Comparison of Reproductive Strategies between Two Sympatric Copsychus Passerines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
2.3. Field Procedure
2.4. Statistical Analyses
3. Results
3.1. Nest Box Selection
3.2. Breeding Stages and Clutch Size
3.3. Egg Incubation and Nestling Heating
3.4. Nestling Feeding and Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Martain, T.E. Food as a limit on breeding birds: A life-history perspective. Annu. Rev. 1987, 18, 453–487. [Google Scholar] [CrossRef]
- Zheng, G. Ornithology; Science Press: Beijing, China, 2012. [Google Scholar]
- Cheng, L.; Zhou, L.; Yu, C.; Wei, Z.; Li, C. Flexible nest site selection of the endangered Oriental Storks (Ciconia boyciana): Trade-off from adaptive strategies. Avian Res. 2023, 14, 100088. [Google Scholar] [CrossRef]
- Loucif, K.; Maazi, M.C.; Houhamdi, M.; Chenchouni, H. Nest site selection and breeding ecology of the Ferruginous Duck (Aythya nyroca) in Algeria. Glob. Ecol. Conserv. 2021, 26, e01524. [Google Scholar] [CrossRef]
- D’Amelio, P.B.; Ferreira, A.C.; Fortuna, R.; Paquet, M.; Silva, L.R.; Theron, F.; Doutrelant, C.; Covas, R. Disentangling climatic and nest predator impact on reproductive output reveals adverse high-temperature effects regardless of helper number in an arid-region cooperative bird. Ecol. Lett. 2022, 25, 151–162. [Google Scholar] [CrossRef]
- Jones, T.M.; Chiavacci, S.J.; Benson, T.J.; Ward, M.P. Nesting and post-fledging predation risk influence diel patterns of songbird fledging. Ibis 2022. [CrossRef]
- Avilés, J.M. Inter-specific brood parasitism and the evolution of avian reproductive strategies. Oikos 2018, 128, 338–346. [Google Scholar] [CrossRef]
- Grames, E.M.; Montgomery, G.A.; Youngflesh, C.; Tingley, M.W.; Elphick, C.S. The effect of insect food availability on songbird reproductive success and chick body condition: Evidence from a systematic review and meta-analysis. Ecol. Lett. 2023, 26, 658–673. [Google Scholar] [CrossRef]
- Ceresa, F.; Belda, E.J.; Brambilla, M.; Gómez, J.; Mompó, C.; Monrós, J.S. Factors Shaping Breeding Phenology in Birds: An Assessment of Two Sympatric Acrocephalus Warblers with Different Life Histories. Ardeola 2020, 67, 371–385. [Google Scholar] [CrossRef]
- Hayes, W.M.; O’Shea, B.J.; Pierre, M.A.; Wilson, A.; Bicknell, J.E. Bird communities across different levels of human settlement: A comparative analysis from two northern Amazonian ecoregions. Sci. Total Environ. 2023, 903, 166535. [Google Scholar] [CrossRef]
- Mereta, S.T.; Lemmens, P.; De Meester, L.; Goethals, P.L.M.; Boets, P. The Relative Importance of Human Disturbance, Environmental and Spatial Factors on the Community Composition of Wetland Birds. Water 2021, 13, 3448. [Google Scholar] [CrossRef]
- Martin, T.E. Avian life-history evolution has an eminent past: Does it have a bright future? Auk 2004, 121, 289–301. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Naikatini, A.N.; Keppel, G.; Brodie, G.; Kleindorfer, S. Interspecific Competition and Vertical Niche Partitioning in Fiji’s Forest Birds. Diversity 2022, 14, 223. [Google Scholar] [CrossRef]
- Sastranegara, H.; Mardiastuti, A.; Mulyani, Y.A. Guild Composition and Niche Overlap of Insectivorous Birds in Evergreen Rainforest. J. Manaj. Hutan Trop. (J. Trop. For. Manag.) 2020, 26, 13–20. [Google Scholar] [CrossRef]
- Ye, Y.; Hu, C.; Jiang, Y.; Davison, G.W.H.; Ding, C. Three-dimensional niche partitioning between two colonially nesting ardeid species in central China. Avian Res. 2021, 12, 33. [Google Scholar] [CrossRef]
- Yijin, Z.; Mingqin, S.; Quanjiang, L. Wading Bird Habitat, Water Depth Utilization and Niche Separation in Poyang Lake, China. Pak. J. Zool. 2020, 52, 2243–2250. [Google Scholar] [CrossRef]
- Atiénzar, F.; Belda, E.J.; Barba, E. Coexistence of Mediterranean tits: A multidimensional approach. Écoscience 2015, 20, 40–47. [Google Scholar] [CrossRef]
- Vrezec, A.; Tome, D. Altitudinal segregation between Ural OwlStrix uralensisand Tawny OwlS. aluco: Evidence for competitive exclusion in raptorial birds. Bird Study 2010, 51, 264–269. [Google Scholar] [CrossRef]
- Brazill-Boast, J.; Van Rooij, E.; Pryke, S.R.; Griffith, S.C. Interference from long-tailed finches constrains reproduction in the endangered Gouldian finch. J. Anim. Ecol. 2011, 80, 39–48. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Yin, J.; Liu, Z.; Liu, H.; Wan, D. Coexistence mechanism of two species passerines in man-made nest boxes. Acta Ecol. Sin. 2013, 33, 150–158. [Google Scholar] [CrossRef]
- Ye, P.; Yang, C.; Liang, W. Nest site availability and niche differentiation between two cavity-nesting birds in time and space. Ecol. Evol. 2019, 9, 11904–11910. [Google Scholar] [CrossRef]
- Poirazidis, K.; Goutner, V.; Tsachalidis, E.; Kati, V. Comparison of nest-site selection patterns of different sympatric raptor species as a tool for their conservation. Anim. Biodivers. Conserv. 2007, 30, 131–145. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, H.; Han, H.; Wei, W.; Zhou, H.; Zhang, Z. Comparison of breeding strategies of two sympatric thrush species in an alpine environment. Front. Ecol. Evol. 2022, 10, 1049983. [Google Scholar] [CrossRef]
