Effect of Regular Training on Platelet Function in Untrained Thoroughbreds
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Sample Collection
2.3. Light Transmission Aggregometry (LTA)
2.4. Platelet Function Analyzer
2.5. Nitrite-Nitrate (NOx)
2.6. RT-qPCR Analyses
2.7. Statistical Analysis
3. Results
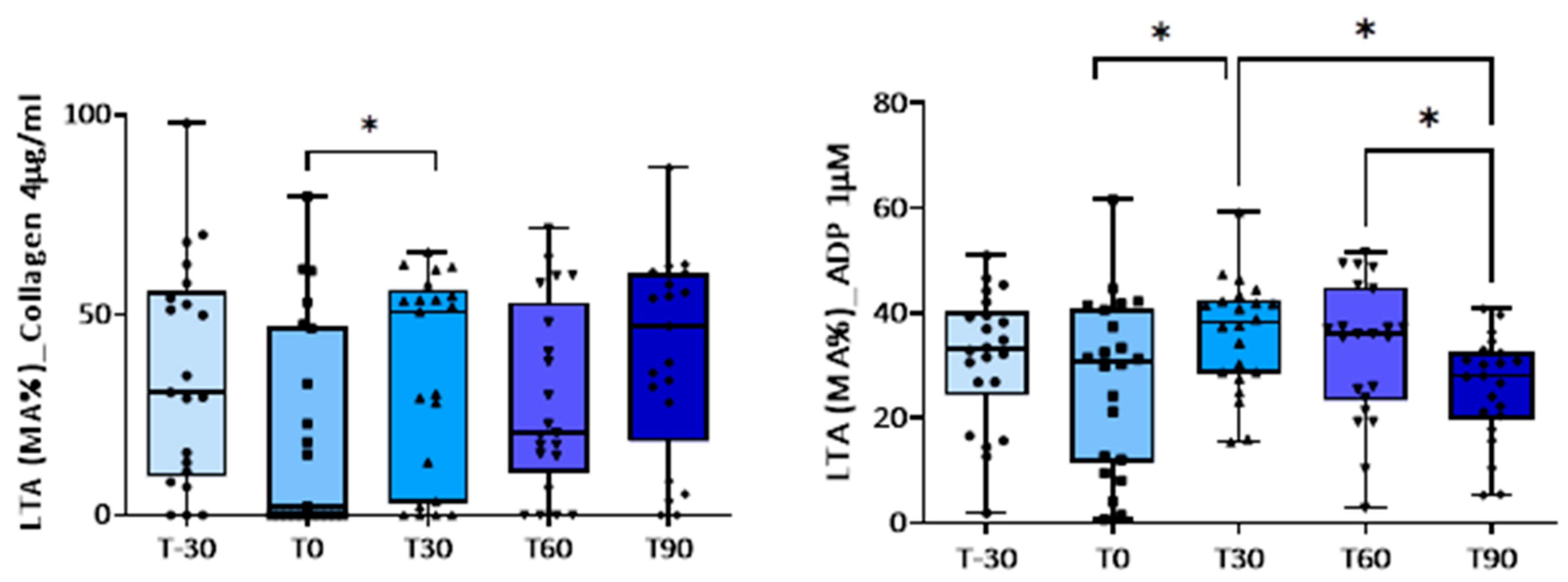
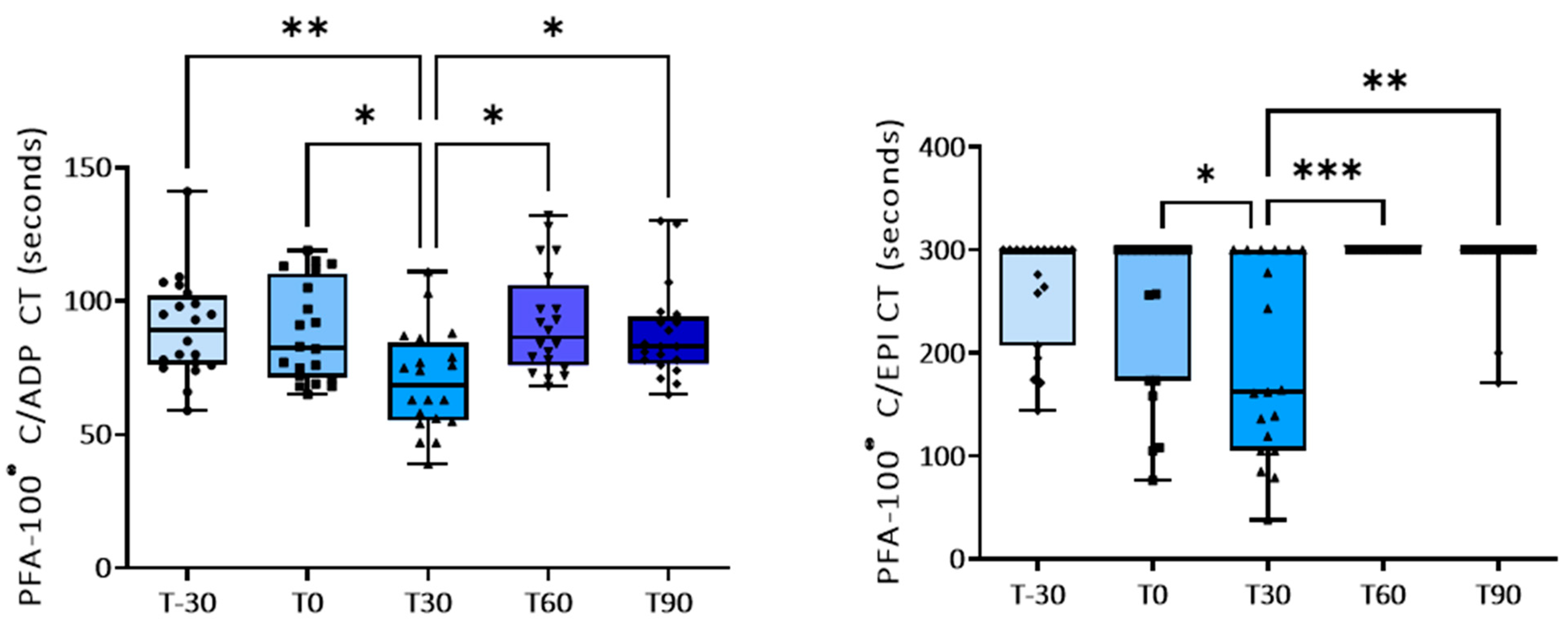
3.1. Effect of Training on Platelet Activation
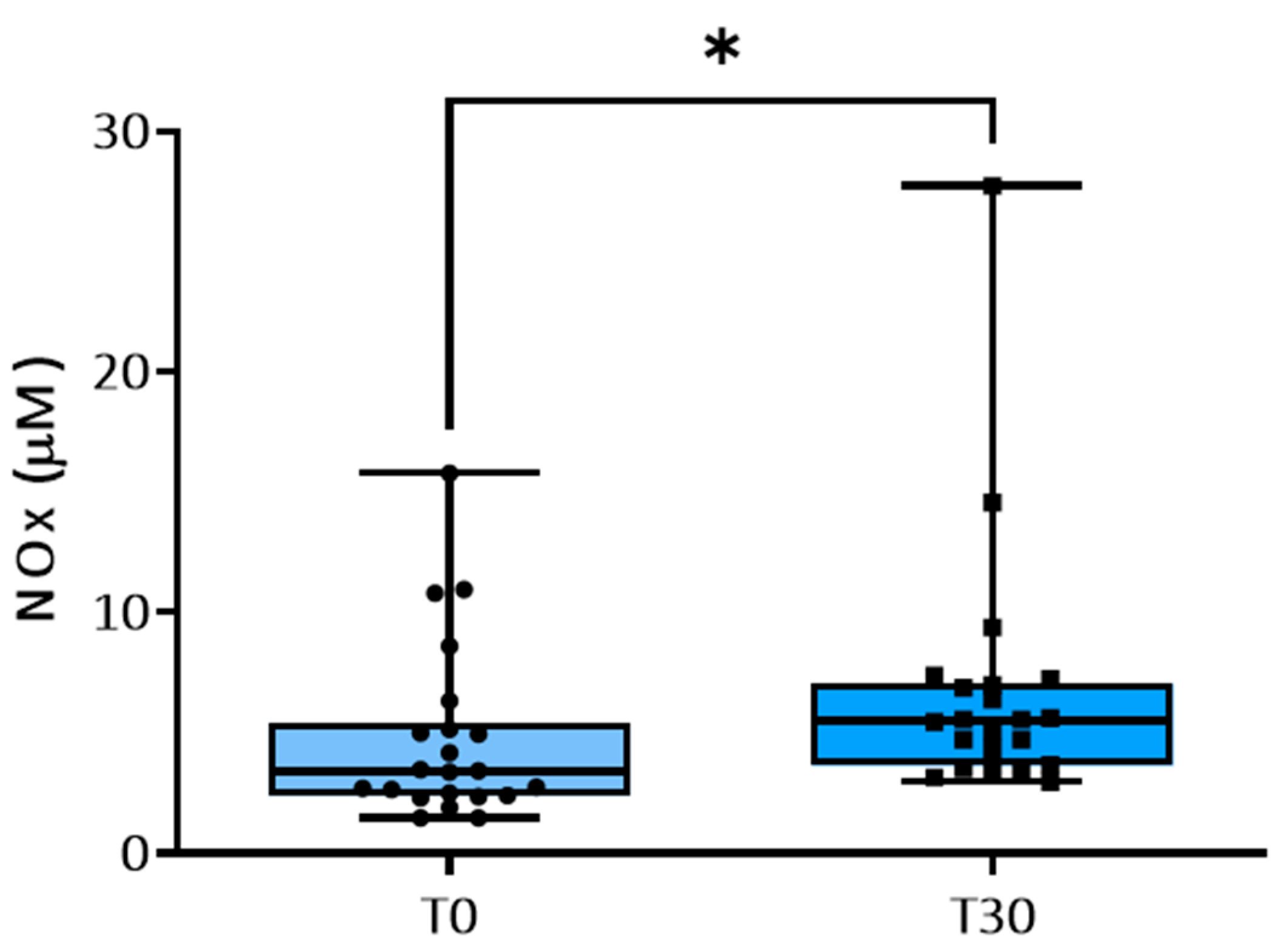
3.2. NO Metabolism
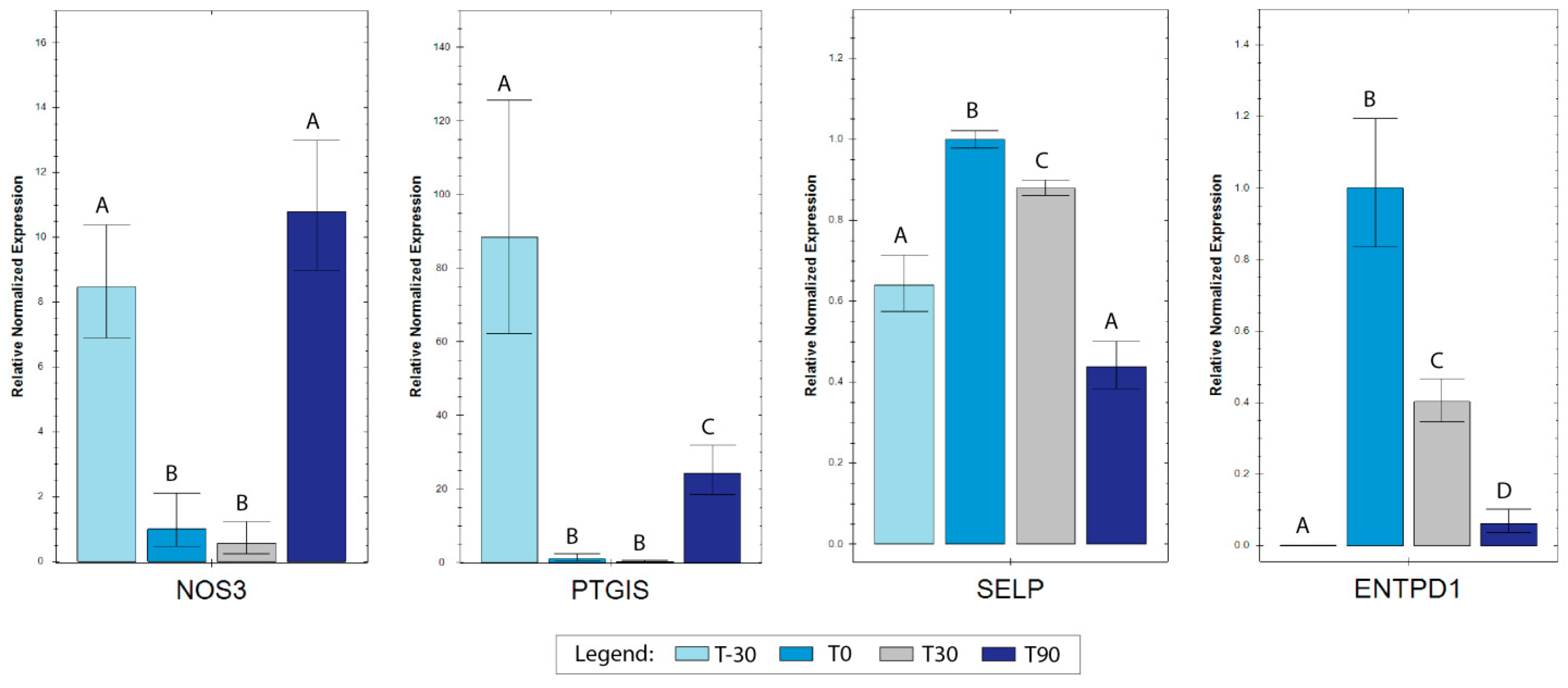
3.3. RT-qPCR Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assenza, A.; Tosto, F.; Casella, S.; Fazio, F.; Giannetto, C.; Piccione, G. Changes in Blood Coagulation Induced by Exercise Training in Young Athletic Horses. Res. Vet. Sci. 2013, 95, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Fazio, F.; Giudice, E.; Pietro, S.D.; Bruschetta, D.; Piccione, G. Different Training Schedules Influence Platelet Aggregation in Show Jumping Horses. Pol. J. Vet. Sci. 2017, 20, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kingston, J.K.; Sampson, S.N.; Beard, L.A.; Meyers, K.M.; Sellon, D.C.; Bayly, W.M. The Effect of Supramaximal Exercise on Equine Platelet Function. Equine Vet. J. Suppl. 1999, 31, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Bazzano, M.; Giannetto, C.; Marafioti, S.; Fazio, F. Training-Induced Changes in Clotting Parameters of Athletic Horses. J. Vet. Sci. 2014, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Grasso, F.; Fazio, F.; Giudice, E. The Effect of Physical Exercise on the Daily Rhythm of Platelet Aggregation and Body Temperature in Horses. Vet. J. 2008, 176, 216–220. [Google Scholar] [CrossRef]
- Piccione, G.; Fazio, F.; Giudice, E.; Grasso, F.; Caola, G. Exercise-Induced Changes in the Clotting Times and Fibrinolytic Activity during Official 1600 and 2000 Meters Trot Races in the Standardbred Horses. Acta Vet. Brno 2005, 74, 509–514. [Google Scholar] [CrossRef][Green Version]
- McKeever, K.H.; Hinchcliff, K.W.; Kociba, G.J.; Reed, S.M.; Muir, W.W. Changes in Coagulation and Fibrinolysis in Horses during Exercise. Am. J. Vet. Res. 1990, 51, 1335–1339. [Google Scholar] [CrossRef]
