Supplementation with Silybum marianum Extract, Synbiotics, Omega-3 Fatty Acids, Vitamins, and Minerals: Impact on Biochemical Markers and Fecal Microbiome in Overweight Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Laboratory Analysis
2.2.1. Blood Analysis
2.2.2. Microbiome Analysis
2.3. Bioinformatic and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krawczyk, M.; Grąt, M.; Barski, K.; Ligocka, J.; Antczak, A.; Kornasiewicz, O.; Skalski, M.; Patkowski, W.; Nyckowski, P.; Zieniewicz, K.; et al. 1000 liver transplantations at the Department of General, Transplant and Liver Surgery, Medical University of Warsaw—Analysis of indications and results. Pol. Przegl. Chir. 2012, 84, 304–312. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Andrade, F.; Schlechta Portella, C.F. Research methods in complementary and alternative medicine: An integrative review. J. Integr. Med. 2018, 16, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Martello, E.; Perondi, F.; Bisanzio, D.; Lippi, I.; Meineri, G.; Gabriele, V. Antioxidant Effect of a Dietary Supplement Containing Fermentative S-Acetyl-Glutathione and Silybin in Dogs with Liver Disease. Vet. Sci. 2023, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Perondi, F.; Bisanzio, D.; Adami, R.; Lippi, I.; Meineri, G.; Cutrignelli, M.I.; Massa, S.; Martello, E. The effect of a diet supplement containing S-acetyl-glutathione (SAG) and other antioxidant natural ingredients on glutathione peroxidase in healthy dogs: A pilot study. Ital. J. Anim. Sci. 2023, 22, 589–593. [Google Scholar] [CrossRef]
- Sgorlon, S.; Stefanon, B.; Sandri, M.; Colitti, M. Nutrigenomic activity of plant derived compounds in health and disease: Results of a dietary intervention study in dog. Res. Vet. Sci. 2016, 109, 142–148. [Google Scholar] [CrossRef]
- Murai, T.; Kawasumi, K.; Tominaga, K.; Okada, Y.; Kobayashi, M.; Arai, T. Effects of astaxanthin supplementation in healthy and obese dogs. Vet. Med. 2019, 10, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Barić Rafaj, R.; Kuleš, J.; Marinculić, A.; Tvarijonaviciute, A.; Ceron, J.; Mihaljević, Ž.; Tumpa, A.; Mrljak, V. Plasma markers of inflammation and hemostatic and endothelial activity in naturally overweight and obese dogs. BMC Vet. Res. 2017, 13, 13. [Google Scholar] [CrossRef]
- Bahmani, M.; Shirzad, H.; Rafieian, S.; Rafieian-Kopaei, M. Silybum marianum: Beyond Hepatoprotection. J. Evid. Based Complement. Altern. Med. 2015, 20, 292–301. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood reflects tissue oxidative stress: A systematic review. Biomarkers 2015, 20, 97–108. [Google Scholar] [CrossRef]
- Li, Q.; Lauber, C.L.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S.S. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio 2017, 8, e01703-16. [Google Scholar] [CrossRef]
- Barry-Heffernan, C.; Ekena, J.; Dowling, S.; Pinkerton, M.E.; Viviano, K. Biomarkers of oxidative stress as an assessment of the redox status of the liver in dogs. J. Vet. Intern. Med. 2019, 33, 611–617. [Google Scholar] [CrossRef]
- Habermaass, V.; Olivero, D.; Gori, E.; Mariti, C.; Longhi, E.; Marchetti, V. Intestinal Microbiome in Dogs with Chronic Hepatobiliary Disease: Can We Talk about the Gut-Liver Axis? Animals 2023, 13, 3174. [Google Scholar] [CrossRef]
- Webster, C.R.L.; Center, S.A.; Cullen, J.M.; Penninck, D.G.; Richter, K.P.; Twedt, D.C.; Watson, P.J. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J. Vet. Intern. Med. 2019, 33, 1173–1200. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Sherriff, J.L.; O’Sullivan, T.A.; Properzi, C.; Oddo, J.L.; Adams, L.A. Choline, Its Potential Role in Nonalcoholic Fatty Liver Disease, and the Case for Human and Bacterial Genes. Adv. Nutr. 2016, 7, 5–13. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ko, K.S. Protective Effects of S-Adenosylmethionine and Its Combinations with Taurine and/or Betaine Against Lipopolysaccharide or Polyinosinic-polycytidylic Acid-induced Acute Hepatotoxicity. J. Cancer Prev. 2016, 21, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Kusakabe, M.; Imanishi, I.; Hisano, T.; Fukamachi, T.; Taguchi, N.; Iyori, K.; Hsiao, Y.H. A randomised, double-blinded, controlled trial to determine the efficacy of combined therapy of oclacitinib and marine oil extract PCSO-524 in dogs with atopic dermatitis. Vet. Dermatol. 2023, 34, 523–531. [Google Scholar] [CrossRef]