- Alambiaga, I.; Álvarez, E.; Diez-Méndez, D.; Verdejo, J.; Barba, E. “The tale of the three little tits”: Different nest building solutions under the same environmental pressures. Avian Biol. Res. 2020, 13, 49–56. [Google Scholar] [CrossRef]
- Granroth-Wilding, H.M.V.; Phillips, R.A. Segregation in space and time explains the coexistence of two sympatric sub-Antarctic petrels. Ibis 2018, 161, 101–116. [Google Scholar] [CrossRef]
- Sottas, C.; Reif, J.; Kreisinger, J.; Schmiedová, L.; Sam, K.; Osiejuk, T.S.; Reifová, R. Tracing the early steps of competition-driven eco-morphological divergence in two sister species of passerines. Evol. Ecol. 2020, 34, 501–524. [Google Scholar] [CrossRef]
- Holt, R.F.; Martin, K. Landscape modification and patch selection: The demography of two secondary cavity nesters colonizing clearcuts. Auk 1997, 114, 443–455. [Google Scholar]
- Quader, S. What makes a good nest? Benefits of nest choice to female Baya Weavers (Ploceus philippinus). Auk 2006, 123, 475–486. [Google Scholar] [CrossRef]
- Wesolowski, T. On the origin of parental care and the early evolution of male and female parental roles in birds. Am. Nat. 1994, 143, 39–58. [Google Scholar] [CrossRef]
- Batisteli, A.F.; De Souza, L.B.; Santieff, I.Z.; Gomes, G.; Soares, T.P.; Pini, M.; Guillermo-Ferreira, R.; Pizo, M.A.; Sarmento, H. Buildings promote higher incubation temperatures and reduce nest attentiveness in a Neotropical thrush. Ibis 2020, 163, 79–89. [Google Scholar] [CrossRef]
- Cresswell, W. The energetic costs of egg heating constrain incubation attendance but do not determine daily energy expenditure in the pectoral sandpiper. Behav. Ecol. 2004, 15, 498–507. [Google Scholar] [CrossRef]
- Sladecek, M.; Vozabulova, E.; Salek, M.E.; Bulla, M. Diversity of incubation rhythms in a facultatively uniparental shorebird—The Northern Lapwing. Sci. Rep. 2019, 9, 4706. [Google Scholar] [CrossRef] [PubMed]
- Matysioková, B.; Remeš, V. Evolution of parental activity at the nest is shaped by the risk of nest predation and ambient temperature across bird species. Evolution 2018, 72, 2214–2224. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Best, L.B. Factors affecting feeding and brooding of Gray Catbird nestlings. Auk 1982, 99, 148–156. [Google Scholar] [CrossRef]
- Wright, J.; Both, C.; Cotton, P.A.; Bryant, D. Quality vs. quantity: Energetic and nutritional trade-offs in parental provisioning strategies. J. Anim. Ecol. 1998, 67, 620–634. [Google Scholar] [CrossRef]
- Guigueno, M.F.; Sealy, S.G. Nest sanitation in passerine birds: Implications for egg rejection in hosts of brood parasites. J. Ornithol. 2011, 153, 35–52. [Google Scholar] [CrossRef]
- Luro, A.B.; Hauber, M.E. A test of the nest sanitation hypothesis for the evolution of foreign egg rejection in an avian brood parasite rejecter host species. Sci. Nat. 2017, 104, 14. [Google Scholar] [CrossRef]
- Moskat, C.; Szekely, T.; Kisbenedek, T.; Karcza, Z.; Bartol, I. The importance of nest cleaning in egg rejection behaviour of great reed warblers Acrocephalus arundinaceus. J. Avian Biol. 2003, 34, 16–19. [Google Scholar] [CrossRef]
- Angelier, F.; Chastel, O. Stress, prolactin and parental investment in birds: A review. Gen. Comp. Endocrinol. 2009, 163, 142–148. [Google Scholar] [CrossRef]
- Aguon, C.F.; Conant, S. Breeding biology of the White-rumped Shama on Oahu, Hawaii. Wilson Bull. 1994, 106, 311–328. [Google Scholar]
- Angkaew, R.; Sankamethawee, W.; Pierce, A.J.; Savini, T.; Gale, G.A. Nesting near road edges improves nest success and post-fledging survival of White-rumped Shamas (Copsychus malabaricus) in northeastern Thailand. Condor 2019, 121, duy013. [Google Scholar] [CrossRef]
- Chotprasertkoon, T.; Pierce, A.J.; Savini, T.; Round, P.D.; Sankamethawee, W.; Gale, G.A. Influence of vegetation cover on nest cavity selection and nesting success of White-rumped Shamas (Copsychus malabaricus): An experimental test. Wilson J. Ornithol. 2017, 129, 727–741. [Google Scholar] [CrossRef]
- Sethi, V.K.; Bhatt, D. Provisioning of young by the Oriental Magpie-Robin (Copsychus saularis). Wilson J. Ornithol. 2007, 119, 356–360. [Google Scholar] [CrossRef]
- Singh, A.; Bhatt, D.; Sethi, V.K.; Dadwal, N. Nesting success of the Oriental Magpie Robin Copsychus saularis in nest boxes and tree cavities. Wildl. Biol. 2016, 22, 277–283. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Zhou, Q.; Ma, G.; Huang, C. Daily activity pattern in Assamese macaques inhabiting limestone forest, southwest Guangxi, China. Glob. Ecol. Conserv. 2019, 20, e00709. [Google Scholar] [CrossRef]
- Feng, C.; Yang, C.; Liang, W. Nest sanitation facilitates egg recognition in the common tailorbird, a plaintive cuckoo host. Zool. Res. 2019, 40, 466–470. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Q.; Wang, L.; Jiang, A.; Stokke, B.G.; Fossøy, F.; Tunheim, O.H.; Røskaft, E.; Liang, W.; Møller, A.P. Plaintive cuckoos do not select tailorbird hosts that match the phenotypes of their own eggs. Behav. Ecol. 2016, 27, 835–841. [Google Scholar] [CrossRef]
- Feng, C. Comparison of Breeding Strategies in Two Sympatric Tropical Birds. Ph.D. Thesis, Hainan Normal University, Haikou, China, 2019. Available online: https://link.cnki.net/doi/10.27719/d.cnki.ghnsf.2019.000005 (accessed on 2 February 2024).