- Monreal, L.; Angles, A.M.; Monreal, M.; Espada, Y.; Monasterio, J. Changes in Haemostasis in Endurance Horses: Detection by Highly Sensitive ELISA-Tests. Equine Vet. J. 1995, 27, 120–123. [Google Scholar] [CrossRef]
- Piccione, G.; Assenza, A.; Casella, S.; Giannetto, C.; Tosto, F.; Caola, G. Modifications of Platelet Aggregation during Treadmill Section and Obstacle Course in Athletic Horse. Acta Vet. 2010, 60, 165–172. [Google Scholar] [CrossRef]
- El-Sayed, M.S.; Ali, N.; El-Sayed Ali, Z. Aggregation and Activation of Blood Platelets in Exercise and Training. Sports Med. 2005, 35, 11–22. [Google Scholar] [CrossRef]
- Giordano, A.; Meazza, C.; Salvadori, M.; Paltrinieri, S. Thromboelastometric Profiles of Horses Affected by Exercise-Induced Pulmonary Hemorrhages. Vet. Med. Int. 2010, 2010, 945789. [Google Scholar] [CrossRef] [PubMed]
- Kingston, J.K.; Bayly, W.M.; Meyers, K.M.; Sellon, D.C.; Wardrop, K.J. Evaluation of Binding of Fibrinogen and Annexin V to Equine Platelets in Response to Supramaximal Treadmill Exercise. Equine Vet. J. Suppl. 2002, 34, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Grzędzicka, J.; Dąbrowska, I.; Malin, K.; Witkowska-Piłaszewicz, O. Exercise-Related Changes in the Anabolic Index (Testosterone to Cortisol Ratio) and Serum Amyloid A Concentration in Endurance and Racehorses at Different Fitness Levels. Front. Vet. Sci. 2023, 10, 1148990. [Google Scholar] [CrossRef]
- Karolczak, K.; Konieczna, L.; Soltysik, B.; Kostka, T.; Witas, P.J.; Kostanek, J.; Baczek, T.; Watala, C. Plasma Concentration of Cortisol Negatively Associates with Platelet Reactivity in Older Subjects. Int. J. Mol. Sci. 2022, 24, 717. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Falcinelli, E.; Sebastiano, M.; Momi, S. Matrix Metalloproteinases and Platelet Function. Prog. Mol. Biol. Transl. Sci. 2017, 147, 133–165. [Google Scholar] [CrossRef] [PubMed]
