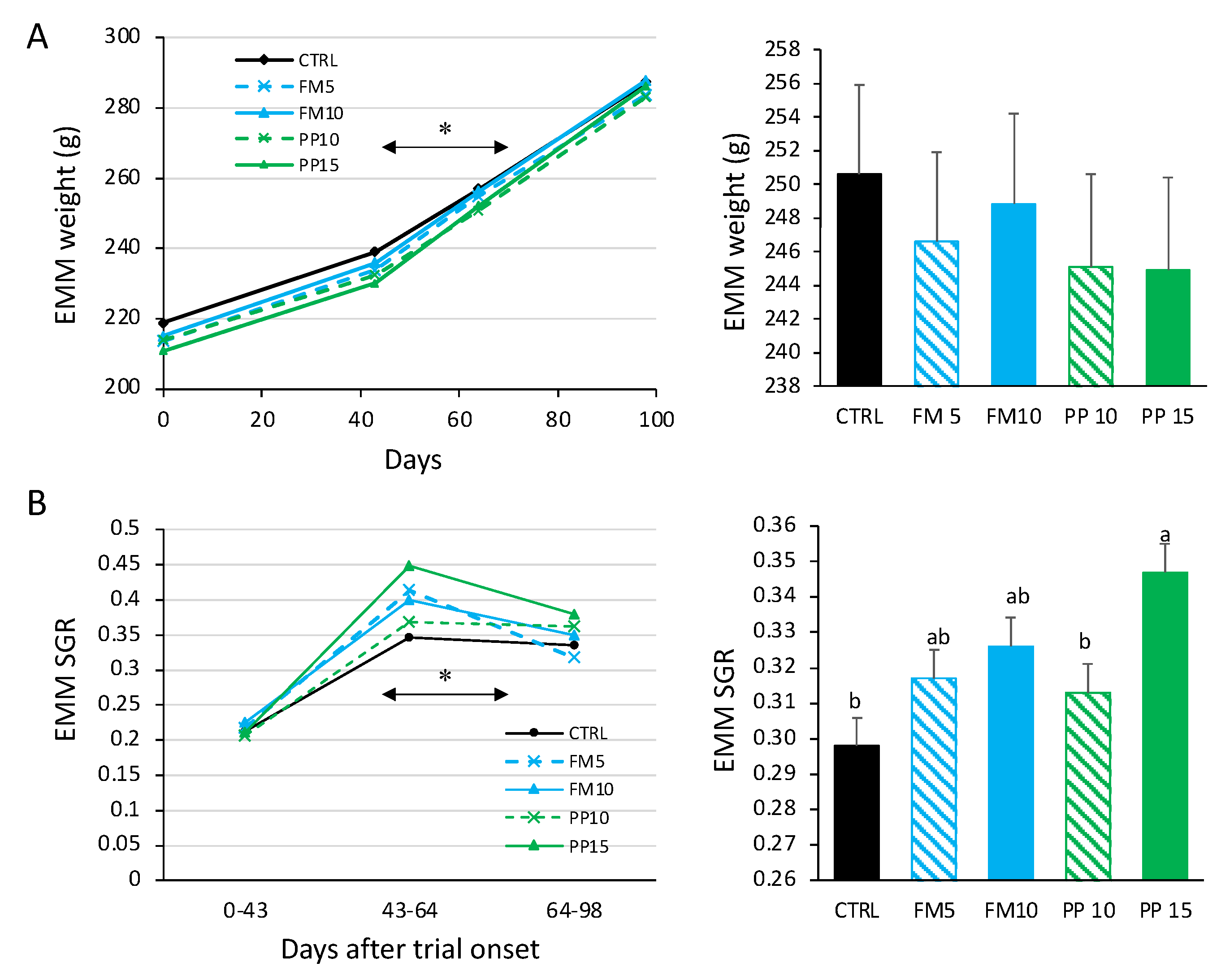
| Initial body weight (IBW, g) | 219.2 ± 5.3 | 213.7 ± 4.7 | 215.1 ± 5.2 | 214.2 ± 5.3 | 210.8 ± 4.6 | 0.979 |
| Final body weight (FBW, g) | 288.6 ± 6.5 | 284.5 ± 5.8 | 288.6 ± 6.1 | 283.8 ± 6.2 | 288.3 ± 5.5 | 0.887 |
| Specific Growth Rate (SGR, % d−1) | 0.30 ± 0.01 b | 0.32 ± 0.01 ab | 0.33 ± 0.01 ab | 0.31 ± 0.01 b | 0.35 ± 0.01 a | 0.012 |
| Feed Conversion Ratio (FCR) | 1.76 ± 0.20 | 1.58 ± 0.14 | 1.61 ± 0.02 | 1.71 ± 0.17 | 1.42 ± 0.23 | 0.426 |
| Protein efficiency ratio (PER) | 1.00 ± 0.08 | 1.11 ± 0.08 | 1.09 ± 0.08 | 1.04 ± 0.08 | 1.26 ± 0.08 | 0.299 |
| CTRL | FM5 | FM10 | PP10 | PP15 | p (Diet) | |
|---|---|---|---|---|---|---|
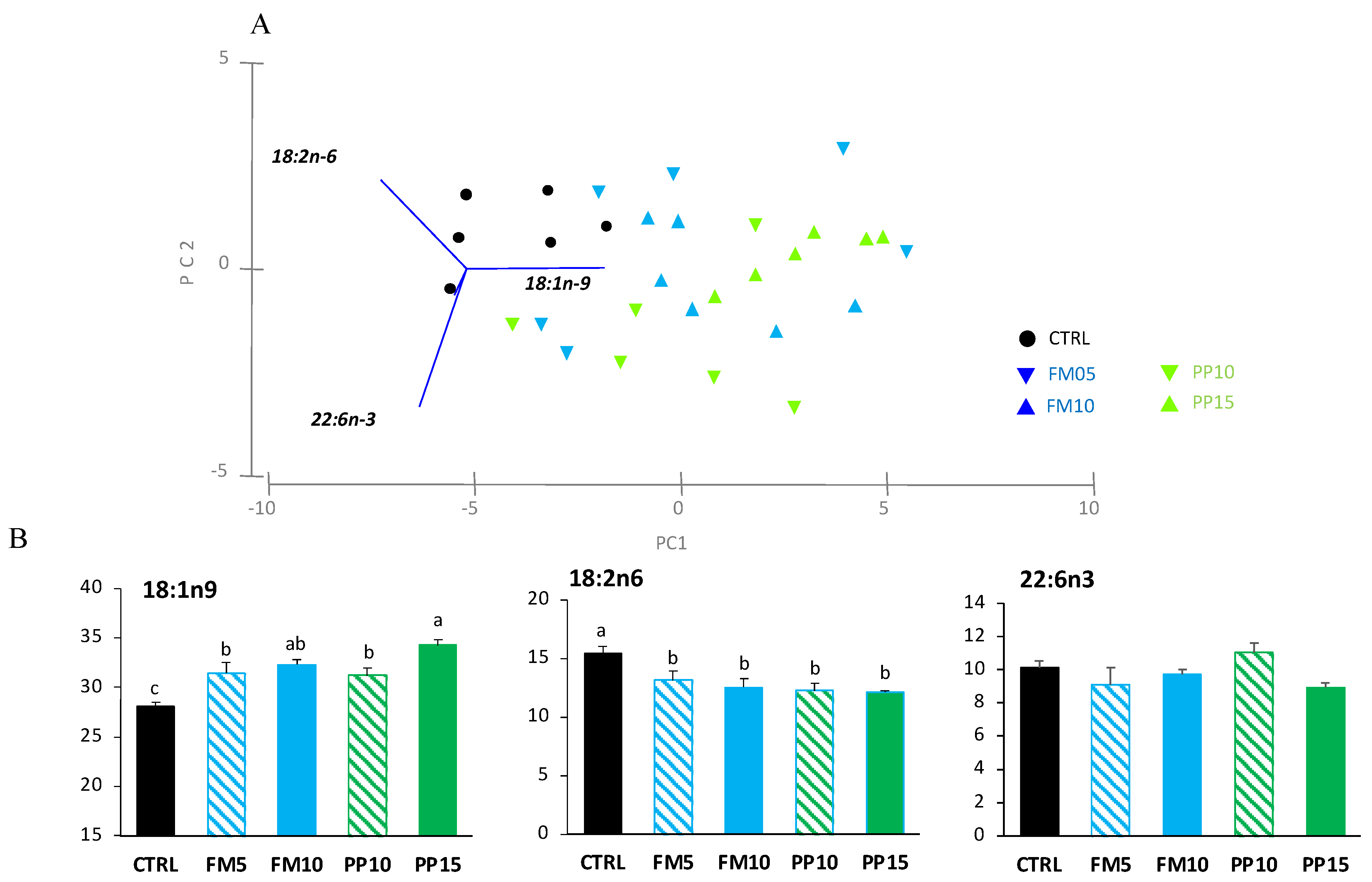
| 14:0 | 3.14 ± 0.21 | 3.46 ± 0.19 | 3.42 ± 0.07 | 3.40 ± 0.16 | 3.69 ± 0.09 | 0.122 |
| 15:0 | 0.45 ± 0.02 | 0.38 ± 0.03 | 0.41 ± 0.03 | 0.46 ± 0.03 | 0.40 ± 0.01 | 0.092 |
| 16:0 | 17.29 ± 0.29 | 17.44 ± 0.32 | 16.88 ± 0.31 | 16.69 ± 0.30 | 16.89 ± 0.15 | 0.351 |
| 18:0 | 3.93 ± 0.38 | 3.92 ± 0.27 | 3.72 ± 0.37 | 3.39 ± 0.24 | 3.25 ± 0.19 | 0.347 |
| 20:0 | 0.20 ± 0.02 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.00 | 0.974 |
| 22:0 | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.095 |
| 24:0 | 0.10 ± 0.02 | 0.15 ± 0.04 | 0.11 ± 0.02 | 0.13 ± 0.02 | 0.11 ± 0.03 | 0.640 |