- Zheng, G. A Checklist on the Classification and Distribution of the Birds of China, 4th ed.; Science Press: Beijing, China, 2023. [Google Scholar]
- Bhattacharya, H.; Cirillo, J.; Subba, B.R.; Todt, D. Song performance rules in the Oriental Magpie Robin (Copsychus salauris). Our Nat. 2007, 5, 1–13. [Google Scholar] [CrossRef]
- Danmek, A.; Sitasuwan, N. Persistence and alteration of the song structure of the Oriental Magpie Robin (Copsychus saularis) in some areas of northern Thailand. Chiang Mai J. Sci. 2017, 44, 478–486. [Google Scholar]
- Hanafi, S.; Chong, L.P.; Maruthaveeran, S.; Yeong, K.L. Vocal response of Oriental Magpie Robin (Copsychus saularis) to urban environmental factors in Peninsular Malaysia. Sains Malays. 2019, 48, 2061–2069. [Google Scholar] [CrossRef]
- Peng, L.F.; Yang, D.C.; Lu, C.H. Complete mitochondrial genome of Oriental Magpie-Robin Copsychus saularis (Aves: Muscicapidae). Mitochondrial DNA Part B 2016, 1, 21–22. [Google Scholar] [CrossRef]
- Hong, Y. Reproductive Ecology of White—Rumped Shama Copsychus malabaricus. J. Green Sci. Technol. 2019, 16, 30–32. [Google Scholar]
- Leupen, B.T.C.; Krishnasamy, K.; Sheoherd, C.R.; Chng, S.C.L.; Bergin, D.; Eaton, J.A.; Yukin, D.A.; Hue, S.; Miller, A.; Nekaris, K.; et al. Trade in White-rumped Shamas Kittacincla malabarica demands strong national and international responses. Forktail 2018, 34, 1–8. [Google Scholar]
- Palko, I.V.; Kalyakin, M.V.; Thinh, K.V. Nesting of the White-rumped Shama (Copsychus malabaricus) in southern Vietnam. Bonn. Zool. Monogr. 2011, 57, 185–191. [Google Scholar]
- Quan, R.C.; Li, H. Nest defense by a White-rumped Shama (Copsychus malabaricus) against snakes. Wilson J. Ornithol. 2015, 127, 538–542. [Google Scholar] [CrossRef]
- Fan, M.W.; Lin, R.L.; Fang, W.; Lin, Y. The distribution and abundance of the alien invasive White-rumped Shama (Copsychus malabaricus) in Taiwan. Taiwania 2009, 54, 248–254. [Google Scholar]