- Plisak, U.; Szczepaniak, J.; Żmigrodzka, M.; Giercuszkiewicz-Hecold, B.; Witkowska-Piłaszewicz, O. Changes in Novel Anti-Infalmmatory Cytokine Concetration in the Bood of Endurance and Race Horses at Different Levels of Training. Comput. Struct. Biotechnol. J. 2023, 21, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Piknova, B.; Nghiem, K.; Lozier, J.N.; Schechter, A.N. Inhibitory Effect of Nitrite on Coagulation Processes Demonstrated by Thrombelastography. Nitric Oxide 2014, 40, 45–51. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Carlström, M.; Weitzberg, E. Metabolic Effects of Dietary Nitrate in Health and Disease. Cell Metab. 2018, 28, 9–22. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. NO-Synthase Independent NO Generation in Mammals. Biochem. Biophys. Res. Commun. 2010, 396, 39–45. [Google Scholar] [CrossRef]
- Arefirad, T.; Seif, E.; Sepidarkish, M.; Mohammadian Khonsari, N.; Mousavifar, S.A.; Yazdani, S.; Rahimi, F.; Einollahi, F.; Heshmati, J.; Qorbani, M. Effect of Exercise Training on Nitric Oxide and Nitrate/Nitrite (NOx) Production: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 953912. [Google Scholar] [CrossRef]
- Lo Feudo, C.M.; Stucchi, L.; Conturba, B.; Stancari, G.; Zucca, E.; Ferrucci, F. Medical Causes of Poor Performance and Their Associations with Fitness in Standardbred Racehorses. J. Vet. Intern. Med. 2023, 37, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Hinchcliff, K.W.; Couetil, L.L.; Knight, P.K.; Morley, P.S.; Robinson, N.E.; Sweeney, C.R.; van Erck, E. Exercise Induced Pulmonary Hemorrhage in Horses: American College of Veterinary Internal Medicine Consensus Statement. J. Vet. Intern. Med. 2015, 29, 743–758. [Google Scholar] [CrossRef]
- Poole, D.C.; Erickson, H.H. Exercise-Induced Pulmonary Hemorrhage: Where Are We Now? Vet. Med. 2016, 7, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, N.; Gatica, S.; Olivares, P.; Cabello-Verrugio, C.; Simon, F. Endothelial Cells Exhibit Two Waves of P-Selectin Surface Aggregation Under Endotoxic and Oxidative Conditions. Protein J. 2019, 38, 667–674. [Google Scholar] [CrossRef]
- Yadav, V.; Chi, L.; Zhao, R.; Tourdot, B.E.; Yalavarthi, S.; Jacobs, B.N.; Banka, A.; Liao, H.; Koonse, S.; Anyanwu, A.C.; et al. Ectonucleotidase Tri(Di)Phosphohydrolase-1 (ENTPD-1) Disrupts Inflammasome/Interleukin 1β-Driven Venous Thrombosis. J. Clin. Investig. 2019, 129, 2872–2877. [Google Scholar] [CrossRef]