- Ephraim, E.; Brockman, J.A.; Jewell, D.E. A Diet Supplemented with Polyphenols, Prebiotics and Omega-3 Fatty Acids Modulates the Intestinal Microbiota and Improves the Profile of Metabolites Linked with Anxiety in Dogs. Biology 2022, 11, 976. [Google Scholar] [CrossRef]
- Giannetto, C.; Arfuso, F.; Giudice, E.; Rizzo, M.; Piccione, G.; Mhalhel, K.; Levanti, M. Antioxidant and Hepatoprotective Effect of a Nutritional Supplement with Silymarin Phytosome, Choline Chloride, l-Cystine, Artichoke, and Vitamin E in Dogs. Antioxidants 2022, 11, 2339. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Wu, J.; Ye, T.; Wang, S.; Wang, P.; Xing, D. Butyrate-producing bacteria, and the gut-heart axis in atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 507, 236–241. [Google Scholar] [CrossRef]
- Oba, P.M.; Kelly, J.; Kostiuk, D.; Swanson, K.S. Effects of weight loss and feeding specially formulated diets on the body composition, blood metabolite profiles, voluntary physical activity, and fecal metabolites and microbiota of obese dogs. J. Anim. Sci. 2023, 101, skad073. [Google Scholar] [CrossRef] [PubMed]
- Phungviwatnikul, T.; Lee, A.H.; Belchik, S.E.; Suchodolski, J.S.; Swanson, K.S. Weight loss and high-protein, high-fiber diet consumption impact blood metabolite profiles, body composition, voluntary physical activity, fecal microbiota, and fecal metabolites of adult dogs. J. Anim. Sci. 2022, 100, skab379. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Shalaby, N.; Samocha-Bonet, D.; Kaakoush, N.O.; Danta, M. The Role of the Gastrointestinal Microbiome in Liver Disease. Pathogens 2023, 12, 1087. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-liver axis in hepatic disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef]
- Konturek, P.C.; Harsch, I.A.; Konturek, K.; Schink, M.; Konturek, T.; Neurath, M.F.; Zopf, Y. Gut–Liver Axis: How Do Gut Bacteria Influence the Liver? Med. Sci. 2018, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Minemura, M.; Shimizu, Y. Gut microbiota and liver diseases. World J. Gastroenterol. 2015, 21, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Desdouets, C. Oxidative stress promotes pathologic polyploidization in non-alcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 981–992. [Google Scholar]
- Wan, M.L.Y.; El-Nezami, H. Targeting gut microbiota in hepatocellular carcinoma: Probiotics as a novel therapy. Hepatobiliary Surg. Nutr. 2018, 7, 11–20. [Google Scholar] [CrossRef]
- Laflamme, D.P. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food. Available online: https://europeanpetfood.org (accessed on 31 December 2023).
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Subcommittee on Dog and Cat Nutrition; Committee on Animal Nutrition. Available online: https://nap.nationalacademies.org/catalog/10668 (accessed on 31 December 2023).
- Marchegiani, A.; Fruganti, A.; Gavazza, A.; Mangiaterra, S.; Candellone, A.; Fusi, E.; Rossi, G.; Cerquetella, M. Evidence on Molecules Most Frequently Included in Canine and Feline Complementary Feed to Support Liver Function. Vet. Med. Int. 2020, 9, 9185759. [Google Scholar] [CrossRef] [PubMed]
- Scarsella, E.; Cintio, M.; Iacumin, L.; Ginaldi, F.; Stefanon, B. Interplay between Neuroendocrine Biomarkers and Gut Microbiota in Dogs Supplemented with Grape Proanthocyanidins: Results of Dietary Intervention Study. Animals 2020, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable, and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Greengene Database. Available online: https://greengenes.secondgenome.com (accessed on 31 December 2023).
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knigh, R. Quantitative and qualitative diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.microbiomeanalyst.ca/ (accessed on 31 December 2023).
- IBM SPSS Statistics for Windows; Version 25.0; IBM Corp.: Armonk, NY, USA, 2017; Available online: https://www.ibm.com (accessed on 31 December 2023).