| Total Saturated FAs | 25.29 ± 0.75 | 25.70 ± 0.46 | 24.89 ± 0.56 | 24.41 ± 0.55 | 24.69 ± 0.32 | 0.396 |
| 16:1n-9 | 0.78 ± 0.03 b | 0.90 ± 0.05 ab | 1.02 ± 0.07 a | 1.09 ± 0.05 a | 1.05 ± 0.03 a | 0.002 |
| 16:1n-7 | 4.94 ± 0.18 | 5.54 ± 0.33 | 5.23 ± 0.12 | 5.33 ± 0.17 | 5.48 ± 0.11 | 0.306 |
| 18:1n-9 | 28.09 ± 0.37 c | 31.44 ± 1.11 b | 32.28 ± 0.54 ab | 31.18 ± 0.80 b | 34.24 ± 0.59 a | 0.000 |
| 18:1n-7 | 3.34 ± 0.06 | 3.19 ± 0.09 | 3.11 ± 0.05 | 3.19 ± 0.08 | 3.01 ± 0.10 | 0.103 |
| 20:1n-11 | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.00 | 0.15 ± 0.00 | 0.15 ± 0.01 | 0.069 |
| 20:1n-9 | 1.38 ± 0.06 | 1.44 ± 0.05 | 1.44 ± 0.05 | 1.435 ± 0.07 | 1.68 ± 0.19 | 0.172 |
| 20:1n-7 | 0.20 ± 0.01 | 0.20 ± 0.00 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.566 |
| 22:1n-11 | 0.51 ± 0.04 | 0.46 ± 0.03 | 0.49 ± 0.03 | 0.54 ± 0.02 | 0.54 ± 0.04 | 0.391 |
| 22:1n-9cis | 0.27 ± 0.01 | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.28 ± 0.01 | 0.27 ± 0.02 | 0.986 |
| 24:1n-9 | 0.31 ± 0.06 | 0.33 ± 0.03 | 0.34 ± 0.03 | 0.35 ± 0.02 | 0.26 ± 0.06 | 0.658 |
| Total Monounsaturated FAs | 40.00 ± 0.56 c | 43.92 ± 1.37 ab | 44.52 ± 0.48 ab | 43.74 ± 0.87 b | 46.85 ± 0.47 a | 0.000 |
| 18:2n-6 | 15.47 ± 0.57 a | 13.16 ± 0.76 b | 12.57 ± 0.73 b | 12.26 ± 0.66 b | 12.12 ± 0.13 b | 0.001 |