- Boudreaux, M.K.; Koehler, J.; Habecker, P.L.; Piero, F.D. Evaluation of the Genes Encoding CD39/NTPDase-1 and CD39L1/NTPDase-2 in Horses with and without Abnormal Hemorrhage and in Horses with Pathologic Evidence of Exercise-Induced Pulmonary Hemorrhage. Vet. Clin. Pathol. 2015, 44, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Yabuki, T.; Inoue, H.; Tone, Y.; Hara, S.; Hatae, T.; Nagata, M.; Takahashi, E.I.; Tanabe, T. Human Gene Encoding Prostacyclin Synthase (PTGIS): Genomic Organization, Chromosomal Localization, and Promoter Activity. Genomics 1996, 36, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.T.-V.; Elbatreek, M.H.; Fuchß, T.; Grädler, U.; Schmidt, H.H.H.W.; Shah, A.M.; Wallace, A.; Knowles, R. Nitric Oxide Synthase Inhibitors into the Clinic at Last. In Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Vedmedovska, N.; Bokucava, D.; Kivite-Urtane, A.; Rovite, V.; Zake-Nikitina, L.; Klovins, J.; Fodina, V.; Donders, G.G.G. The Correlation Between Abnormal Uterine Artery Flow in the First Trimester and Genetic Thrombophilic Alteration: A Prospective Case-Controlled Pilot Study. Diagnostics 2020, 10, 654. [Google Scholar] [CrossRef]
- Miglio, A.; Falcinelli, E.; Mezzasoma, A.M.; Cappelli, K.; Mecocci, S.; Gresele, P.; Antognoni, M.T. Effect of First Long-Term Training on Whole Blood Count and Blood Clotting Parameters in Thoroughbreds. Animals 2021, 11, 447. [Google Scholar] [CrossRef]
- Miglio, A.; Cappelli, K.; Capomaccio, S.; Mecocci, S.; Silvestrelli, M.; Antognoni, M.T. Metabolic and Biomolecular Changes Induced by Incremental Long-Term Training in Young Thoroughbred Racehorses during First Workout Season. Animals 2020, 10, 317. [Google Scholar] [CrossRef]
- Segura, D.; Monreal, L.; Espada, Y.; Pastor, J.; Mayós, I.; Homedes, J. Assessment of a Platelet Function Analyser in Horses: Reference Range and Influence of a Platelet Aggregation Inhibitor. Vet. J. 2005, 170, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Guglielmini, G.; Torti, M.; Gresele, P. Intraplatelet Signaling Mechanisms of the Priming Effect of Matrix Metalloproteinase-2 on Platelet Aggregation. J. Thromb. Haemost. 2005, 3, 2526–2535. [Google Scholar] [CrossRef]