- Hoyt, D.F. Practical methods of estimating volume and fresh weight of bird eggs. Auk 1979, 96, 73–77. [Google Scholar]
- Cantoni, E.; Ronchetti, E. Robust inference for generalized linear models. J. Am. Stat. Assoc. 2001, 96, 1022–1030. [Google Scholar] [CrossRef]
- Jurasinski, G.; Retzer, V. simba: A Collection of Functions for Similarity Analysis of Vegetation Data. R Package Version 0.3–5. 2012. Available online: http://cran.nexr.com/web/packages/simba/index.html (accessed on 2 February 2024).
- Wang, H.; Gao, W. Utilization of natural cavities by secondary cavity-nesting birds in secondary forest. Zool. Res. 2002, 23, 136–140. [Google Scholar]
- Collar, N.; Christie, D.; Kirwan, G.M. Oriental Magpie-Robin (Copsychus saularis); Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., Juana, E., Eds.; Birds of the World, Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Liang, W.; Yang, C.; Wang, L.; Møller, A.P. Avoiding parasitism by breeding indoors: Cuckoo parasitism of hirundines and rejection of eggs. Behav. Ecol. Sociobiol. 2013, 67, 913–918. [Google Scholar] [CrossRef]
- Yao, X.; Cai, Y.; Ye, p.; Liang, W.; Yang, C. Nesting preferences of two cavity-nesting passerines in human houses. Ornithol. Sci. 2023, 22, 179–182. [Google Scholar] [CrossRef]
- Weisner, E. An Inordinate Fondness for Beetles: A Study of Insect Species Diversity and Abundance in Mazumbai Forest Reserve Versus Nearby Agricultural Areas. Indep. Study Proj. (ISP) Collect. 2018, 2872. Available online: https://digitalcollections.sit.edu/isp_collection/2872 (accessed on 2 February 2024).
- Darsono, D.; Riwidiharso, E.D.Y.; Santoso, S.; Sudiana, E.; Yani, E.D.Y.; Nasution, E.K.; Aprilliana, H.; Chasanah, T. Insect diversity in various distances to forest edge in small nature reserve: A case study of Bantarbolang Nature Reserve, Central Java, Indonesia. Biodiversitas J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Smith, J.A.; Harrison, T.J.E.; Martin, G.R.; Reynolds, S.J. Feathering the Nest: Food Supplementation Influences Nest Construction by Blue (Cyanistes caeruleus) and Great Tits (Parus major). Avian Biol. Res. 2013, 6, 18–25. [Google Scholar] [CrossRef]