| 18:3n-6 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.00 | 0.10 ± 0.01 | 0.09 ± 0.00 | 0.252 |
| 20:2n-6 | 1.18 ± 0.065 ab | 1.21 ± 0.06 a | 1.03 ± 0.03 ab | 0.94 ± 0.04 b | 0.94 ± 0.02 b | 0.000 |
| 20:3n-6 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.14 ± 0.01 | 0.480 |
| 20:4n-6 | 0.65 ± 0.07 | 0.66 ± 0.09 | 0.62 ± 0.06 | 0.76 ± 0.10 | 0.61 ± 0.05 | 0.698 |
| 22:4n-6 | 0.27 ± 0.01 | 0.26 ± 0.03 | 0.25 ± 0.01 | 0.26 ± 0.02 | 0.21 ± 0.01 | 0.269 |
| 22:5n-6 | 0.74 ± 0.03 | 0.70 ± 0.07 | 0.71 ± 0.02 | 0.74 ± 0.05 | 0.61 ± 0.02 | 0.226 |
| Total n-6 Polyunsaturated FAs | 18.57 ± 0.57 a | 16.24 ± 1.00 ab | 15.42 ± 0.81 b | 15.22 ± 0.72 b | 14.71 ± 0.14 b | 0.001 |
| 18:3n-3 | 1.39 ± 0.06 a | 1.09 ± 0.05 b | 1.03 ± 0.07 b | 1.01 ± 0.08 b | 0.91 ± 0.05 b | 0.000 |
| 18:4n-3 | 0.24 ± 0.02 ab | 0.18 ± 0.01 b | 0.22 ± 0.02 ab | 0.25 ± 0.02 a | 0.21 ± 0.01 ab | 0.035 |
| 20:3n-3 | 0.56 ± 0.021 a | 0.55 ± 0.02 a | 0.45 ± 0.01 b | 0.41 ± 0.02 b | 0.39 ± 0.02 b | 0.000 |
| 20:4n-3 | 0.35 ± 0.02 ab | 0.29 ± 0.01 b | 0.33 ± 0.02 ab | 0.36 ± 0.03 a | 0.31 ± 0.01 ab | 0.034 |
| 20:5n-3 | 0.36 ± 0.05 ab | 0.25 ± 0.03 b | 0.30 ± 0.03 ab | 0.40 ± 0.06 a | 0.31 ± 0.02 ab | 0.024 |
| 22:5n-3 | 2.34 ± 0.31 | 1.83 ± 0.15 | 2.24 ± 0.06 | 2.34 ± 0.32 | 1.96 ± 0.12 | 0.260 |