- Kundu, S.K.; Heilmann, E.J.; Sio, R.; Garcia, C.; Davidson, R.M.; Ostgaard, R.A. Description of an in Vitro Platelet Function Analyzer--PFA-100. Semin. Thromb. Hemost. 1995, 21 (Suppl. 2), 106–112. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Bury, L.; Mezzasoma, A.M.; Falcinelli, E. Platelet Function Assays in Diagnosis: An Update. Expert Rev. Hematol. 2019, 12, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, E.; Francisci, D.; Schiaroli, E.; Minuz, P.; Orsini, S.; Malincarne, L.; Sebastiano, M.; Mezzasoma, A.M.; Pasticci, M.B.; Guglielmini, G.; et al. Effect of Aspirin Treatment on Abacavir-Associated Platelet Hyperreactivity in HIV-Infected Patients. Int. J. Cardiol. 2018, 263, 118–124. [Google Scholar] [CrossRef]
- Momi, S.; Caracchini, R.; Falcinelli, E.; Evangelista, S.; Gresele, P. Stimulation of Platelet Nitric Oxide Production by Nebivolol Prevents Thrombosis. Arter. Thromb. Vasc. Biol. 2014, 34, 820–829. [Google Scholar] [CrossRef]
- Marañón, G.; Muñoz-Escassi, B.; Manley, W.; García, C.; Cayado, P.; de la Muela, M.S.; Olábarri, B.; León, R.; Vara, E. The Effect of Methyl Sulphonyl Methane Supplementation on Biomarkers of Oxidative Stress in Sport Horses Following Jumping Exercise. Acta Vet. Scand. 2008, 50, 45. [Google Scholar] [CrossRef]
- Cappelli, K.; Amadori, M.; Mecocci, S.; Miglio, A.; Antognoni, M.T.; Razzuoli, E. Immune Response in Young Thoroughbred Racehorses under Training. Animals 2020, 10, 1809. [Google Scholar] [CrossRef]
- Cappelli, K.; Felicetti, M.; Capomaccio, S.; Nocelli, C.; Silvestrelli, M.; Verini-Supplizi, A. Effect of Training Status on Immune Defence Related Gene Expression in Thoroughbred: Are Genes Ready for the Sprint? Vet. J. 2013, 195, 373–376. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Karampour, S.; Gaeini, A.A. Response of Coagulation and Anti-Coagulant Factors of Elite Athletes Following Acute Resistance and High-Intensity Interval Training. J. Sports Med. Phys. Fit. 2018, 58, 120–126. [Google Scholar] [CrossRef]
