Contribution of elovl5a to Docosahexaenoic Acid (DHA) Synthesis at the Transcriptional Regulation Level in Common Carp, Cyprinus carpio
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Fish Sample and Fatty Acid Content Determination
2.3. RNA Isolation and qRT-PCR
2.4. Plasmid Construction
2.5. Cell Culture, Transfection, and Dual-Luciferase Report Assay
2.6. CRISPR/Cas9 Disruption
2.7. Verification after Gene Disruption
2.8. Gene Expression Analysis
2.9. Data Analysis
3. Results
3.1. Fatty Acid Content of Yellow River Carp in Muscle Tissue
3.2. The Expression of Candidate Genes in Yellow River Carp
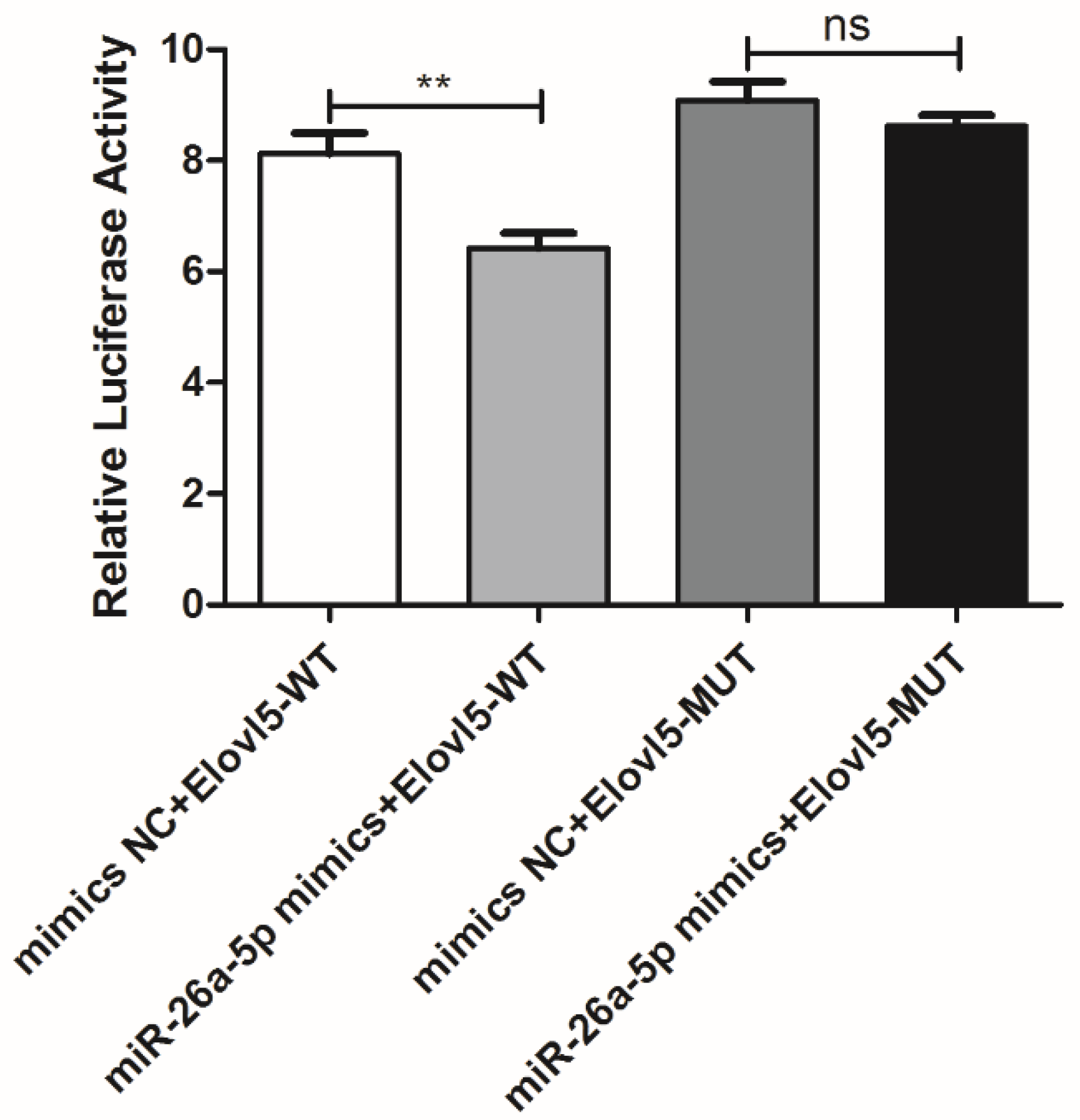
3.3. elovl5a Is the Target Gene of miR-26a-5p
3.4. CRISPR/Cas9 Disruption of elovl5a in Yellow River Carp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep. Technol. Assess. (Full Rep.) 2016, 224, 1–826. [Google Scholar]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Zheng, W.H.; Degrace, P.; Tian, L.X.; Liu, Y.J. Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp (Ctenopharyngodon idella) fed high-fat diets. Br. J. Nutr. 2006, 95, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, X.; Wang, X.; Li, J.; Liu, G.; Kuang, Y.; Xu, J.; Zheng, X.; Ren, L.; Wang, G.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.M.; Wang, Q.; Li, Q.S.; Sun, X.Q.; Li, J.T. The Promoter SNPs Were Associated with Both the Contents of Poly-Unsaturated Fatty Acids (PUFAs) and the Expressions of PUFA-Related Genes in Common Carp. Biology 2023, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, C.R.; Wang, Y.X.; Xu, Z.M.; Li, S.Q.; Li, J.C.; Sun, X.Q.; Wang, H.W.; Wang, Q.; Zhang, Y.; et al. Fads2b Plays a Dominant Role in ∆6/∆5 Desaturation Activities Compared with Fads2a in Common Carp (Cyprinus carpio). Int. J. Mol. Sci. 2023, 24, 10638. [Google Scholar] [CrossRef]
- Leonard, A.E.; Kelder, B.; Bobik, E.G.; Chuang, L.T.; Lewis, C.J.; Kopchick, J.J.; Mukerji, P.; Huang, Y.S. Identification and expression of mammalian long-chain PUFA elongation enzymes. Lipids 2002, 37, 733–740. [Google Scholar] [CrossRef]
- Monroig, O.; Wang, S.; Zhang, L.; You, C.; Tocher, D.R.; Li, Y. Elongation of long-chain fatty acids in rabbitfish Siganus canaliculatus: Cloning, functional characterisation and tissue distribution of Elovl5- and Elovl4-like elongases. Aquaculture 2012, 350, 63–70. [Google Scholar] [CrossRef]
- Castro, L.F.; Tocher, D.R.; Monroig, O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Cook, H.W.; McMaster, C.R. Chapter 7 Fatty acid desaturation and chain elongation in eukaryotes. New Compr. Biochem. 2002, 36, 181–204. [Google Scholar]
- Agaba, M.; Tocher, D.R.; Dickson, C.A.; Dick, J.R.; Teale, A.J. Zebrafish cDNA encoding multifunctional Fatty Acid elongase involved in production of eicosapentaenoic (20:5n-3) and docosahexaenoic (22:6n-3) acids. Mar. Biotechnol. 2004, 6, 251–261. [Google Scholar] [CrossRef]
- Agaba, M.K.; Tocher, D.R.; Zheng, X.; Dickson, C.A.; Dick, J.R.; Teale, A.J. Cloning and functional characterisation of polyunsaturated fatty acid elongases of marine and freshwater teleost fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 142, 342–352. [Google Scholar] [CrossRef]