| 22:6n-3 | 10.13 ± 0.41 | 9.13 ± 1.00 | 9.73 ± 0.29 | 11.05 ± 0.58 | 8.91 ± 0.33 | 0.140 |
| Total n-3 Polyunsaturated FA | 15.41 ± 0.80 | 13.33 ± 1.16 | 14.32 ± 0.28 | 15.83 ± 0.89 | 13.00 ± 0.48 | 0.061 |
| n-3PUFA/n-6PUFA | 0.83 ± 0.04 | 0.82 ± 0.06 | 0.94 ± 0.05 | 1.05 ± 0.07 | 0.88 ± 0.03 | 0.048 |
| CTRL | FM5 | FM10 | PP10 | PP15 | p (Diet) | |
|---|---|---|---|---|---|---|
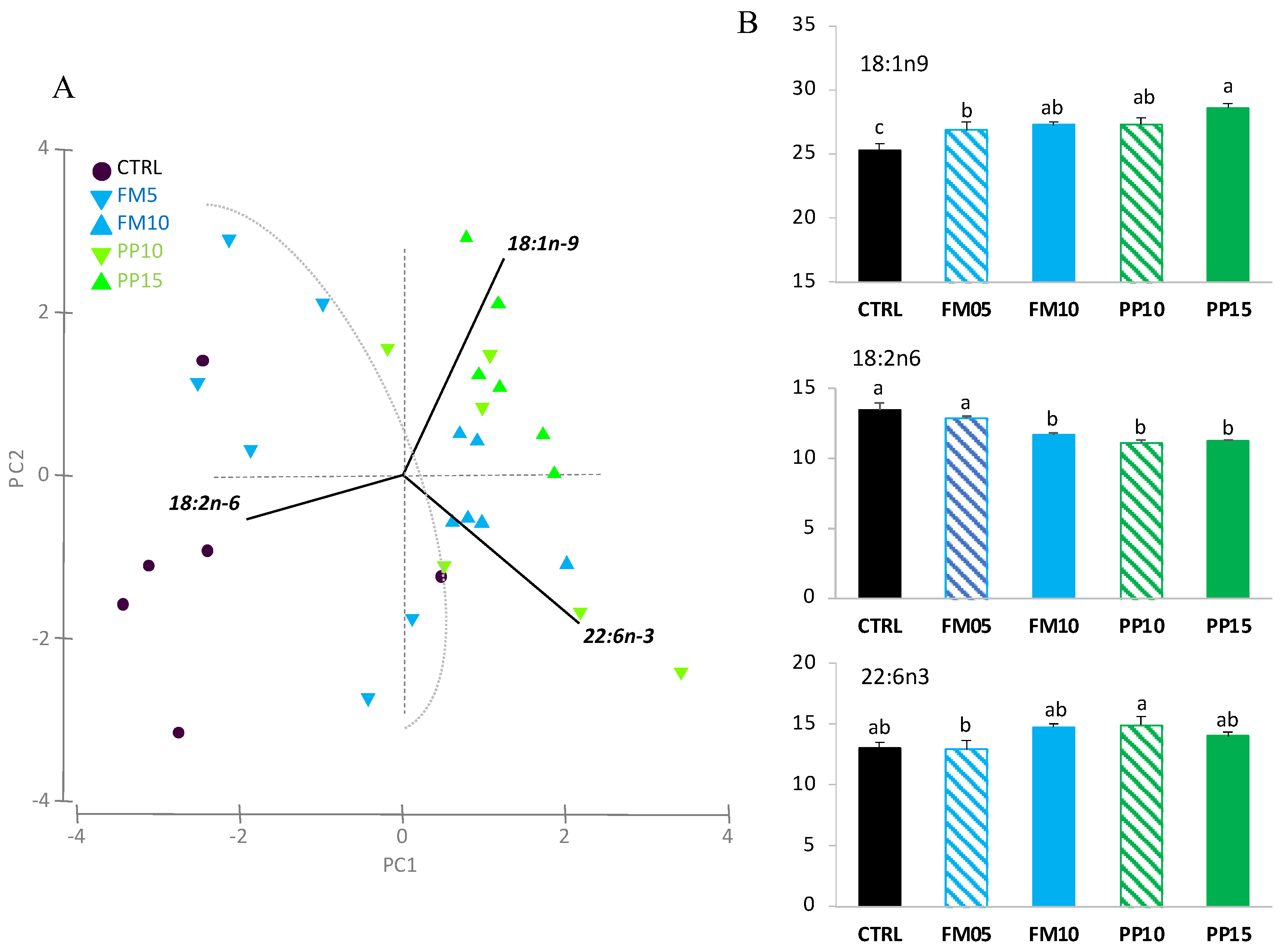
| 14:0 | 2.30 ± 0.13 | 2.36 ± 0.12 | 1.86 ± 0.16 | 1.99 ± 0.21 | 2.16 ± 0.11 | 0.190 |
| 15:0 | 0.48 ± 0.02 a | 0.47 ± 0.02 ab | 0.40 ± 0.02 b | 0.40 ± 0.02 ab | 0.40 ± 0.01 b | 0.009 |
| 16:0 | 17.79 ± 0.25 | 18.11 ± 0.12 | 17.97 ± 0.21 | 18.08 ± 0.17 | 18.43 ± 0.10 | 0.223 |
| 18:0 | 3.54 ± 0.08 b | 3.63 ± 0.17 ab | 4.10 ± 0.13 a | 3.82 ± 0.15 ab | 3.90 ± 0.03 ab | 0.035 |
| 20:0 | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.26 ± 0.01 | 0.26 ± 0.01 | 0.457 |
| 22:0 | 0.19 ± 0.01 b | 0.19 ± 0.00 ab | 0.18 ± 0.01 ab | 0.17 ± 0.01 b | 0.18 ± 0.00 ab | 0.035 |
| 24:0 | 0.08 ± 0.03 | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.10 ± 0.01 | 0.07 ± 0.02 | 0.657 |
| Total Saturated FAs | 24.65 ± 0.41 | 25.11 ± 0.19 | 24.88 ± 0.38 | 24.83 ± 0.34 | 25.39 ± 0.19 | 0.568 |
| 16:1n-9 | 0.47 ± 0.01 b | 0.54 ± 0.01 a | 0.55 ± 0.02 a | 0.56 ± 0.01 a | 0.58 ± 0.01 a | 0.000 |