- Piccione, G.; Marafioti, S.; Giannetto, C.; Panzera, M.; Fazio, F. Effect of Dietary Supplementation with Omega 3 on Clotting Time, Fibrinogen Concentration and Platelet Aggregation in the Athletic Horse. Livest. Sci. 2014, 161, 109–113. [Google Scholar] [CrossRef]
- Kornreich, B.; Enyeart, M.; Jesty, S.A.; Nydam, D.V.; Divers, T. The Effects of Pentoxifylline on Equine Platelet Aggregation. J. Vet. Intern. Med. 2010, 24, 1196–1202. [Google Scholar] [CrossRef]
- Lanier, C.J.; Taintor, J.S.; Christopherson, P.W.; Spangler, E.A. Effect of Lactic Acid Addition to Equine Whole Blood on Platelet Aggregation Measured by Impedance Aggregometry. Vet. Clin. Pathol. 2022, 51, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Giannetto, C.; Fazio, F.; Assenza, A.; Piccione, G. Nictemeral profile of platelet aggregation and clotting parameters in horses during training. Bull. Vet. Inst. Puławy 2009, 53, 801–806. [Google Scholar]
- Norris, J.W.; Watson, J.L.; Tablin, F.; Kozikowski, T.A.; Knych, H.K. Pharmacokinetics and Competitive Pharmacodynamics of ADP-Induced Platelet Activation after Oral Administration of Clopidogrel to Horses. Am. J. Vet. Res. 2019, 80, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.B.; Divers, T.J.; Watts, A.E.; Ness, S.L.; Frye, A.H.; Stokol, T.; Fubini, S.L. Effects of Clopidogrel on the Platelet Activation Response in Horses. Am. J. Vet. Res. 2013, 74, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Roscher, K.A.; Failing, K.; Moritz, A. Inhibition of Platelet Function with Clopidogrel, as Measured with a Novel Whole Blood Impedance Aggregometer in Horses. Vet. J. 2015, 203, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Heber, S.; Volf, I. Effects of Physical (In)Activity on Platelet Function. BioMed Res. Int. 2015, 2015, 165078. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Piłaszewicz, O.; Pingwara, R.; Winnicka, A. The Effect of Physical Training on Peripheral Blood Mononuclear Cell Ex Vivo Proliferation, Differentiation, Activity, and Reactive Oxygen Species Production in Racehorses. Antioxidants 2020, 9, 1155. [Google Scholar] [CrossRef] [PubMed]