- Grundy, S.M.; Hansen, B.; Smith, S.C.; Cleeman, J.I.; Kahn, R.A.; American Heart Association, National Heart, Lung, and Blood Institute, American Diabetes Association. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 19–24. [Google Scholar]
- Verkest, K.R. Is the metabolic syndrome a useful clinical concept in dogs? review of the evidence. Vet. J. 2014, 199, 24–30. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Barić-Rafaj, R.; Horvatic, A.; Muñoz-Prieto, A.; Guillemin, N.; Lamy, E.; Tumpa, A.; Ceron, J.J.; Martinez-Subiela, S.; Mrljak, V. Identification of changes in serum analytes and possible metabolic pathways associated with canine obesity-related metabolic dysfunction. Vet. J. 2019, 244, 51–59. [Google Scholar] [CrossRef]
- Gommeren, K.; Desmas, I.; Garcia, A.; Bauer, N.; Moritz, A.; Roth, J.; Peeters, D. Inflammatory cytokine and C-reactive protein concentrations in dogs with systemic inflammatory response syndrome. J. Vet. Emerg. Crit. Care 2018, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Torrente, C.; Manzanilla, E.G.; Bosch, L.; Fresno, L.; Rivera Del Alamo, M.; Andaluz, A.; Saco, Y.; Ruiz de Gopegui, R. Plasma iron, C-reactive protein, albumin, and plasma fibrinogen concentrations in dogs with systemic inflammatory response syndrome. J. Vet. Emerg. Crit. Care 2015, 25, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.C.; Girish, C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 2006, 124, 491–504. [Google Scholar] [PubMed]
- Trappoliere, M.; Caligiuri, A.; Schmid, M.; Bertolani, C.; Failli, P.; Vizzutti, F.; Novo, E.; di Manzano, C.; Marra, F.; Loguercio, C.; et al. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J. Hepatol. 2009, 50, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Thuluvath, P.J. Complementary and alternative medicine in hepatology: Review of the evidence of efficacy. Clin. Gastroenterol. Hepatol. 