Local and Systemic Inflammation in Finnish Dairy Cows with Digital Dermatitis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Herds
2.2. Study Population and Sampling
2.3. Analysis of Blood Samples
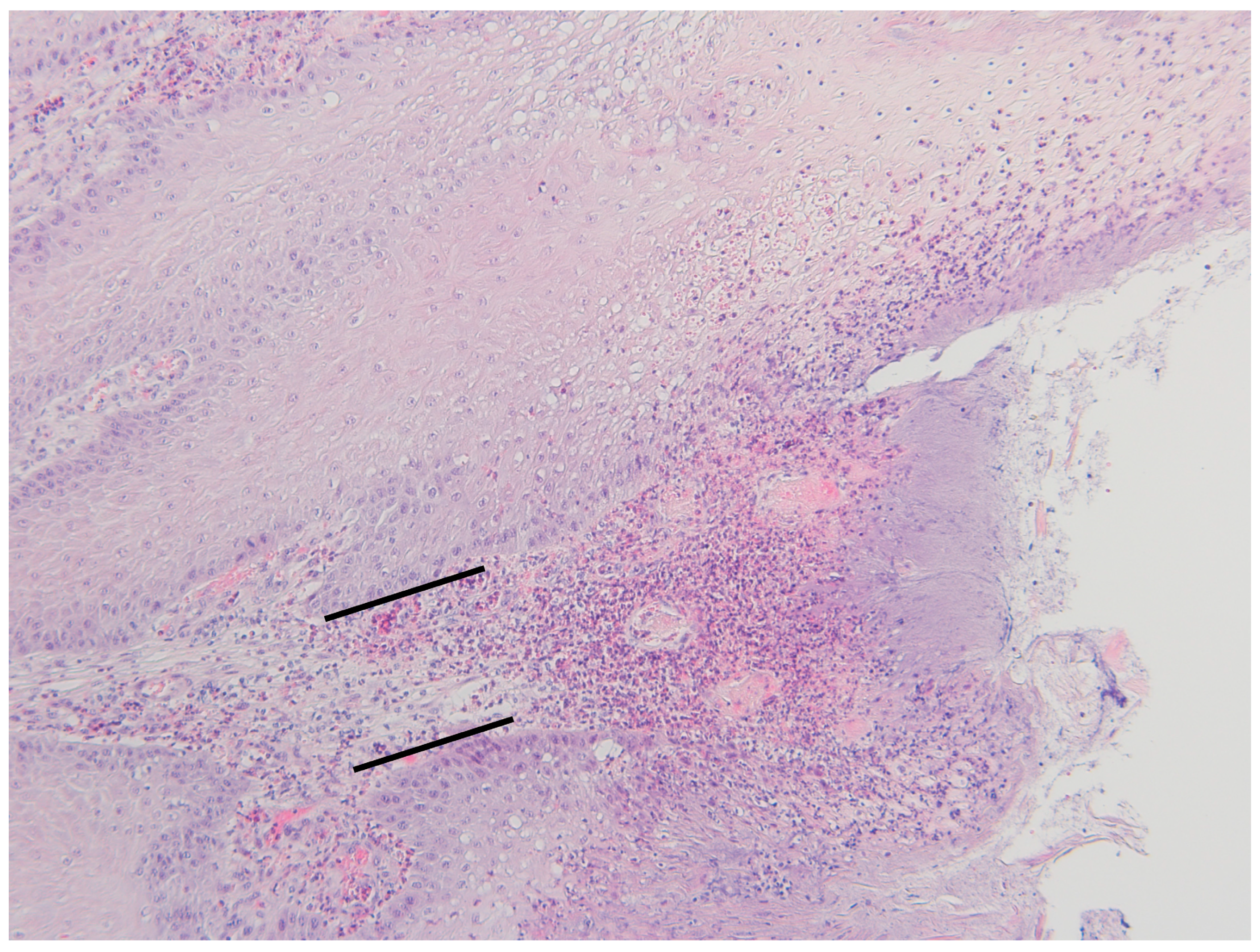
2.4. Histopathological Evaluation
2.5. Statistical Analysis
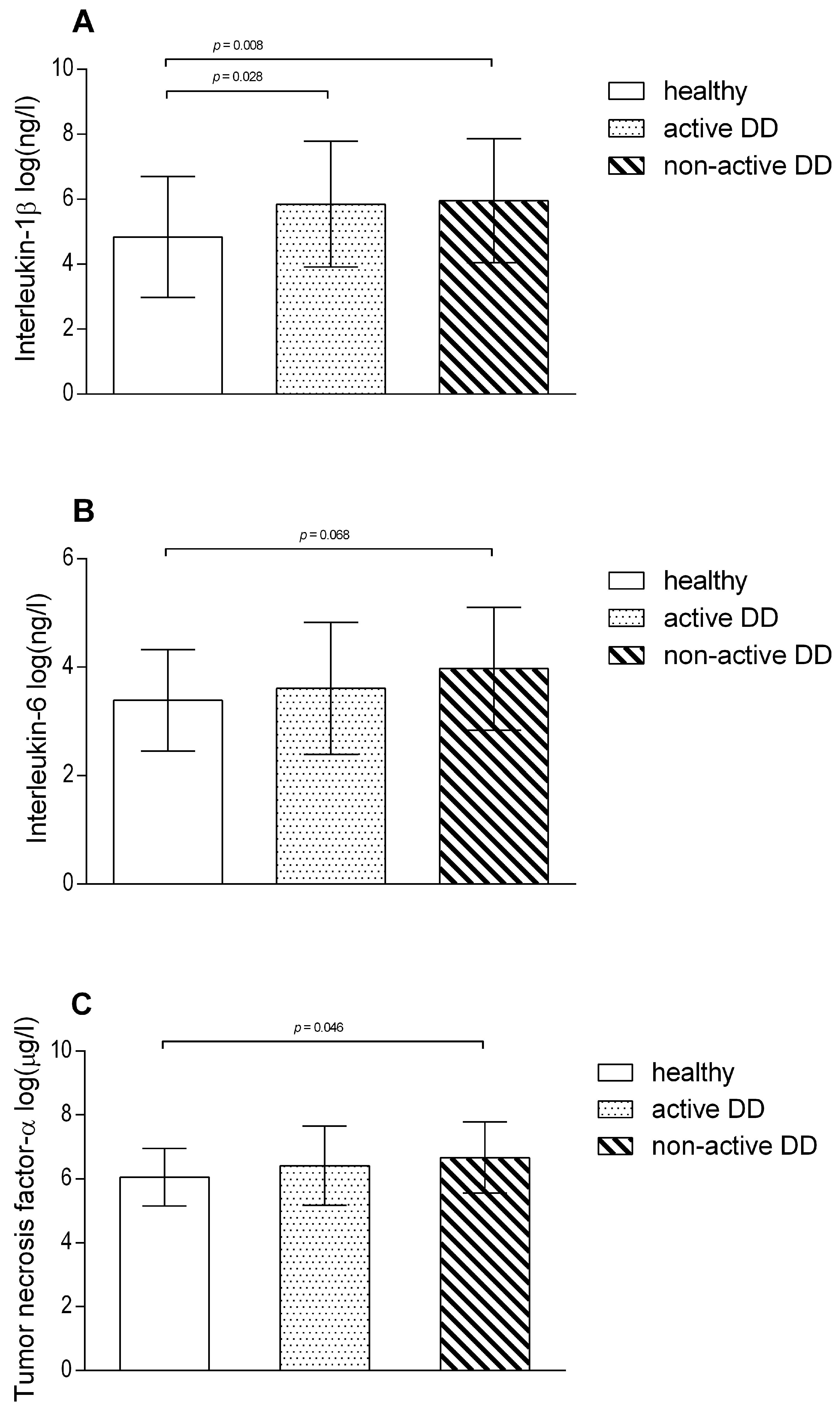
3. Results
3.1. Systemic and Local Inflammatory Response to DD Lesions
3.2. Histopathological Changes
4. Discussion
4.1. Acute Phase Proteins and Cytokines
4.2. Histopathology
4.3. Pain
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson-Welder, J.H.; Alt, D.P.; Nally, J.E. Digital Dermatitis in Cattle: Current Bacterial and Immunological Findings. Animals 2015, 5, 1114–1135. [Google Scholar] [CrossRef] [PubMed]
- Relun, A.; Lehebel, A.; Chesnin, A.; Guatteo, R.; Bareille, N. Association between digital dermatitis lesions and test-day milk yield of Holstein cows from 41 French dairy farms. J. Dairy Sci. 2013, 96, 2190–2200. [Google Scholar] [CrossRef]
- Mellado, M.; Saavedra, E.; Gaytán, L.; Veliz, F.G.; Macías-Cruz, U.; Avendaño-Reyes, L.; García, E. The effect of lameness-causing lesions on milk yield and fertility of primiparous Holstein cows in a hot environment. Livest Sci. 2018, 217, 8–14. [Google Scholar] [CrossRef]
- Evans, N.J.; Murray, R.D.; Carter, S.D. Bovine digital dermatitis: Current concepts from laboratory to farm. Vet. J. 2016, 211, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.L.; Read, D.H.; Famula, T.R.; Mongini, A.; Döpfer, D. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 2012, 193, 654–658. [Google Scholar] [CrossRef]
- Döpfer, D.; ter Huurne, A.A.H.M.; Cornelisse, J.L.; van Asten, A.J.A.M.; Koopmans, A.; Meijer, F.A.; Schukken, Y.H.; Szakáll, I.; Klee, W.; Bosma, R.B. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 1997, 140, 620–623. [Google Scholar] [CrossRef]
- Biemans, F.; Bijma, P.; Boots, N.M.; de Jong, M.C.M. Digital Dermatitis in dairy cattle: The contribution of different disease classes to transmission. Epidemics 2018, 23, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Relun, A.; Guatteo, R.; Roussel, P.; Bareille, N. A simple method to score digital dermatitis in dairy cows in the milking parlor. J. Dairy Sci. 2011, 94, 5424–5434. [Google Scholar] [CrossRef]
- Solano, L.; Barkema, H.W.; Jacobs, C.; Orsel, K. Validation of the M-stage scoring system for digital dermatitis on dairy cows in the milking parlor. J. Dairy Sci. 2017, 100, 1592–1603. [Google Scholar] [CrossRef]