- Di Francescomarino, S.; Sciartilli, A.; Di Valerio, V.; Di Baldassarre, A.; Gallina, S. The Effect of Physical Exercise on Endothelial Function. Sports Med. 2009, 39, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Maiorana, A.; O’Driscoll, G.; Taylor, R. Effect of Exercise Training on Endothelium-Derived Nitric Oxide Function in Humans. J. Physiol. 2004, 561, 1–25. [Google Scholar] [CrossRef]
- Atkinson, B.; Dwyer, K.; Enjyoji, K.; Robson, S.C. Ecto-Nucleotidases of the CD39/NTPDase Family Modulate Platelet Activation and Thrombus Formation: Potential as Therapeutic Targets. Blood Cells Mol. Dis. 2006, 36, 217–222. [Google Scholar] [CrossRef]
- Meyer-Lindemann, U.; Moggio, A.; Dutsch, A.; Kessler, T.; Sager, H.B. The Impact of Exercise on Immunity, Metabolism, and Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 3394. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.-L.L.; Ruz, A.; Martí-Korff, S.; Estepa, J.-C.; Aguilera-Tejero, E.; Werkman, J.; Sobotta, M.; Lindner, A. Effects of Intensity and Duration of Exercise on Muscular Responses to Training of Thoroughbred Racehorses. J. Appl. Physiol. 2007, 102, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
| March (T-30) | April (T0) | May (T30) | June (T60) | July (T90) |
|---|---|---|---|---|
| Untrained horses | Start of training | Incremental training | Incremental training | Incremental training |
| 15 min Walk | 15 min Walk | 15 min Walk | 15 min Walk | 15 min Walk |
| 10 min Trot | 10 min Trot | 10 min Trot | 10 min Trot | 10 min Trot |
| Rest | 6 min Canter | 6 min Canter | 6 min Canter | 6 min Canter |
| 10 min Trot | Every Tuesday: | Every Tuesday: | Every Tuesday: | Every Tuesday: |
| Walk | 1 min Gallop | 2 min Gallop | 3 min Gallop | 4 min Gallop |
| Gene | Primer Forward | Primer Reverse | Amp. Length | Accession |
|---|---|---|---|---|
| SELP | GCTGACAATCCAGGAAGCCC | CGCTTTGAGCAGTCAAGGGA | 146 | NM_001081792 |
| ENTPD1 | TTGAGCCACCAAGACCAGAAG | ATTCTGGGTCAACCCCACAG | 125 | XM_001500628 |
| PTGIS | TTCCTGAGTCCGCAGAAGGA | TCGCTTCCCGTCCTTGTAAA | 117 | XM_001501166 |
| NOS3 | TTCGGGAGAGTGAGCTGGTA | CAATCCCGCGCATCAAAGAC | 109 | XM_001504650 |