- der Weduwen, D.; Keogan, K.; Samplonius, J.M.; Phillimore, A.B.; Shutt, J.D. The correlates of intraspecific variation in nest height and nest building duration in the Eurasian blue tit Cyanistes caeruleus. J. Avian Biol. 2021, 52, e02528. [Google Scholar] [CrossRef]
- Mahesh, V.; Lanka, S. Nest Construction Mechanism of House Sparrow (Passer domesticus): A comparative study between Open and Inbox nests. Ambient Sci. 2022, 9, 68–74. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Parker, G.A.; Haig, D. Clutch size, fecundity and parent-offspring conflict. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1997, 332, 67–79. [Google Scholar]
- Dunn, P.O.; Thusius, K.J.; Kimber, K.; Winkler, D.W. Geographic and ecological variation in clutch size of tree swallows. Auk 2000, 117, 215–221. [Google Scholar] [CrossRef]
- Doligez, B.; Clobert, J. Clutch size reduction as a response to increased to increased flycatche. Ecology 2003, 84, 2582–2588. [Google Scholar] [CrossRef]
- Yang, C.; Liang, W.; Cai, Y.; Wu, J.; Shi, S.; Antonov, A. Variation in Russet Sparrow (Passer cinnamomeus) breeding biology in relation to small-scale altitudinal differences in China. Zool. Sci. 2012, 29, 419–422. [Google Scholar] [CrossRef]
- Greeney, H.F.; Sánchez, C.; Sánchez, J.E.; Carman, E. A review of nest and egg descriptions for the genus Myrmeciza, with the first description of nests and eggs of the dull-mantled antbird (M. laemosticta). J. Ornithol. 2013, 154, 1049–1056. [Google Scholar] [CrossRef]
- Atanasov, A.T. Allometric relationship between volume to surface ratio and incubation days in bird’s eggs. In Proceedings of the International Conference of Computational Methods in Sciences and Engineering (ICCMSE-2019), Rhodes, Greece, 1–5 May 2019. [Google Scholar]