- Vanhoudt, A.; Yang, D.A.; Armstrong, T.; Huxley, J.N.; Laven, R.A.; Manning, A.D.; Newsome, R.F.; Nielen, M.; van Werven, T.; Bell, N.J. Interobserver agreement of digital dermatitis M-scores for photographs of the hind feet of standing dairy cattle. J. Dairy Sci. 2019, 102, 5466–5474. [Google Scholar] [CrossRef] [PubMed]
- Krull, A.C.; Shearer, J.K.; Gorden, P.J.; Cooper, V.L.; Phillips, G.J.; Plummera, P.J. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 2014, 82, 3359–3373. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Demirkan, I.; Singh, P.; Getty, B.; Timofte, D.; Vink, W.D.; Murray, R.D.; Blowey, R.W.; Birtles, R.J.; et al. Association of unique, isolated treponemes with bovine digital dermatitis lesions. J. Clin. Microbiol. 2009, 47, 689–696. [Google Scholar] [CrossRef]
- Zinicola, M.; Lima, F.; Lima, S.; Machado, V.; Gomez, M.; Döpfer, D.; Guard, C.; Bicalho, R. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. PLoS ONE 2015, 10, e0120504. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.W.; Strube, M.L.; Isbrand, A.; Al-Medrasi, W.D.H.M.; Boye, M.; Jensen, T.K.; Klitgaard, K. Potential bacterial core species associated with digital dermatitis in cattle herds identified by molecular profiling of interdigital skin samples. Vet. Microbiol. 2016, 186, 139–149. [Google Scholar] [CrossRef]
- Mumba, T.; Dopfer, D.; Kruitwagen, C.; Dreher, M.; Gaastra, W.; van der Zeijst, B.A. Detection of spirochetes by polymerase chain reaction and its relation to the course of digital dermatitis after local antibiotic treatment in dairy cattle. J. Vet. Med. 1999, 46, 117–126. [Google Scholar] [CrossRef]
- Refaai, W.; Ducatelle, R.; Geldhof, P.; Mihi, B.; El-Shair, M.; Opsomer, G. Digital dermatitis in cattle is associated with an excessive innate immune response triggered by the keratinocytes. BMC Vet. Res. 2013, 9, 193. [Google Scholar] [CrossRef]
- Moreira, T.F.; Facury Filho, E.J.; Carvalho, A.U.; Strube, M.L.; Nielsen, M.V.; Klitgaard, K.; Jensen, T.K. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. PLoS ONE 2018, 13, e0193870. [Google Scholar] [CrossRef]
- Klitgaard, K.; Boye, M.; Capion, N.; Jensen, T.K. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 2008, 46, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Kushner, I. The phenomenon of the acute phase response. Ann. N. Y. Acad. Sci. 1982, 389, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.; Gauldie, J. The acute phase response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Suojala, L.; Orro, T.; Järvinen, H.; Saatsi, J.; Pyörälä, S. Acute phase response in two consecutive experimentally induced E. coli intramammary infections in dairy cows. Acta Vet. Scand. 