- Gregory, M.K.; James, M.J. Rainbow trout (Oncorhynchus mykiss) ELovl5 and Elovl2 differ in selectivity for elongation of omega-3 docosapentaenoic acid. Biochim. Biophys. Acta 2014, 1841, 1656–1660. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, P.; Dong, L.; Zhang, Y.; Han, Z.; Wang, Q.; Wang, Z. Whole-genome single-nucleotide polymorphism (SNP) marker discovery and association analysis with the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) content in Larimichthys crocea. PeerJ 2016, 4, e2664. [Google Scholar] [CrossRef]
- Lei, C.X.; Li, M.M.; Tian, J.J.; Wen, J.K.; Li, Y.Y. Transcriptome analysis of golden pompano (Trachinotus ovatus) liver indicates a potential regulatory target involved in HUFA uptake and deposition. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 33, 100633. [Google Scholar] [CrossRef]
- Xue, X.; Feng, C.Y.; Hixson, S.M.; Johnstone, K.; Anderson, D.M.; Parrish, C.C.; Rise, M.L. Characterization of the fatty acyl elongase (elovl) gene family, and hepatic elovl and delta-6 fatty acyl desaturase transcript expression and fatty acid responses to diets containing camelina oil in Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 175, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, J.; Zhang, M.; You, C.; Liu, Y.; Wang, S.; Li, Y. miR-146a is involved in the regulation of vertebrate LC-PUFA biosynthesis by targeting elovl5 as demonstrated in rabbitfish Siganus canaliculatus. Gene 2018, 676, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.T.; Huang, Y.; Tang, Y.K.; Yu, J.H.; XU, P. Two Elovl5-like elongase genes in Cyprinus carp var. Jian: Gene characterization, mRNA expression, and nutritional regulation. Mol. Biol. 2015, 49, 527–534. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, P.; Jiang, Y.; Zhao, Z.; Feng, J.; Tai, R.; Dong, C.; Xu, J. Genomic, transcriptomic, and epigenomic features differentiate genes that are relevant for muscular polyunsaturated fatty acids in the common carp. Front. Genet. 2019, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- GB/T 22223-2008; Determination of Total Fat, Saturated Fat, and Unsaturated Fat in Foods—Hydrolytic Extraction-Gas Chromatography. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- Zhang, W.; Jia, Y.; Ji, X.; Zhang, R.; Liang, T.; Du, Q.; Chang, Z. Optimal reference genes in different tissues, gender, and gonad of Yellow River carp (Cyprinus carpio var) at various developmental periods. Pak. J. Zool. 2016, 48, 1615–1622. [Google Scholar]
- Dael, P.V. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: Review of recent studies and recommendations. Nutr. Res. Pract. 2021, 15, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Bou, M.; Berge, G.M.; Baeverfjord, G.; Sigholt, T.; Østbye, T.; Romarheim, O.H.; Hatlen, B.; Leeuwis, R.; Venegas, C.; Ruyter, B. Requirements of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L): Effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. Br. J. Nutr. 2017, 117, 30–47. [Google Scholar] [CrossRef]