| B2M | TCCTGCTCGGGCTACTCTC | TGCTGGGTGACGTGAGTAAA | 83 | NM_001082502 |
| SDHA | GCGCGCTTCAGACGATTTAT | CCAGTGCTCCTCAAATGGCT | 146 | XM_014734954 |
| Maximal Amplitude (%) | T-30 | T0 | T30 | T60 | T90 |
|---|---|---|---|---|---|
| Collagen (4 µg/mL) | 35.4 ± 6.0 | 21.0 ± 5.7 | 35.0 ± 5.0 p = 0.05 vs. T0 | 28.8 ± 5.1 | 40.4 ± 5.4 |
| ADP (1 µM) | 31.5 ± 2.7 | 26.9 ± 3.4 | 35.9 ± 2.2 p = 0.008 vs. T0; p = 0.013 vs. T90 | 33.1 ± 2.8 p = 0.029 vs. T90 | 25.7 ± 2.1 |
| ADP (5 µM) | 58.4 ± 3.7 | 57.8 ± 1.9 | 61.0 ± 3.0 | 60.8 ± 4.6 | 60.5 ± 3.6 |
| ADP (10 µM) | 63.5 ± 2.3 | 63.3 ± 1.6 | 64.4 ± 2.2 | 59.3 ± 2.2 | 62.3 ± 2.1 |
| A23187 (5 µM) | Not done | 24.2 ± 5.1 | 30.9 ± 5.2 | 41.8 ± 5.3 p = 0.03 vs. T0; p = 0.04 vs. T90 | 22.0 ± 4.9 |
| PFA-100® (sec) | T-30 | T0 | T30 | T60 | T90 |
|---|---|---|---|---|---|
| C-ADP | 89.8 ± 4.1 | 88.2 ± 4.1 | 70.0 ± 4.2 p = 0.03 vs. T0; p = 0.0021 vs. T-30 | 92.0 ± 4.4 p = 0.011 vs. T30 | 87.6 ± 3.9 p = 0.019 vs. T30 |
| C-EPI | 262.0 ± 12.5 | 242.4 ± 18.0 | 190.0 ± 21.0 p = 0.045 vs. T0 | 300 ± 0.0 p = 0.0006 vs. T30 | 287.0 ± 8.3 p = 0.0021 vs. T30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglio, A.; Falcinelli, E.; Cappelli, K.; Mecocci, S.; Mezzasoma, A.M.; Antognoni, M.T.; Gresele, P. Effect of Regular Training on Platelet Function in Untrained Thoroughbreds. Animals 2024, 14, 414. https://doi.org/10.3390/ani14030414
Miglio A, Falcinelli E, Cappelli K, Mecocci S, Mezzasoma AM, Antognoni MT, Gresele P. Effect of Regular Training on Platelet Function in Untrained Thoroughbreds. Animals. 2024; 14(3):414. https://doi.org/10.3390/ani14030414
Chicago/Turabian StyleMiglio, Arianna, Emanuela Falcinelli, Katia Cappelli, Samanta Mecocci, Anna Maria Mezzasoma, Maria Teresa Antognoni, and Paolo Gresele. 2024. "Effect of Regular Training on Platelet Function in Untrained Thoroughbreds" Animals 14, no. 3: 414. https://doi.org/10.3390/ani14030414
APA StyleMiglio, A., Falcinelli, E., Cappelli, K., Mecocci, S., Mezzasoma, A. M., Antognoni, M. T., & Gresele, P. (2024). Effect of Regular Training on Platelet Function in Untrained Thoroughbreds. Animals, 14(3), 414. https://doi.org/10.3390/ani14030414