2008, 50, 18. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.C.; Geraghty, T.; Simões, P.B.A.; Mshelbwala, F.M.; Haining, H.; Eckersall, P.D. A pilot study of acute phase proteins as indicators of bovine mastitis caused by different pathogens. Res. Vet. Sci. 2018, 119, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Prohl, A.; Schroedl, W.; Rhode, H.; Reinhold, P. Acute phase proteins as local biomarkers of respiratory infection in calves. BMC Vet. Res. 2015, 11, m167. [Google Scholar] [CrossRef] [PubMed]
- Bagga, A.; Randhawa, S.S.; Sharma, S.; Bansal, B.K. Acute phase response in lame crossbred dairy cattle. Vet. World 2016, 9, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Ilievska, K.; Atanasov, B.; Dovenski, T.; Smolac, O.; Stojanov, B.; Trojachanec, P. Acute Phase Proteins—As Indicators of Claw Diseases in Dairy Cattle. Maced. Vet. Rev. 2019, 42, 95–100. [Google Scholar] [CrossRef]
- Kontturi, M.; Junni, R.; Kujala-Wirth, M.; Malinen, E.; Seuna, E.; Pelkonen, S.; Soveri, T.; Simojoki, H. Acute phase response and clinical manifestation in outbreaks of interdigital phlegmon in dairy herds. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101375. [Google Scholar] [CrossRef]
- Pirkkalainen, H.; Talvio, I.; Kujala-Wirth, M.; Soveri, T.; Orro, T. Acute phase response of sole ulcer, white line disease and digital dermatitis in dairy cows. Vet. Anim. Sci. 2022, 17, 100253. [Google Scholar] [CrossRef]
- Pirkkalainen, H.; Riihimäki, A.; Simojoki, H.; Soveri, T.; Rajala-Schultz, P.J.; Hintikka, T.; Pelkonen, S.; Kontturi, M.; Kujala-Wirth, M. Prevalence of digital dermatitis using mirror scoring in Finnish freestall dairy herds. J. Dairy Sci. 2021, 104, 9173–9184. [Google Scholar] [CrossRef] [PubMed]
- Epitools—Epidemiological Calculators. Available online: https://epitools.ausvet.com.au/ (accessed on 18 September 2020).
- Makimura, S.; Suzuki, N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Jpn. J. Vet. Res. 1982, 44, 15–21. [Google Scholar] [CrossRef]
- Middleton, J.E. Wound Healing: Process, Phases and Promoting; Nova Science Publishers: New York, NY, USA, 2011; pp. 77–93. [Google Scholar]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Phys. Rev. 2003, 83, 581–632. [Google Scholar] [CrossRef]