- Jin, Y.; Datsomor, A.K.; Olsen, R.E.; Vik, J.O.; Torgersen, J.S.; Edvardsen, R.B.; Wargelius, A.; Winge, P.; Grammes, F. Targeted mutagenesis of ∆5 and ∆6 fatty acyl desaturases induce dysregulation of lipid metabolism in Atlantic salmon (Salmo salar). BMC Genom. 2020, 21, 805. [Google Scholar] [CrossRef]
- Xie, D.; Liu, X.; Wang, S.; You, C.; Li, Y. Effects of dietary LNA/LA ratios on growth performance, fatty acid composition and expression levels of elovl5, Δ4 fad and Δ6/Δ5 fad in the marine teleost Siganus canaliculatus. Aquaculture 2018, 484, 309–316. [Google Scholar] [CrossRef]
- Sun, S.; Ren, T.; Li, X.; Cao, X.; Gao, J. Polyunsaturated fatty acids synthesized by freshwater fish: A new insight to the roles of elovl2 and elovl5 in vivo. Biochem. Biophys. Res. Commun. 2020, 532, 414–419. [Google Scholar] [CrossRef]
- Liu, C.; Ye, D.; Wang, H.; He, M.; Sun, Y. Elovl2 But Not Elovl5 Is Essential for the Biosynthesis of Docosahexaenoic Acid (DHA) in Zebrafish: Insight from a Comparative Gene Knockout Study. Mar. Biotechnol. 2020, 22, 613–619. [Google Scholar] [CrossRef]
- Monroig, O.; Shu-Chien, A.C.; Kabeya, N.; Tocher, D.R.; Castro, L.F.C. Desaturases and elongases involved in long-chain polyunsaturated fatty acid biosynthesis in aquatic animals: From genes to functions. Prog. Lipid Res. 2022, 86, 101157. [Google Scholar] [CrossRef]
- Kabeya, N.; Takeuchi, Y.; Yamamoto, Y.; Yazawa, R.; Haga, Y.; Satoh, S.; Yoshizaki, G. Modification of the n-3 HUFA biosynthetic pathway by transgenesis in a marine teleost, nibe croaker. J. Biotechnol. 2014, 172, 46–54. [Google Scholar] [CrossRef]
- Monroig, O.; Rotllant, J.; Sanchez, E.; Cerda-Reverter, J.M.; Tocher, D.R. Expression of long-chain polyunsaturated fatty acid (LC-PUFA) biosynthesis genes during zebrafish Danio rerio early embryogenesis. BBA Mol. Cell Biol. Lipids 2009, 1791, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Marrero, M.; Monroig, O.; Navarro, J.C.; Ribes-Navarro, A.; Perez, J.A.; Galindo, A.; Rodriguez, C. Metabolic and molecular evidence for long-chain PUFA biosynthesis capacity in the grass carp Ctenopharyngodon idella. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 270, 111232. [Google Scholar] [CrossRef] [PubMed]
- Datsomor, A.K.; Zic, N.; Li, K.; Olsen, R.E.; Jin, Y.; Vik, J.O.; Edvardsen, R.B.; Grammes, F.; Wargelius, A.; Winge, P. CRISPR/Cas9-mediated ablation of elovl2 in Atlantic salmon (Salmo salar L.) inhibits elongation of polyunsaturated fatty acids and induces Srebp-1 and target genes. Sci. Rep. 2019, 9, 7533. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Monroig, Ó.; Wang, T.; Yuan, Y.; Navarro, J.C.; Hontoria, F.; Liao, K.; Tocher, D.R.; Mai, K.; Xu, W.; et al. Functional characterization and differential nutritional regulation of putative Elovl5 and Elovl4 elongases in large yellow croaker (Larimichthys crocea). Sci. Rep. 2017, 7, 2303. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Monroig, O.; Zheng, X.; Leaver, M.J.; Tocher, D.R. Highly unsaturated fatty acid synthesis in Atlantic salmon: Characterization of ELOVL5- and ELOVL2-like elongases. Mar. Biotechnol. 2009, 11, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Ye, J.; Lu, M.; Wang, S.; Li, Y. Comparison of activities of fatty acyl desaturases and elongases among six teleosts with different feeding and ecological habits. Front. Mar. Sci. 2020, 7, 117. [Google Scholar] [CrossRef]