- Yi, J.; Lin, Y.; Xu, X.; Nong, Z.; Yu, L. Incubation behavior and rhythm of Greater Coucal (Centropus sinensis) in southwest Guangxi. Chin. J. Zool. 2017, 52, 574–582. [Google Scholar]
- Bueno-Enciso, J.; Barrientos, R.; Sanz, J.J. Incubation behaviour of Blue Cyanistes caeruleus and Great Tits Parus major in a Mediterranean Habitat. Acta Ornithol. 2017, 52, 21–34. [Google Scholar] [CrossRef]
- Ye, P.; Yao, X.; Bi, J.; Li, G.; Liang, W.; Yang, C. Breeding biology of the Green-backed Tit (Parus monticolus) in southwest China. Avian Res. 2021, 12, 60. [Google Scholar] [CrossRef]
- Conway, C.J.; Martin, A.T.E. Evolution of passerine incubation behavior: Influence of food, temperature, and nest predation. Evolution 2000, 54, 670–685. [Google Scholar] [PubMed]
- Weathers, W.W.; Sullivan, K.A. Nest attentiveness and egg temperature in the yellow-eyed junco. Condor 1989, 91, 628–633. [Google Scholar] [CrossRef]
- Yu, J.; Wang, P.; Lv, L.; Zhang, Z.; Wang, Y.; Xu, J.; Li, J.; Xi, B.; Zhu, J.; Du, Z. Diurnal brooding behavior of long-tailed tits (Aegithalos caudatus glaucogularis). Zool. Res. 2016, 37, 84–89. [Google Scholar]
- Ding, Z.; Liang, J.; Pan, X.; Hu, H. Feeding behavior and nestling growth of Yellow-bellied Prinia (Prinia flaviventris). Chin. J. Zool. 2016, 51, 969–976. [Google Scholar]
- Bi, J.; Jiang, Y.; Yang, C. Breeding ecology of the Yellow-bellied Warbler (Abroscopus superciliaris). Avian Res. 2020, 11, 41. [Google Scholar] [CrossRef]
- Wu, Z.; Han, L.; Kuang, Z. Breeding behaviors of blue Tailed Bee-eater of Nujiang Valley. Zool. Res. 2009, 30, 429–432. [Google Scholar] [CrossRef][Green Version]
- Zhu, F.; Zhou, C.; Yang, Z.; Li, X. Observation on the breeding habit of Garrulax sannio in Nanchong, Sichuan. Chin. J. Zool. 2010, 45, 150–155. [Google Scholar]
- Romanoff, A.L. Avian spare yolk and its assimilation. Auk 1944, 61, 235–241. [Google Scholar] [CrossRef]