- Hantash, B.M.; Zhao, L.; Knowles, J.A.; Lorenz, H.P. Adult and fetal wound healing. Front. Biosci. 2008, 1, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Krull, A.C.; Shearer, J.K.; Gorden, P.J.; Scott, H.M.; Plummer, P.J. Digital dermatitis: Natural lesion progression and regression in Holstein dairy cattle over 3 years. J. Dairy Sci. 2016, 99, 3718–3731. [Google Scholar] [CrossRef]
- Scholey, R.A.; Evans, N.J.; Blowey, R.W.; Massey, J.P.; Murray, R.D.; Smith, R.F.; Ollier, W.E.; Carter, S.D. Identifying host pathogenic pathways in bovine digital dermatitis by RNA-Seq analysis. Vet. J. 2013, 197, 699–706. [Google Scholar] [CrossRef]
- Zuerner, R.L.; Heidari, M.; Elliott, M.K.; Alt, D.P.; Neill, J.D. Papillomatous digital dermatitis spirochetes suppress the bovine macrophage innate immune response. Vet. Microbiol. 2007, 125, 256–264. [Google Scholar] [CrossRef]
- Watts, K.M.; Fodor, C.; Beninger, C.; Lahiri, P.; Arrazuria, R.; De Buck, J.; Knight, C.G.; Orsel, K.; Barkema, H.W.; Cobo, E.R. A Differential Innate Immune Response in Active and Chronic Stages of Bovine Infectious Digital Dermatitis. Front. Microbiol. 2018, 9, 1586. [Google Scholar] [CrossRef]
- Evans, N.J.; Brown, J.M.; Scholey, R.; Murray, R.D.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Differential inflammatory responses of bovine foot skin fibroblasts and keratinocytes to digital dermatitis treponemes. Vet. Immunol. Immunopathol. 2014, 161, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.; Capion, N.; Klitgaard, K.; Rogdo, T.; Fjeldaas, T.; Boye, M.; Jensen, T.K. Bovine digital dermatitis: Possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet. Microbiol. 2012, 160, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Moreira, T.F.; Nicolino, R.R.; de Andrade, L.S.; Filho, E.J.F.; de Carvalho, A.U. Prevalence of lameness and hoof lesions in all year-round grazing cattle in Brazil. Trop. Anim. Health Prod. 2018, 50, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Alsaaod, M.; Jensen, T.K.; Miglinci, L.; Gurtner, C.; Brandt, S.; Plüss, J.; Studer, E.; Steiner, A. Proof of an optimized salicylic acid paste-based treatment concept of ulcerative M2-stage digital dermatitis lesions in 21 dairy cows. PLoS ONE 2022, 17, e0269521. [Google Scholar] [CrossRef] [PubMed]
- Autio, T.; Tuunainen, E.; Nauholz, H.; Pirkkalainen, H.; London, L.; Pelkonen, S. Overview of Control Programs for Cattle Diseases in Finland. Front. Vet. Sci. 2021, 8, 688936. [Google Scholar] [CrossRef]
- Animal Health ETT. Available online: https://www.ett.fi/en/animal-health-ett/ (accessed on 14 October 2023).