| 16:1n-7 | 4.79 ± 0.12 | 4.76 ± 0.19 | 4.18 ± 0.12 | 4.40 ± 0.21 | 4.35 ± 0.07 | 0.031 |
| 18:1n-9 | 25.33 ± 0.47 c | 26.88 ± 0.62 b | 27.29 ± 0.22 ab | 27.31 ± 0.48 ab | 28.62 ± 0.30 a | 0.000 |
| 18:1n-7 | 2.80 ± 0.12 a | 2.70 ± 0.19 ab | 2.56 ± 0.12 bc | 2.58 ± 0.21 bc | 2.43 ± 0.07 c | 0.000 |
| 20:1n-11 | 0.21 ± 0.01 a | 0.20 ± 0.01 ab | 0.19 ± 0.01 ab | 0.201 ± 0.00 ab | 0.19 ± 0.01 b | 0.023 |
| 20:1n-9 | 1.51 ± 0.05 | 1.45 ± 0.04 | 1.40 ± 0.02 | 1.43 ± 0.04 | 1.36 ± 0.02 | 0.084 |
| 20:1n-7 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.860 |
| 22:1n-11 | 0.76 ± 0.03 | 0.71 ± 0.02 ab | 0.62 ± 0.02 b | 0.69 ± 0.03 ab | 0.64 ± 0.01 b | 0.002 |
| 22:1n-9cis | 0.29 ± 0.01 | 0.29 ± 0.01 | 0.26 ± 0.03 | 0.28 ± 0.01 | 0.27 ± 0.02 | 0.157 |
| 24:1n-9 | 0.40 ± 0.01 | 0.22 ± 0.10 | 0.41 ± 0.03 | 0.43 ± 0.01 | 0.41 ± 0.02 | 0.046 |
| Total Monounsaturated FAs | 36.72 ± 0.68 | 37.89 ± 0.92 | 37.61 ± 0.35 | 38.05 ± 0.67 | 39.01 ± 0.43 | 0.100 |
| 18:2n-6 | 13.50 ± 0.52 a | 12.86 ± 0.19 a | 11.69 ± 0.15 b | 11.15 ± 0.19 b | 11.25 ± 0.06 b | 0.000 |
| 18:3n-6 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.117 |
| 20:2n-6 | 0.66 ± 0.01 abc | 0.68 ± 0.01 a | 0.67 ± 0.00 ab | 0.62 ± 0.01 bc | 0.62 ± 0.01 c | 0.001 |
| 20:3n-6 | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.341 |