2007, 5, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Vandeweerd, J.M.; Cambier, C.; Gustin, P. Nutraceuticals for canine liver disease: Assessing the evidence. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1171–1179. [Google Scholar] [CrossRef]
- Gogulski, M.; Cieślak, A.; Grabska, J.; Ardois, M.; Pomorska-Mól, M.; Kołodziejski, P.A.; Libera, K.; Strompfová, V.; Szumacher-Strabel, M. Effects of silybin supplementation on nutrient digestibility, hematological parameters, liver function indices, and liver-specific mi-RNA concentration in dogs. BMC Vet. Res. 2021, 17, 228. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef]
- Jump, D.B.; Lytle, K.A.; Depner, C.M.; Tripathy, S. Omega-3 polyunsaturated fatty acids as a treatment strategy for non-alcoholic fatty liver disease. Pharmacol. Ther. 2018, 181, 108–125. [Google Scholar] [CrossRef]
- Tu, T.; Li, B.; Li, X.; Zhang, B.; Xiao, Y.; Li, J.; Qin, F.; Liu, N.; Sun, C.; Liu, Q.; et al. Dietary ω-3 fatty acids reduced atrial fibrillation vulnerability via attenuating myocardial endoplasmic reticulum stress and inflammation in a canine model of atrial fibrillation. J. Cardiol. 2022, 79, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Meineri, G.; Cocolin, L.; Morelli, G.; Schievano, C.; Atuahene, D.; Ferrocino, I. Effect of an Enteroprotective Complementary Feed on Faecal Markers of Inflammation and Intestinal Microbiota Composition in Weaning Puppies. Vet. Sci. 2023, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Schauf, S.; Nakamura, N.; Castrillo, C. Effect of Calsporin (Bacillus subtilis C-3102) addition to the diet on faecal quality and nutrient digestibility in healthy adult dogs. J. Appl. Anim. Nutr. 2019, 7, e3. [Google Scholar] [CrossRef]