- Rehling, A.; Spiller, I.; Krause, E.T.; Nager, R.G.; Monaghan, P.; Trillmich, F. Flexibility in the duration of parental care: Zebra finch parents respond to offspring needs. Anim. Behav. 2012, 83, 35–39. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Yin, L.; Li, L.; Chang, P.; Wan, D. The behavior of parental feeding and offspring begging by Parus varius. Chin. J. Zool. 2012, 47, 19–27. [Google Scholar]
- Quan, R.; Li, H.; Wang, B.; Goodale, E. The relationship between defecation and feeding in nestling birds: Observational and experimental evidence. Front. Zool. 2015, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Leffelaar, D.; Raleigh, J.R. Equality of feeding roles and the maintenance of monogamy in tree swallows. Behav. Ecol. Sociobiol. 1986, 18, 199–206. [Google Scholar] [CrossRef]
- Moreau, R.E. Relations between number in brood, feeding-rate and nestling period in nine species of birds in Tanganyika Territory. J. Anim. Ecol. 1947, 16, 205–209. [Google Scholar] [CrossRef]
- Bryant, D.M.; Westerterp, K.R. The energy budget of the House Martin (Delichon urbica). Ardea 1980, 38–90, 91–102. [Google Scholar] [CrossRef]
- Allen, R.W.; Nice, M.M. A study of the breeding biology of the Purple Martin (Progne subis). Am. Midl. Nat. 1952, 47, 606–665. [Google Scholar] [CrossRef]
- Nolan, J.V. The Ecology and Behavior of the Prairie Warbler Dendroica Discolor; The American Ornithologists’ Union: Washington, DC, USA, 1978. [Google Scholar]
- Pinkowski, B.C. Feeding of nestling and fledgling Eastern Bluebirds. Wilson Bull. 1978, 90, 84–98. [Google Scholar]
- MacGregor, A.; Cockburn, A. Sex differences in parental response to begging nestlings in superb fairy-wrens. Anim. Behav. 2002, 63, 923–932. [Google Scholar] [CrossRef][Green Version]
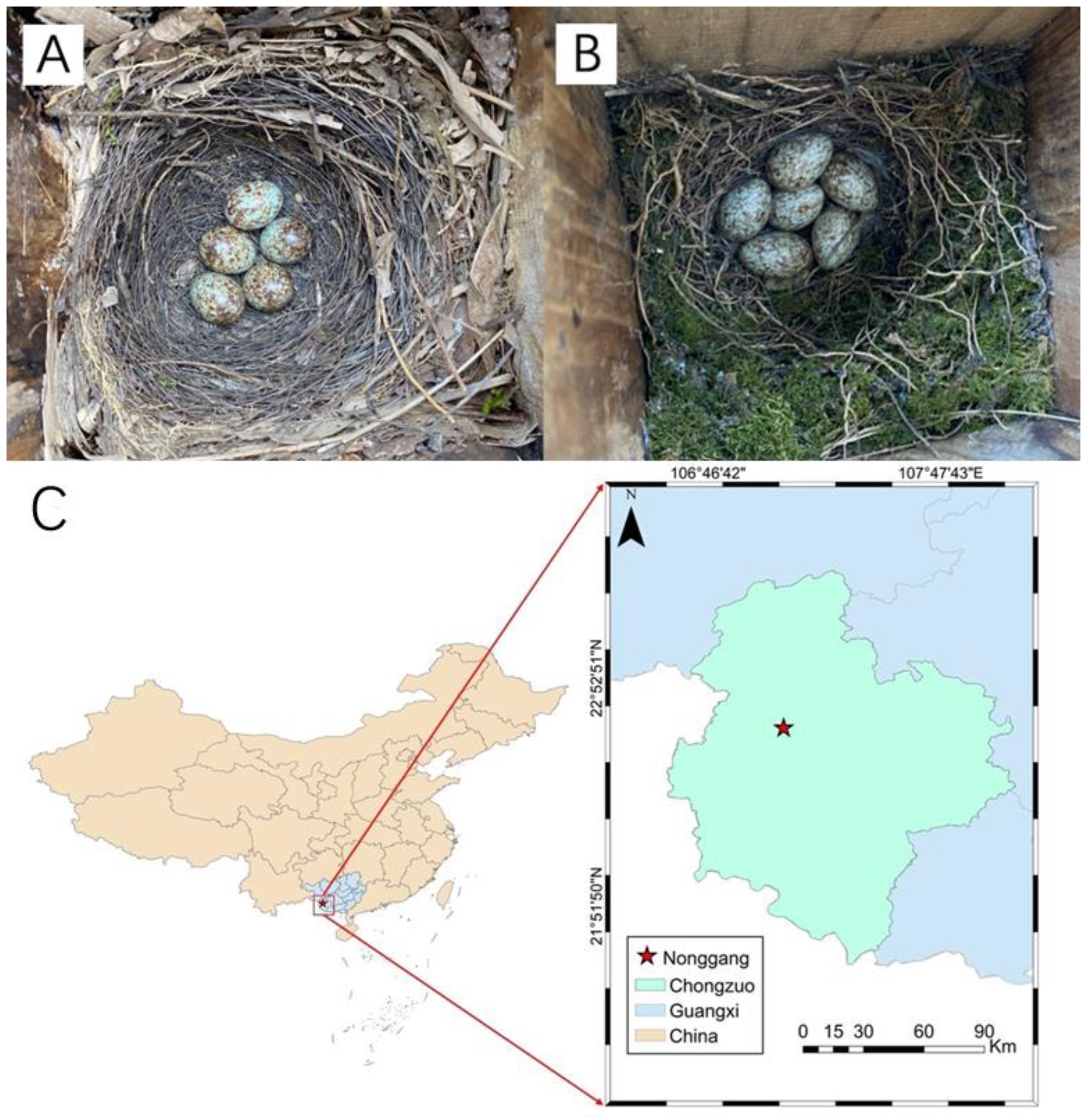
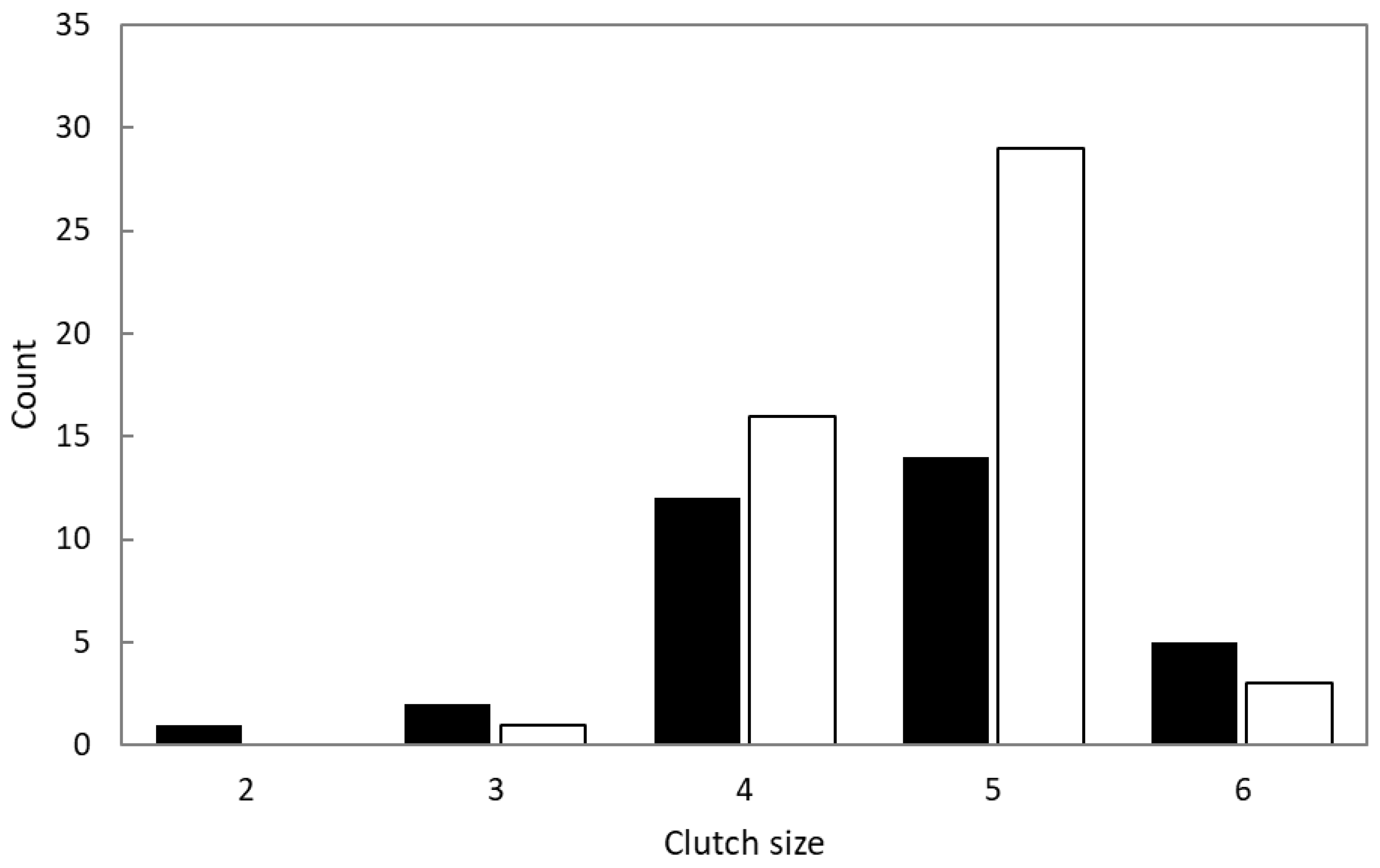
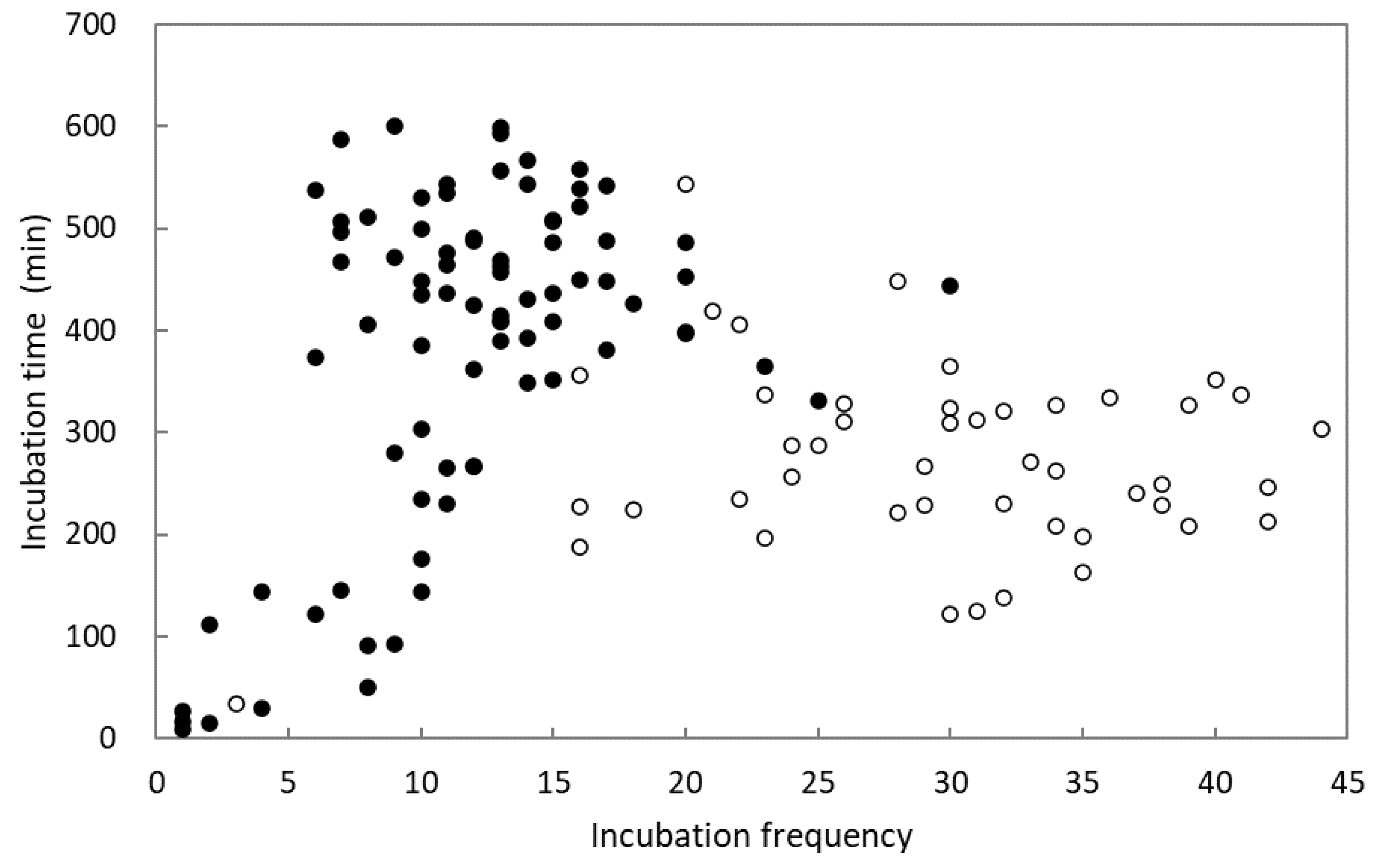

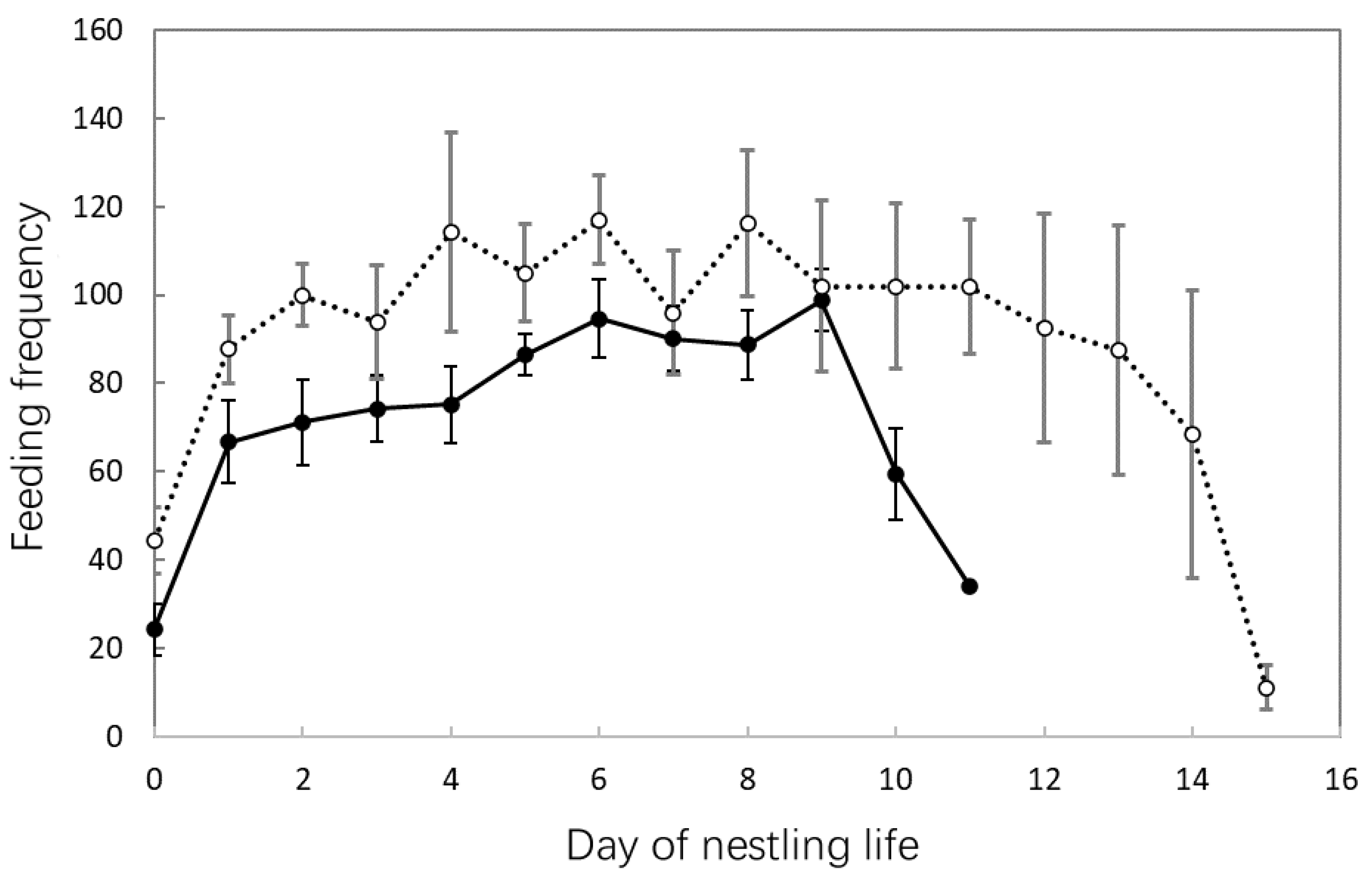
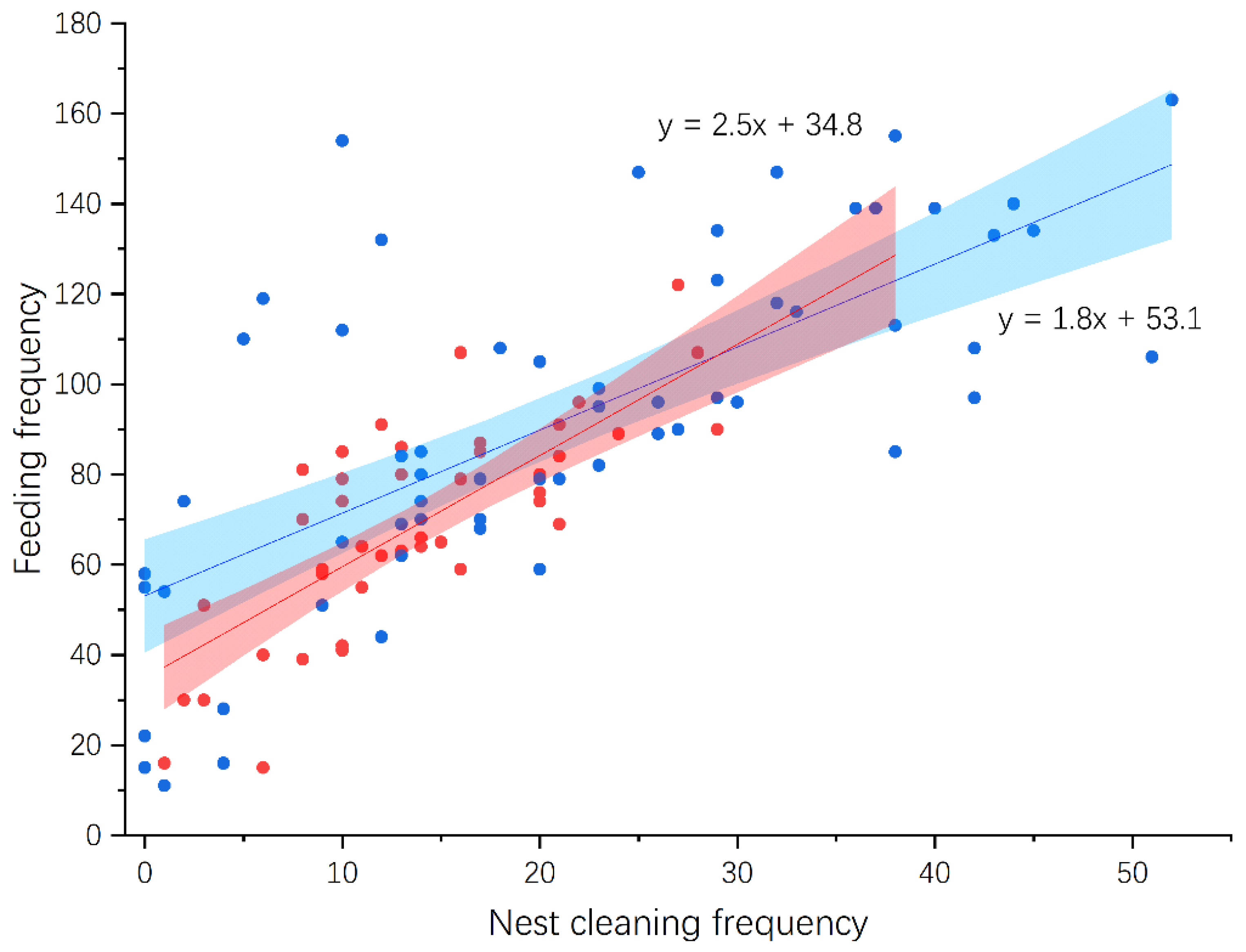
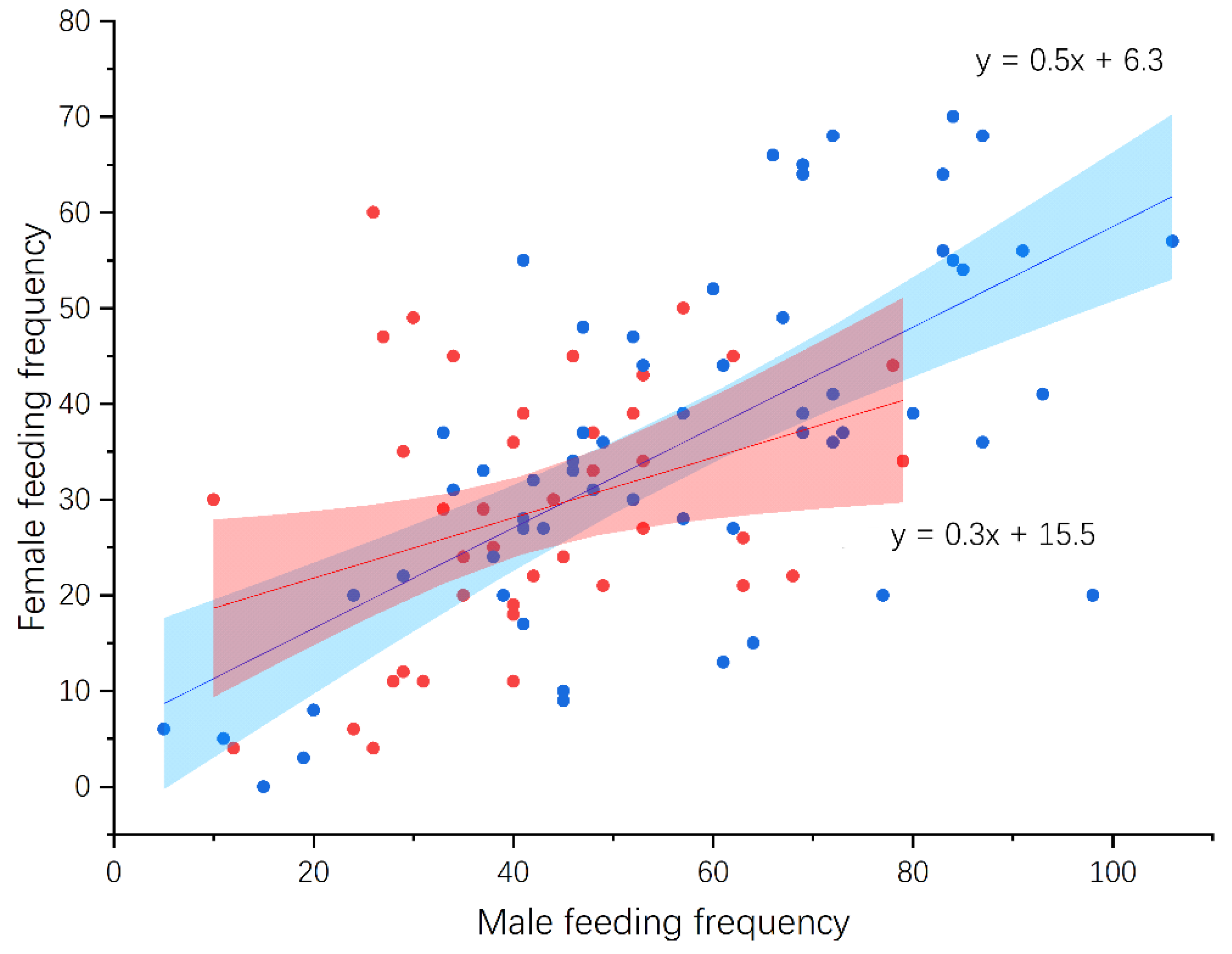