- Rodriguez-Lainz, A.; Melendez-Retamal, P.; Hird, D.; Read, D.; Walker, R. Farm- and host-level risk factors for papillomatous digital dermatitis in Chilean dairy cattle. Prev. Vet. Med. 1999, 42, 87–97. [Google Scholar] [CrossRef]
- Bruijnis, M.R.; Beerda, B.; Hogeveen, H.; Stassen, E.N. Assessing the welfare impact of foot disorders in dairy cattle by a modeling approach. Animal 2012, 6, 962–970. [Google Scholar] [CrossRef]
- Jewell, M.T.; Cameron, M.; McKenna, S.L.; Cockram, M.S.; Sanchez, J.; Keefe, G.P. Relationships between type of hoof lesion and behavioral signs of lameness in Holstein cows housed in Canadian tiestall facilities. J. Dairy Sci. 2021, 104, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D.; Orsel, K.; Pajor, E.A. Impact of digital dermatitis on locomotion and gait traits of beef cattle. J. Anim. Sci. 2022, 100, skac262. [Google Scholar] [CrossRef]
- Read, D.H.; Walker, R.L. Papillomatous digital dermatitis (footwarts) in California dairy cattle: Clinical and gross pathologic findings. J. Vet. Diagn. Investig. 1998, 69, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Frankena, K.; Somers, J.G.C.J.; Schouten, W.G.P.; van Stek, J.V.; Metz, J.H.M.; Stassen, E.N.; Graat, E.A.M. The effect of digital lesions and floor type on locomotion score in Dutch dairy cows. Prev. Vet. Med. 2009, 88, 150–157. [Google Scholar] [CrossRef]