- de Lima, D.C.; Souza, C.M.M.; Nakamura, N.; Mesa, D.; de Oliveira, S.G.; Félix, A.P. Dietary supplementation with Bacillus subtilis C-3102 improves gut health indicators and fecal microbiota of dogs. Anim. Feed Sci. Technol. 2020, 270, 114672. [Google Scholar] [CrossRef]
- Isidori, M.; Rueca, F.; Massacci, F.R.; Diaferia, M.; Giontella, A.; Caldin, M.; Furlanello, T.; Corbee, R.J.; Mannucci, G.; Pezzotti, G.; et al. The Use of Ascophyllum nodosum and Bacillus subtilis C-3102 in the Management of Canine Chronic Inflammatory Enteropathy: A Pilot Study. Animals 2021, 11, 3417. [Google Scholar] [CrossRef] [PubMed]
- Panasevich, M.R.; Daristotle, L.; Quesnell, R.; Reinhart, G.A.; Frantz, N.Z. Altered fecal microbiota, IgA, and fermentative end-products in adult dogs fed prebiotics and a nonviable Lactobacillus acidophilus. J Anim Sci. 2021, 99, skab347. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations, and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Duysburgh, C.; Rakebrandt, M.; Marzorati, M. Dried yeast cell walls high in beta-glucan and mannan-oligosaccharides positively affect microbial composition and activity in the canine gastrointestinal tract in vitro. J. Anim. Sci. 2020, 98, skaa173. [Google Scholar] [CrossRef]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Healy, H.P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C.j.r. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 2002, 132, 980–989. [Google Scholar] [CrossRef]
- Flickinger, E.A.; Schreijen, E.M.; Patil, A.R.; Hussein, H.S.; Grieshop, C.M.; Merchen, N.R.; Fahey, G.C., Jr. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 2003, 81, 2008–2018. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, T.; Luo, P.; Miao, X.; Wang, J.; Chen, Y. Beneficial effects of silibinin on serum lipids, bile acids, and gut microbiota in methionine-choline-deficient diet-induced mice. Front. Nutr. 2023, 10, 1257158. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, 10. [Google Scholar] [CrossRef] [PubMed]