| 20:4n-6 | 0.82 ± 0.04 b | 0.83 ± 0.06 b | 1.01 ± 0.03 a | 0.98 ± 0.06 ab | 0.96 ± 0.03 ab | 0.008 |
| 22:4n-6 | 0.28 ± 0.01 ab | 0.29 ± 0.01 b | 0.30 ± 0.00 a | 0.30 ± 0.01 ab | 0.27 ± 0.01 b | 0.009 |
| 22:5n-6 | 0.61 ± 0.02 | 0.63 ± 0.03 | 0.70 ± 0.02 | 0.69 ± 0.04 | 0.66 ± 0.01 | 0.061 |
| Total n-6 Polyunsaturated FAs | 16.19 ± 0.50 a | 15.60 ± 0.16 b | 14.68 ± 0.15 c | 14.05 ± 0.13 c | 14.03 ± 0.06 c | 0.000 |
| 18:3n-3 | 1.85 ± 0.08 a | 1.60 ± 0.05 b | 1.35 ± 0.03 c | 1.32 ± 0.06 c | 1.20 ± 0.03 c | 0.000 |
| 18:4n-3 | 0.64 ± 0.04 a | 0.57 ± 0.03 ab | 0.49 ± 0.02 b | 0.52 ± 0.03 b | 0.46 ± 0.01 b | 0.001 |
| 20:3n-3 | 0.36 ± 0.01 a | 0.35 ± 0.01 a | 0.32 ± 0.01 ab | 0.29 ± 0.01 b | 0.28 ± 0.01 b | 0.000 |
| 20:4n-3 | 0.44 ± 0.02 a | 0.41 ± 0.01 ab | 0.39 ± 0.00 b | 0.39 ± 0.01 b | 0.37 ± 0.01 b | 0.001 |
| 20:5n-3 | 1.69 ± 0.07 a | 1.43 ± 0.03 ab | 1.46 ± 0.04 b | 1.57 ± 0.06 b | 1.40 ± 0.04 b | 0.001 |
| 22:5n-3 | 3.76 ± 0.08 a | 3.50 ± 0.12 ab | 3.54 ± 0.05 ab | 3.54 ± 0.12 ab | 3.28 ± 0.09 b | 0.043 |
| 22:6n-3 | 13.04 ± 0.48 ab | 12.93 ± 0.70 b | 14.77 ± 0.27 ab | 14.92 ± 0.69 a | 14.05 ± 0.34 ab | 0.012 |
| Total n-3 Polyunsaturated FAs | 21.79 ± 0.44 | 20.80 ± 0.73 | 22.32 ± 0.22 | 22.56 ± 0.63 | 21.07 ± 0.44 | 0.045 |