- Losinger, W.C. Economic impacts of reduced milk production associated with papillomatous digital dermatitis in dairy cows in the USA. J. Dairy Res. 2006, 73, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, E.J.; Hernandez, J.A.; Shearer, J.K.; Risco, C.A.; Thatcher, W.W. Effect of lameness on ovarian activity in postpartum Holstein cows. J. Dairy Sci. 2004, 87, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Saarikoski, R.; Liukkonen, I.; Piispa, S.; Stolt, M. Terveet Jalat, 3rd ed.; Duodecim: Helsinki, Finland, 2010. [Google Scholar]
- Longhurst, N.; Steele, C. Dry heel fissures: Treatment and prevention. Dermatol. Nur. 2016, 15, 46–49. [Google Scholar]
- Capion, N.; Boye, M.; Ekstrøm, C.T.; Jensen, T.K. Infection dynamics of digital dermatitis in first-lactation Holstein cows in an infected herd. J. Dairy Sci. 2012, 95, 6457–6464. [Google Scholar] [CrossRef] [PubMed]

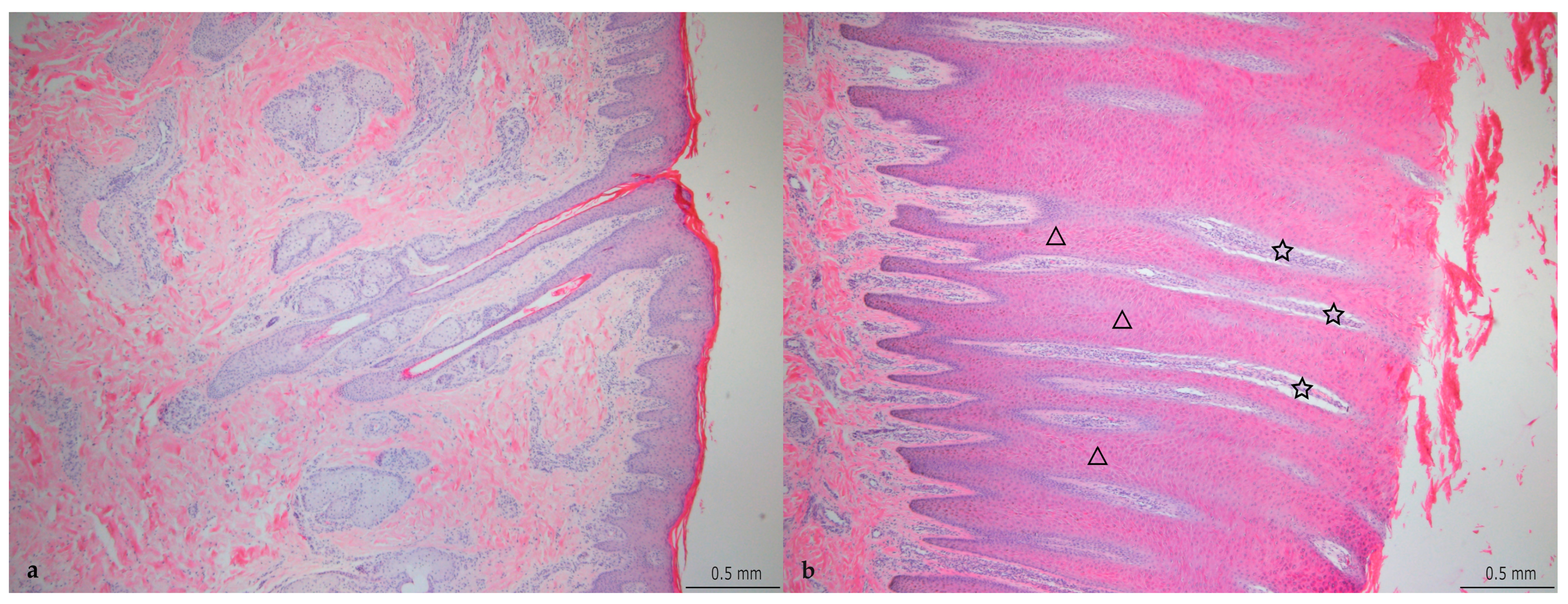

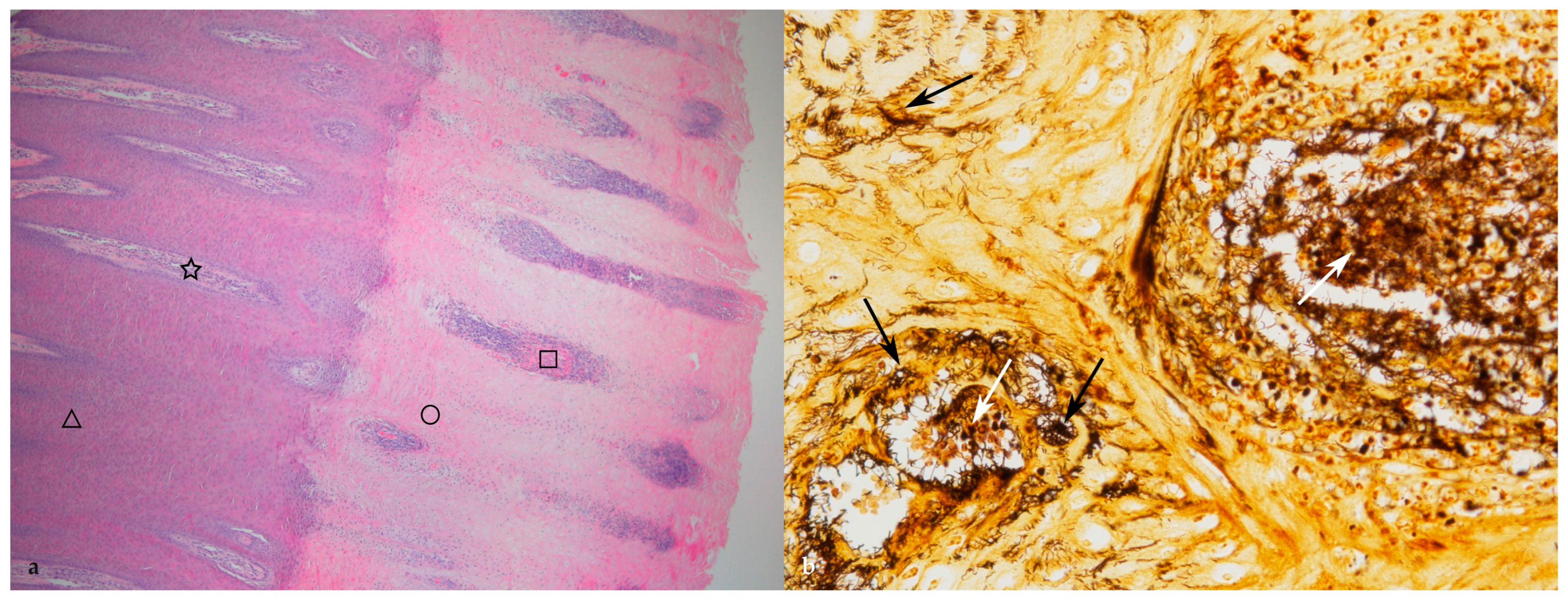
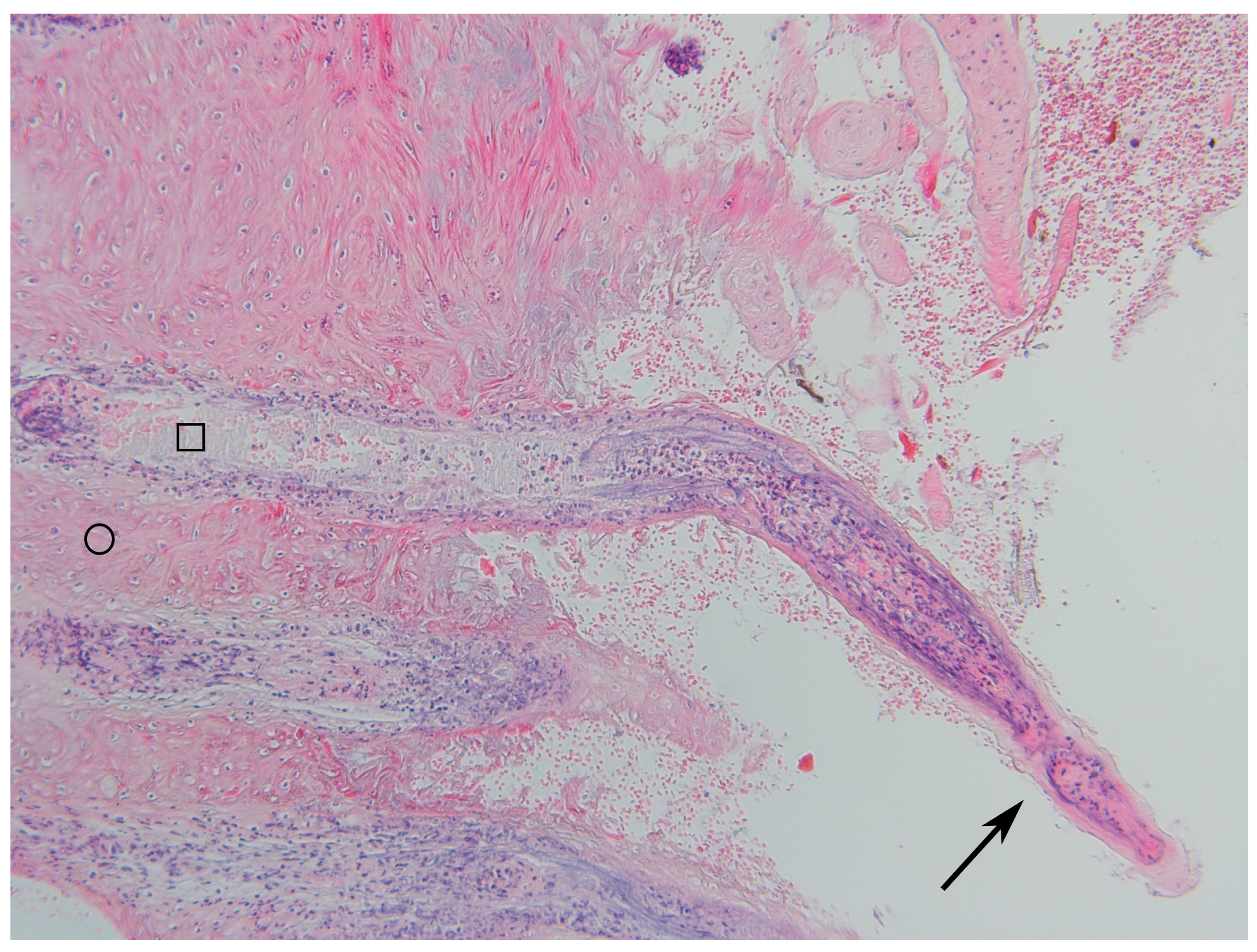
| Grade | Description | Thickness of Epidermis (mm) |
|---|---|---|
| 0 | normal | 0.3–0.8 |
| 1 | mild to moderate hyperplasia and ortokeratotic hyperkeratosis | 1–1.5 |