- Crost, E.H.; Tailford, L.E.; Le Gall, G.; Fons, M.; Henrissat, B.; Juge, N. Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain dependent. PLoS ONE 2013, 8, e76341. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw meat-based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2017, 13, 65. [Google Scholar] [CrossRef]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; Zhong, Y.; Turner, D.; Lan, R.S.; Pack, L.M.; Piccolo, B.D.; Chintapalli, S.V.; Abraham, A.; et al. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr. Obes. 2022, 17, e12921. [Google Scholar] [CrossRef]
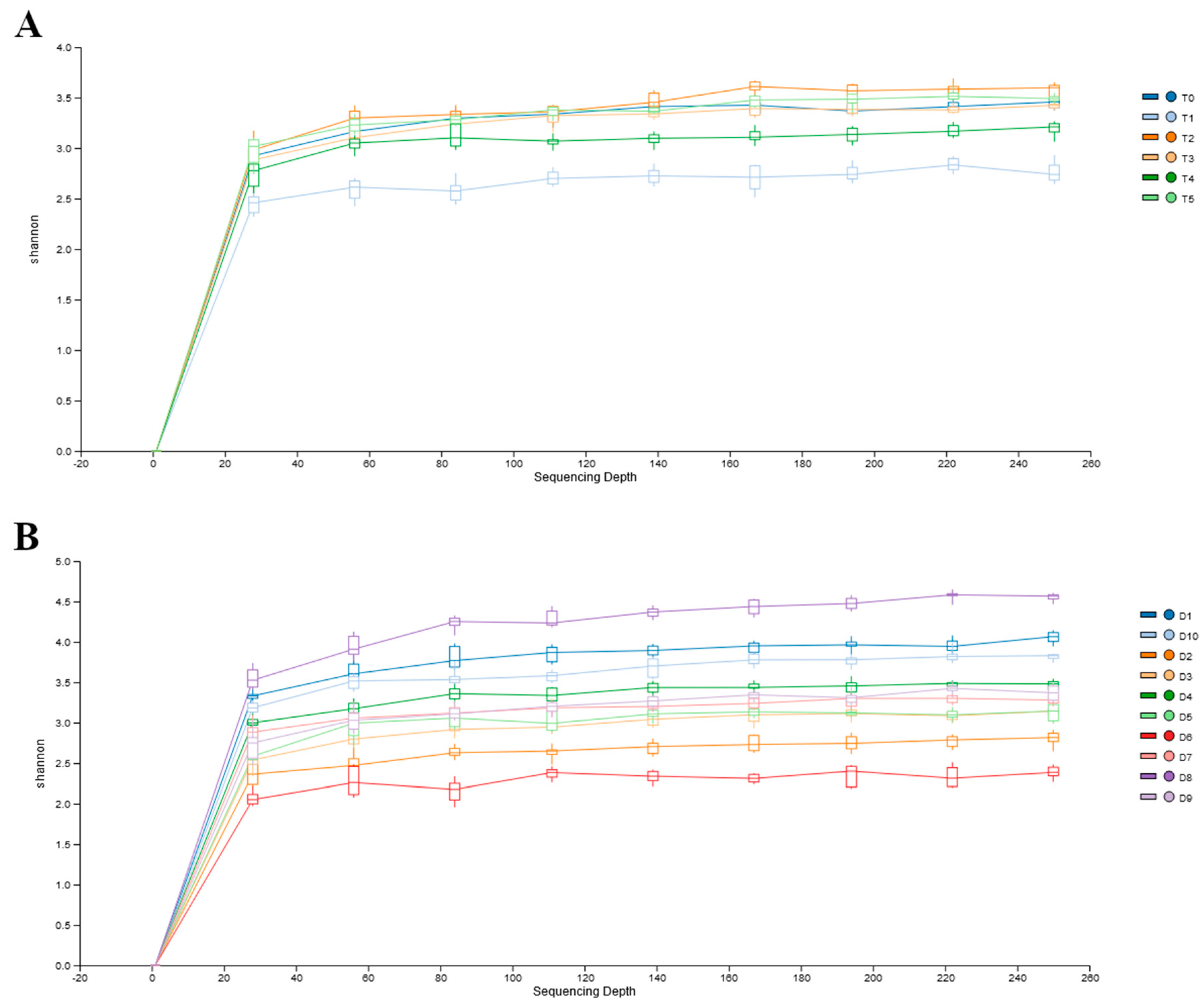
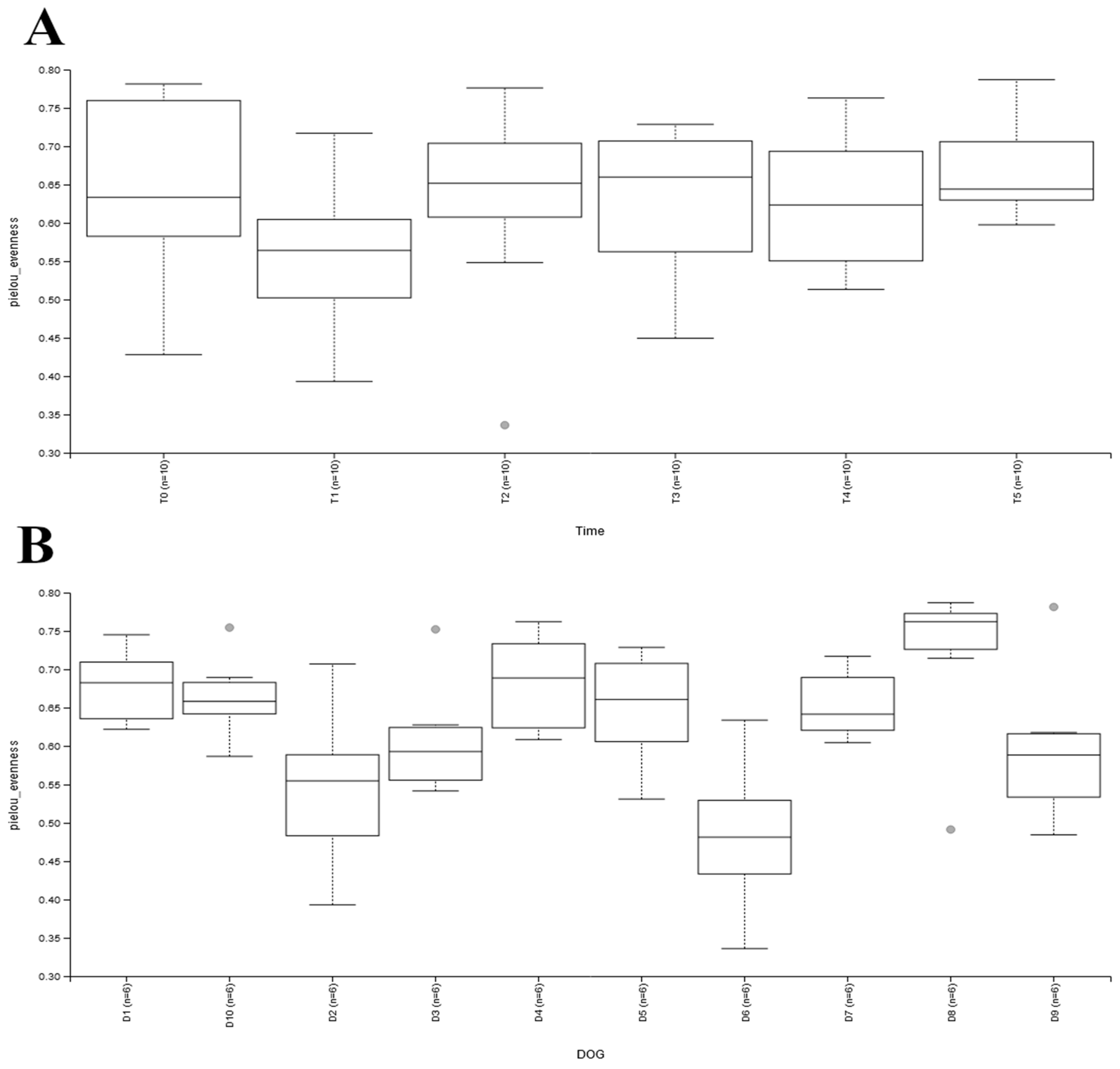
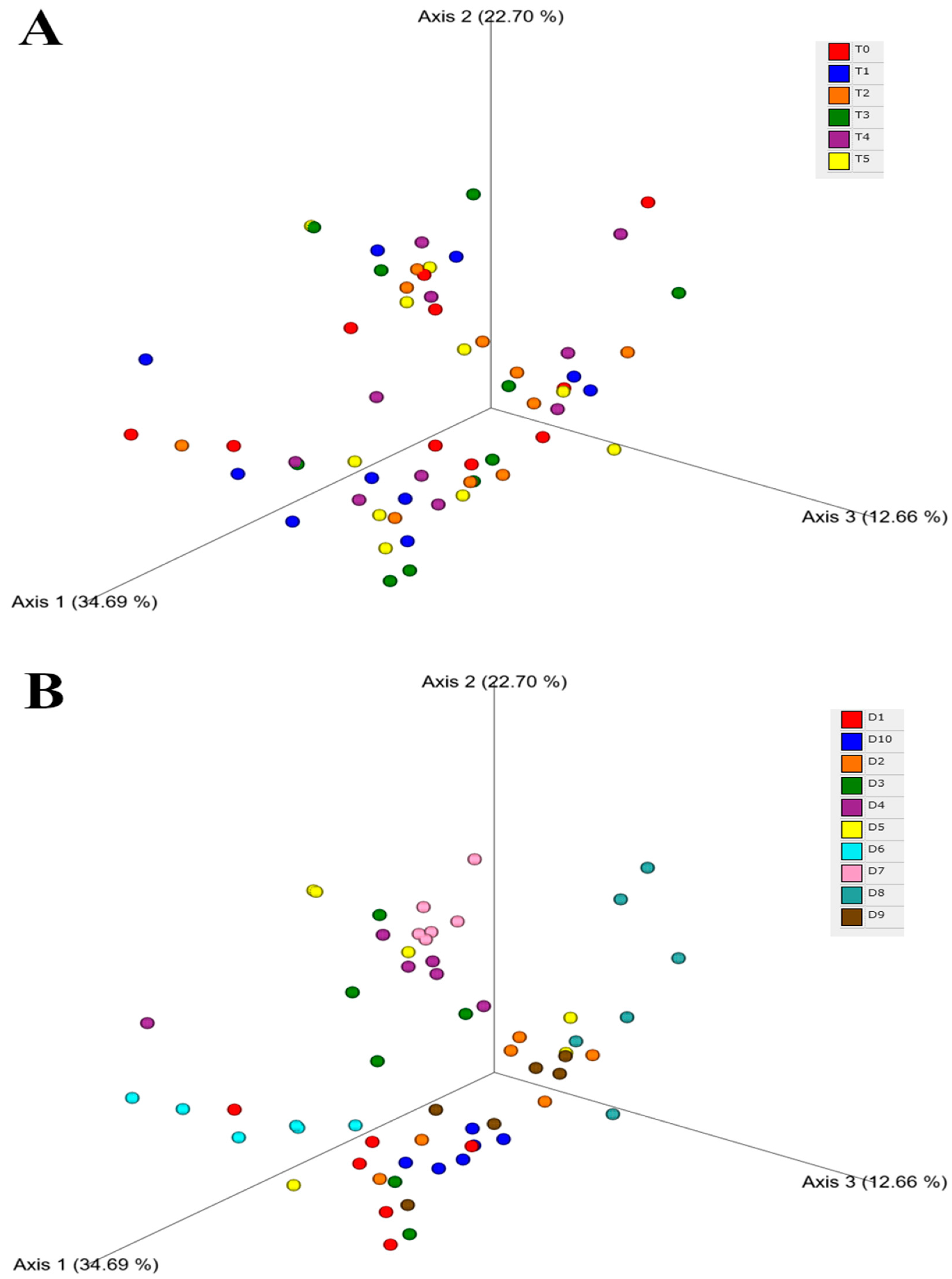
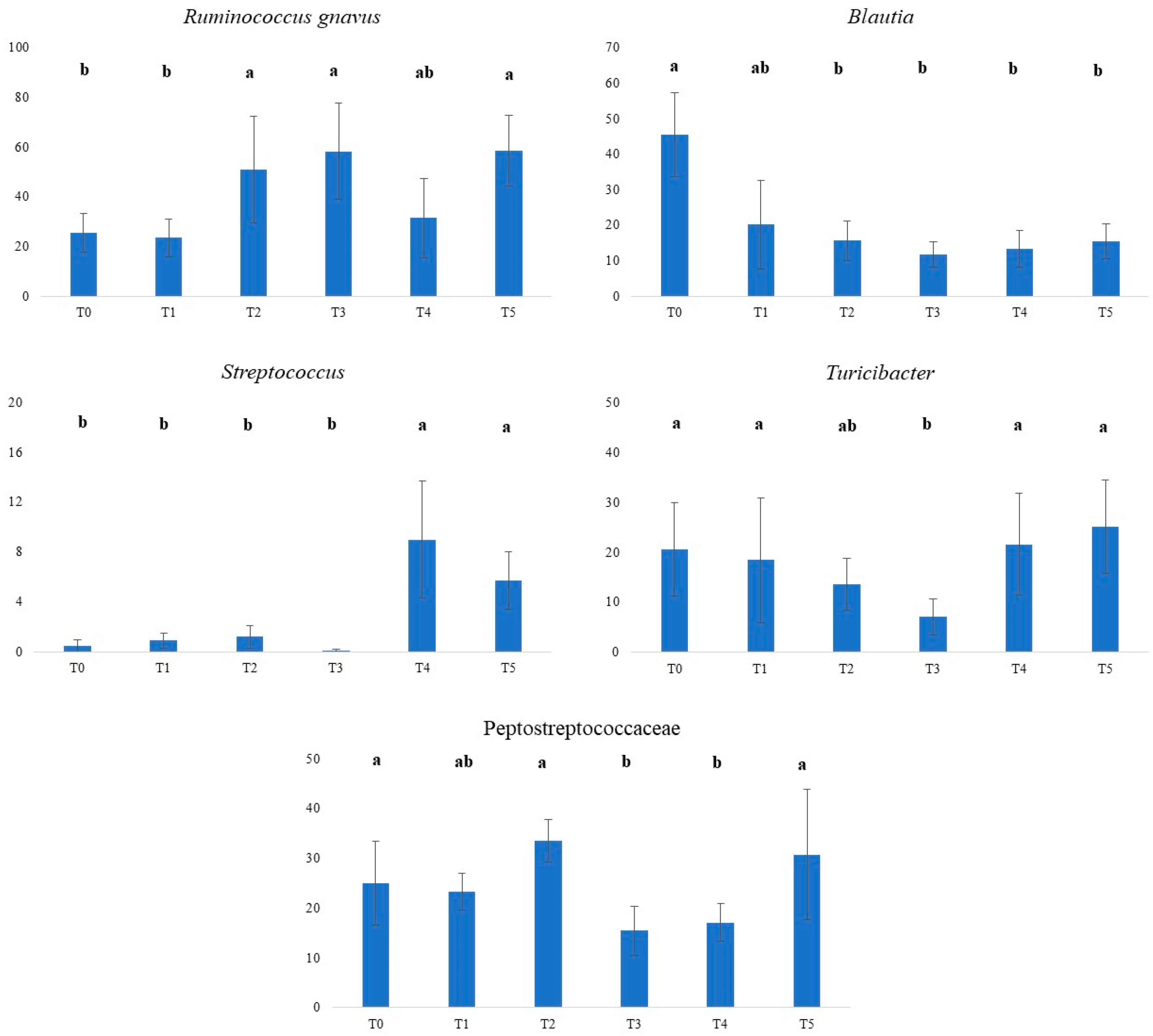
| Dog Number | Age | Sex | Breed | Weight (kg) | BCS | Tablet |
|---|---|---|---|---|---|---|
| D1 | 12 | F | American Staffordshire terrier | 30 | 7 | 2 |
| D2 | 10 | F | Dachshund | 9 | 8 | 0.75 |
| D3 | 8 | M | English Bulldog | 30 | 8 | 2 |
| D4 | 8 | M | Shar Pei | 35.5 | 7 | 2.3 |
| D5 | 12 | M | Staffordshire bull terrier | 22.1 | 7 | 1.5 |
| D6 | 11 | F | Beagle | 17.5 | 8 | 1 |
| D7 | 13 | M | Bullterrier | 38.1 | 7 | 2.3 |
| D8 | 10 | F | Dalmatian | 28.8 | 7 | 2 |
| D9 | 9 | F | German shepherd | 34.8 | 6 | 2 |
| D10 | 8 | M | Basset hound | 30.5 | 8 | 2 |
| Diet | Amount |
|---|---|
| Sylibum marianum extract | 2.52% |
| Choline chloride | 0.80% |
| Bacillus subtilis | 6.40% |
| Fructoligosaccharides | 0.50% |
| Beta glucans from barley | 0.50% |
| Betaine | 0.90% |
| n-3 polyunsaturated fatty acids (n-3 PUFA) in fish oil * | 6.40% |
