eDNA-Based Early Detection Illustrates Rapid Spread of the Non-Native Golden Mussel Introduced into Beijing via Water Diversion
Abstract
Simple Summary
Abstract
1. Introduction
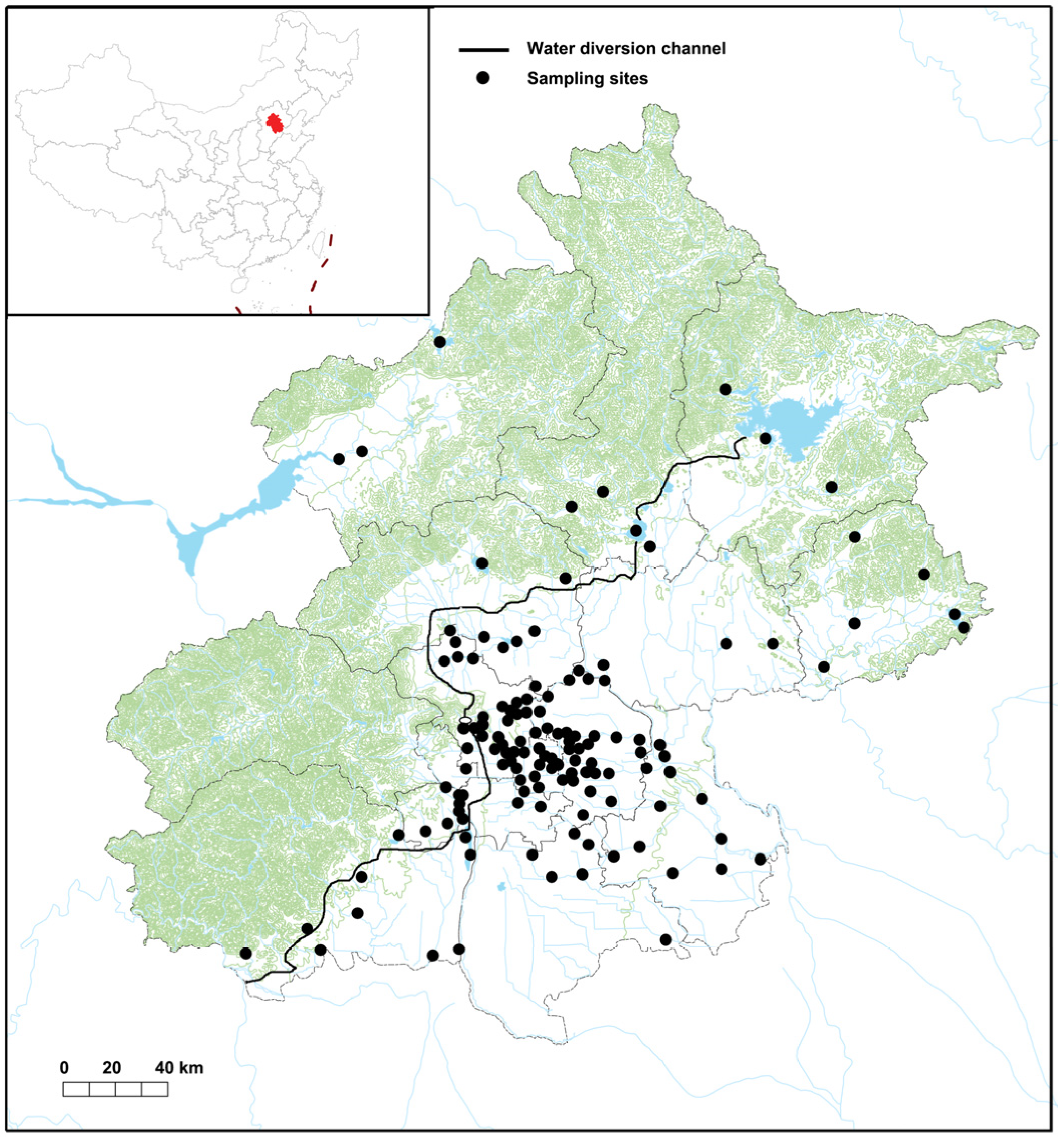
2. Materials and Methods
2.1. eDNA Sampling
2.2. eDNA Extraction and PCR
2.3. Conventional Field Surveys
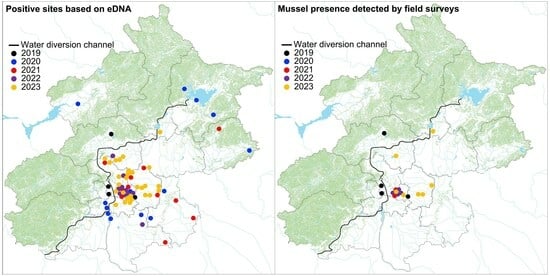
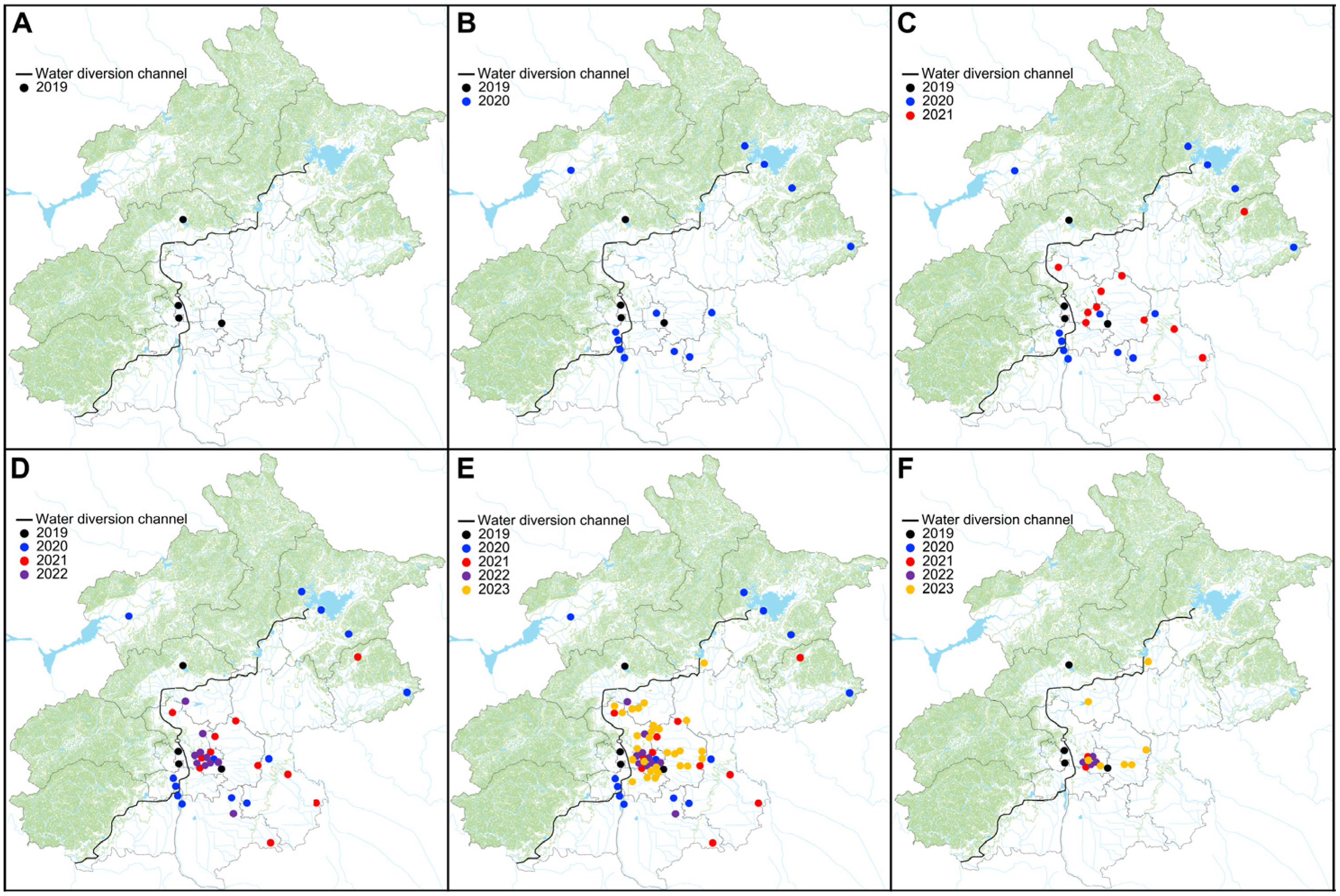
3. Results
3.1. eDNA-Based Early Detection
3.2. Conventional Field Surveys
4. Discussion
4.1. Rapid Spread and Colonization of Golden Mussels in Beijing
4.2. Early Stages of Invasions by Golden Mussels in Beijing
4.3. eDNA-Based Early Detection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shemer, H.; Wald, S.; Semiat, R. Challenges and solutions for global water scarcity. Membranes 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Morante-Carballo, F.; Montalván-Burbano, N.; Quiñonez-Barzola, X.; Jaya-Montalvo, M.; Carrión-Mero, P. What do we know about water scarcity in semi-arid zones? A global analysis and research trends. Water 2022, 14, 2685. [Google Scholar] [CrossRef]
- Xu, Y.; Gun, Z.; Zhao, J.; Chen, J.; Liu, Q.; Cheng, X.; Sutanudjaja, E.H.; Wang, J.; Liu, H.; Zhan, W. Continuing severe water shortage in the water-receiving area of the south-to-north water diversion eastern route project from 2002 to 2020. Water Resour. Res. 2023, 59, e2022WR034365. [Google Scholar] [CrossRef]