- Ren, H.; Yu, J.; Xu, P.; Tang, Y. Single nucleotide polymorphisms of Δ6-desaturase and Elovl5 segments and their associations with common carp (Cyprinus carpio) growth traits. Genet. Mol. Res. 2015, 14, 12848–12854. [Google Scholar] [CrossRef]
- Ren, H.; Yu, J.; Xu, P.; Tang, Y. Influence of dietary fatty acids on muscle fatty acid composition and expression levels of Δ6 desaturase-like and Elovl5-like elongase in common carp (Cyprinus carpio var. Jian). Comp. Biochem. Phys. B 2012, 163, 184–192. [Google Scholar] [CrossRef]


| Gene | Primer Sequences (5′‒3′) | Product Length (bp) | Purpose |
|---|---|---|---|
| acsbg2 | F: CTCAAGTGTAACGTTGATGACAATG | 237 | qRT-PCR |
| R: AGATATGGAGAAGTCTCGTGGAAG | |||
| elovl5a | F: CAGGATGATGAACGTACTTTGGTG | 209 | qRT-PCR |
| R: GGATGAAGCTGTTAAACGTGGC | |||
| fads6 | F: GTGTGTGTGTCTGGGGTTTTTT | 198 | qRT-PCR |
| R: GTCATTTGGTAGATGCGTTTGG | |||
| lpl | F: GAGTCAACAAAATTCGCACACG | 205 | qRT-PCR |
| R: TTCAAAGCAGGCATAATGTAGGG | |||
| elovl5a | F: GCTTTGAACATATTTCCTTCCTGAC | 879 | PCR |
| R: TGCCCAATTCACCACTGCTT |
| Trait | Min (g/kg) | Max (g/kg) | Average (g/kg) | SD | Coefficient of Variation (%) |
|---|---|---|---|---|---|
| ALA—mutant | 0.062 | 0.751 | 0.228 | 0.024 | 103.9 |
| ALA—wildtype | 0.075 | 0.444 | 0.233 | 0.014 | 61 |
| ARA—mutant | 0.228 | 0.802 | 0.403 | 0.019 | 47.88 |
| ARA—wildtype | 0.235 | 0.58 | 0.401 | 0.013 | 31.63 |
| EPA—mutant | 0.135 | 0.322 | 0.235 | 0.016 | 53.12 |
| EPA—wildtype | 0.248 | 0.449 | 0.332 | 0.010 | 20 |
| DHA—mutant | 0.506 | 0.668 | 0.589 | 0.007 | 12.37 |
| DHA—wildtype | 0.343 | 0.537 | 0.472 | 0.007 | 14.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, P.; Zhu, Y.; Jiang, Y.; Feng, J.; Zhao, Z.; Xu, J. Contribution of elovl5a to Docosahexaenoic Acid (DHA) Synthesis at the Transcriptional Regulation Level in Common Carp, Cyprinus carpio. Animals 2024, 14, 544. https://doi.org/10.3390/ani14040544
Zhang H, Li P, Zhu Y, Jiang Y, Feng J, Zhao Z, Xu J. Contribution of elovl5a to Docosahexaenoic Acid (DHA) Synthesis at the Transcriptional Regulation Level in Common Carp, Cyprinus carpio. Animals. 2024; 14(4):544. https://doi.org/10.3390/ani14040544
Chicago/Turabian StyleZhang, Hanyuan, Peizhen Li, Youxiu Zhu, Yanliang Jiang, Jianxin Feng, Zixia Zhao, and Jian Xu. 2024. "Contribution of elovl5a to Docosahexaenoic Acid (DHA) Synthesis at the Transcriptional Regulation Level in Common Carp, Cyprinus carpio" Animals 14, no. 4: 544. https://doi.org/10.3390/ani14040544
APA StyleZhang, H., Li, P., Zhu, Y., Jiang, Y., Feng, J., Zhao, Z., & Xu, J. (2024). Contribution of elovl5a to Docosahexaenoic Acid (DHA) Synthesis at the Transcriptional Regulation Level in Common Carp, Cyprinus carpio. Animals, 14(4), 544. https://doi.org/10.3390/ani14040544