| 2 | low moderate hyperplasia and ortokeratotic hyperkeratosis with necrosis and erosion | 1.5–2 |
| 3 | high moderate hyperplasia and ortokeratotic hyperkeratosis with necrosis and ulceration extending to the nonpapillar dermis | 2–2.4 |
| 4 | marked hyperplasia and (para- and ortokeratotic) hyperkeratosis with some fissures in the thick layer of keratin | 2.5–4.5 |
| 5 | marked hyperplasia and (para- and ortokeratotic) hyperkeratosis with necrosis and erosion | 4–4.5 |
| 6 | marked hyperplasia and (para- and ortokeratotic) hyperkeratosis with necrosis and ulceration extending to the nonpapillar dermis | 4–4.5 |
| DD Lesion | Histopathological Grade 1 | Total (n) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| M0 | 19 | 11 | 0 | 0 | 2 | 3 | 0 | 35 |
| M1 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 7 |
| M2 | 0 | 0 | 0 | 6 | 0 | 7 | 6 | 19 |
| M3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| M4 | 0 | 1 | 2 | 0 | 1 | 22 | 2 | 28 |
| M4.1 | 0 | 0 | 5 | 0 | 0 | 8 | 1 | 14 |
| Total (n) | 21 | 14 | 8 | 6 | 4 | 42 | 9 | 104 |
| Grade 1 | 0 (No Spirochetes) | 1 (Low No. of Spirochetes) | 2 (Moderate No. of Spirochetes) | 3 (Large No. of Spirochetes) |
|---|---|---|---|---|
| 0 | 21 | 0 | 0 | 0 |
| 1 | 14 | 0 | 0 | 0 |
| 2 | 3 | 2 (NZ, MFN) | 1 (NZ) | 2 (NZ) |
| 3 | 0 | 0 | 3 (NZ) | 3 (NZ) |
| 4 | 4 | 0 | 0 | 0 |