| Shama (n = 27) | Magpie Robin (n = 45) | Statistic * | p | |
|---|---|---|---|---|
| Location (count) | 22 trees, 5 poles | 9 trees, 36 poles | χ2 = 26.02 | <0.001 |
| Distance to forest (m) | 0 ± 0 (Median ± IQR) | 5 ± 16 (Median ± IQR) | Z = −5.84 | <0.001 |
| Distance to road (m) | 4 ± 8 (Median ± IQR) | 3 ± 7 (Median ± IQR) | Z = −0.49 | 0.624 |
| Orientation (°) | 222.93 ± 19.87 (Mean ± SE) | 183.62 ± 15.29 (Mean ± SE) | Z = 3.22 | 0.040 |
| Breeding Stage | Shama | Magpie Robin | Statistic * | p |
|---|---|---|---|---|
| Nest building | 3.1 ± 0.2 (19) | 4.3 ± 0.4 (20) | t = −2.34 | 0.025 |
| Egg incubation | 11.8 ± 0.2 (20) | 12.3 ± 0.2 (21) | t = −1.84 | 0.074 |
| Nestling feeding | 11.2 ± 0.2 (17) | 13.3 ± 0.3 (19) | t = −5.79 | <0.001 |
| Response Variable | Effect | Estimate | SE | Z | p |
|---|---|---|---|---|---|
| Body mass | (Intercept) | 1.72 | 0.02 | 75.32 | <0.001 |
| Species | −0.01 | 0.01 | −0.46 | 0.649 | |
| Nestling age | 0.16 | <0.01 | 76.44 | <0.001 | |
| Tarsus length | (Intercept) | 2.38 | 0.02 | 126.78 | <0.001 |
| Species | 0.01 | 0.01 | 0.42 | 0.674 | |
| Nestling age | 0.11 | <0.01 | 65.95 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Bi, J.; Zhao, X.; Cai, Y.; Yang, C. Comparison of Reproductive Strategies between Two Sympatric Copsychus Passerines. Animals 2024, 14, 554. https://doi.org/10.3390/ani14040554
Zhang Z, Bi J, Zhao X, Cai Y, Yang C. Comparison of Reproductive Strategies between Two Sympatric Copsychus Passerines. Animals. 2024; 14(4):554. https://doi.org/10.3390/ani14040554
Chicago/Turabian StyleZhang, Ziqi, Jianli Bi, Xu Zhao, Yan Cai, and Canchao Yang. 2024. "Comparison of Reproductive Strategies between Two Sympatric Copsychus Passerines" Animals 14, no. 4: 554. https://doi.org/10.3390/ani14040554
APA StyleZhang, Z., Bi, J., Zhao, X., Cai, Y., & Yang, C. (2024). Comparison of Reproductive Strategies between Two Sympatric Copsychus Passerines. Animals, 14(4), 554. https://doi.org/10.3390/ani14040554