| Vitamin B12 | 0.00002% |
| Vitamin C | 0.15% |
| Alpha tocopherol | 3.40% |
| Zinc | 0.93% |
| Magnesium | 1.65% |
| Arginine | 0.40% |
| Taurine | 2.40% |
| Hydrolyzed proteins | 24.30% |
| Maltodextrine | 48.75% |
| Item | RV | T0 | T1 | T2 | T3 | T4 | T5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AST | 23–66 | Mean | 49.5 | a | 32.8 | ab | 31.0 | b | 34.3 | ab | 36.0 | ab | 40.5 | ab |
| UI/L | sd | 21.1 | 16.8 | 11.5 | 14.0 | 14.5 | 14.0 | |||||||
| ALT | 21–102 | Mean | 107.2 | ns | 82.4 | ns | 91.2 | ns | 91.6 | ns | 96.0 | ns | 91.1 | ns |
| UI/L | sd | 45.9 | 36.4 | 69.6 | 62.2 | 70.2 | 65.9 | |||||||
| ALP | 20–156 | Mean | 302.1 | a | 246.0 | ab | 242.9 | ab | 208.6 | b | 213.4 | b | 204.6 | b |
| UI/L | sd | 241.5 | 220.3 | 239.2 | 211.8 | 202.7 | 201.7 | |||||||
| GGT, UI/L | 1.2–6.0 | Mean | 6.3 | ns | 6.2 | ns | 6.3 | ns | 6.2 | ns | 5.4 | ns | 5.2 | ns |
| UI/L | sd | 3.5 | 2.3 | 3.8 | 3.2 | 2.8 | 2.8 | |||||||
| Direct Bilirubin | 0.1–0.5 | Mean | 0.6 | a | 0.4 | ab | 0.4 | ab | 0.3 | b | 0.3 | b | 0.3 | b |
| mg/100 mL | sd | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | |||||||
| Glucose | 65–110 | Mean | 106.4 | a | 108.6 | a | 97.4 | ab | 92.9 | b | 93.9 | b | 91.2 | b |
| mg/100 mL | sd | 15.2 | 30.0 | 24.8 | 14.8 | 22.8 | 13.7 | |||||||
| Tryglicerides | 20–112 | Mean | 162.7 | ns | 119.1 | ns | 150.6 | ns | 110.5 | ns | 114.6 | ns | 103.1 | ns |
| mg/100 mL | sd | 94.6 | 61.0 | 103.7 | 54.5 | 80.9 | 48.2 | |||||||
| CRP | <0.5 | Mean | 1.2 | a | 1.0 | a | 0.9 | ab | 0.5 | b | 0.5 | b | 0.5 | b |
| mg/L | sd | 0.8 | 0.5 | 0.6 | 0.1 | 0.2 | 0.1 |
| Time | Total μmol/g | Lactic % | Acetic % | Propionic % | Isobutyric % | Butyric % | Isovaleric % |
|---|---|---|---|---|---|---|---|
| T0 | 213.9 ab | 2.1 ab | 66.7 ns | 15.9 ns | 3.3 ns | 3.5 ns | 8.5 ns |