| 5 | 0 | 14 (1NZ, 13 MFN) | 16 (9MFN, 7NZ) | 12 (NZ) |
| 6 | 0 | 3 (MFN) | 1 (NZ) | 5 (1 NZ, 4 NZ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirkkalainen, H.; Riihimäki, A.; Lienemann, T.; Anttila, M.; Kujala-Wirth, M.; Rajala-Schultz, P.; Simojoki, H.; Soveri, T.; Orro, T. Local and Systemic Inflammation in Finnish Dairy Cows with Digital Dermatitis. Animals 2024, 14, 461. https://doi.org/10.3390/ani14030461
Pirkkalainen H, Riihimäki A, Lienemann T, Anttila M, Kujala-Wirth M, Rajala-Schultz P, Simojoki H, Soveri T, Orro T. Local and Systemic Inflammation in Finnish Dairy Cows with Digital Dermatitis. Animals. 2024; 14(3):461. https://doi.org/10.3390/ani14030461
Chicago/Turabian StylePirkkalainen, Hertta, Aino Riihimäki, Taru Lienemann, Marjukka Anttila, Minna Kujala-Wirth, Päivi Rajala-Schultz, Heli Simojoki, Timo Soveri, and Toomas Orro. 2024. "Local and Systemic Inflammation in Finnish Dairy Cows with Digital Dermatitis" Animals 14, no. 3: 461. https://doi.org/10.3390/ani14030461
APA StylePirkkalainen, H., Riihimäki, A., Lienemann, T., Anttila, M., Kujala-Wirth, M., Rajala-Schultz, P., Simojoki, H., Soveri, T., & Orro, T. (2024). Local and Systemic Inflammation in Finnish Dairy Cows with Digital Dermatitis. Animals, 14(3), 461. https://doi.org/10.3390/ani14030461