| n-3PUFA/n-6PUFA | 1.35 ± 0.05 bc | 1.33 ± 0.040 c | 1.52 ± 0.01 a | 1.61 ± 0.05 a | 1.50 ± 0.03 ab | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachero-Cruzado, I.; Betancor, M.B.; Coronel-Dominguez, A.J.; Manchado, M.; Alarcón-López, F.J. Assessment of Full-Fat Tenebrio molitor as Feed Ingredient for Solea senegalensis: Effects on Growth Performance and Lipid Profile. Animals 2024, 14, 595. https://doi.org/10.3390/ani14040595
Hachero-Cruzado I, Betancor MB, Coronel-Dominguez AJ, Manchado M, Alarcón-López FJ. Assessment of Full-Fat Tenebrio molitor as Feed Ingredient for Solea senegalensis: Effects on Growth Performance and Lipid Profile. Animals. 2024; 14(4):595. https://doi.org/10.3390/ani14040595
Chicago/Turabian StyleHachero-Cruzado, Ismael, Mónica B. Betancor, Antonio Jesús Coronel-Dominguez, Manuel Manchado, and Francisco Javier Alarcón-López. 2024. "Assessment of Full-Fat Tenebrio molitor as Feed Ingredient for Solea senegalensis: Effects on Growth Performance and Lipid Profile" Animals 14, no. 4: 595. https://doi.org/10.3390/ani14040595
APA StyleHachero-Cruzado, I., Betancor, M. B., Coronel-Dominguez, A. J., Manchado, M., & Alarcón-López, F. J. (2024). Assessment of Full-Fat Tenebrio molitor as Feed Ingredient for Solea senegalensis: Effects on Growth Performance and Lipid Profile. Animals, 14(4), 595. https://doi.org/10.3390/ani14040595