| T1 | 173.5 b | 0.4 b | 66.9 ns | 20.9 ns | 3.9 ns | 2.5 ns | 5.4 ns |
| T2 | 227.1 ab | 1.4 ab | 69.6 ns | 16.4 ns | 4.0 ns | 4.5 ns | 4.1 ns |
| T3 | 249.7 a | 2.6 a | 72.1 ns | 14.0 ns | 3.8 ns | 4.2 ns | 3.4 ns |
| T4 | 225.1 ab | 1.9 ab | 66.6 ns | 17.4 ns | 4.6 ns | 3.2 ns | 6.3 ns |
| T5 | 210.0 ab | 2.5 a | 67.4 ns | 16.7 ns | 4.3 ns | 4.1 ns | 5.0 ns |
| RMSE | 8.9 | 0.3 | 1.8 | 1.3 | 0.4 | 0.3 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balouei, F.; Stefanon, B.; Martello, E.; Atuahene, D.; Sandri, M.; Meineri, G. Supplementation with Silybum marianum Extract, Synbiotics, Omega-3 Fatty Acids, Vitamins, and Minerals: Impact on Biochemical Markers and Fecal Microbiome in Overweight Dogs. Animals 2024, 14, 579. https://doi.org/10.3390/ani14040579
Balouei F, Stefanon B, Martello E, Atuahene D, Sandri M, Meineri G. Supplementation with Silybum marianum Extract, Synbiotics, Omega-3 Fatty Acids, Vitamins, and Minerals: Impact on Biochemical Markers and Fecal Microbiome in Overweight Dogs. Animals. 2024; 14(4):579. https://doi.org/10.3390/ani14040579
Chicago/Turabian StyleBalouei, Fatemeh, Bruno Stefanon, Elisa Martello, David Atuahene, Misa Sandri, and Giorgia Meineri. 2024. "Supplementation with Silybum marianum Extract, Synbiotics, Omega-3 Fatty Acids, Vitamins, and Minerals: Impact on Biochemical Markers and Fecal Microbiome in Overweight Dogs" Animals 14, no. 4: 579. https://doi.org/10.3390/ani14040579
APA StyleBalouei, F., Stefanon, B., Martello, E., Atuahene, D., Sandri, M., & Meineri, G. (2024). Supplementation with Silybum marianum Extract, Synbiotics, Omega-3 Fatty Acids, Vitamins, and Minerals: Impact on Biochemical Markers and Fecal Microbiome in Overweight Dogs. Animals, 14(4), 579. https://doi.org/10.3390/ani14040579