- Berhanu, B.; Bisrat, E. Alleviating water scarcity in the central rift valley lakes through an inter-basin water transfer, Ethiopia. Nat. Resour. 2020, 11, 12. [Google Scholar] [CrossRef]
- Zhan, A.; Zhang, L.; Xia, Z.; Ni, P.; Xiong, W.; Chen, Y.; Haffner, D.G.; MacIsaac, H.J. Water diversions facilitate spread of non-native species. Biol. Invasions 2015, 17, 3073–3080. [Google Scholar] [CrossRef]
- Long, D.; Yang, W.; Scanlon, B.R.; Zhao, J.; Liu, D.; Burek, P.; Pan, Y.; You, L.; Wada, Y. South-to-North water diversion stabilizing Beijing’s groundwater levels. Nat. Commun. 2020, 11, 3665. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Z.; Li, S.; MacIsaac, H.J.; Zhan, A. What’s coming eventually comes: A follow-up on an invader’s spread by the world’s largest water diversion in China. Biol. Invasions 2023, 25, 1–5. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Gozlan, R.E.; Liu, H.; Lu, Y.; Qu, X.; Xia, W.; Xiong, F.; Xie, S.; Wang, L. Patterns of fish communities and water quality in impounded lakes of China’s south-to-north water diversion project. Sci. Total Environ. 2020, 713, 136515. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Miao, M.; Kong, Q.; Liu, J. Long-term monitoring of community succession in impoundment lake: Responses of macroinvertebrate to South-to-North water diversion project. Ecol. Indic. 2020, 118, 106734. [Google Scholar] [CrossRef]
- Liu, D.; Wang, R.; Gordon, D.R.; Sun, X.; Chen, L.; Wang, Y. Predicting plant invasions following China’s water diversion project. Environ. Sci. Technol. 2017, 51, 1450–1457. [Google Scholar] [CrossRef]
- Boltovskoy, D.; Karatayev, A.; Burlakova, L.; Cataldo, D.; Karatayev, V.; Sylvester, F.; Mariñelarena, A. Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 2009, 636, 271–284. [Google Scholar] [CrossRef]
- Xu, M. Distribution and Spread of Limnoperna fortunei in China. In Limnoperna Fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel; Boltovskoy, D., Ed.; Springer: Cham, Switzerland, 2015; pp. 313–320. [Google Scholar]
- Morton, B. The colonization of Hong Kong’s raw water supply system by Limnoperna fortunei (Dunker 1857) (Bivalvia: Mytilacea) from China. Malacol. Rew. 1975, 8, 91–105. [Google Scholar]
- Morton, B.; Dinesen, G. Colonization of Asian freshwaters by the Mytilidae (Bivalvia): A comparison of Sinomytilus harmandi from the Tonle-Sap River, Phnom Penh, Cambodia, with Limnoperna fortunei. Molluscan Res. 2010, 30, 57–72. [Google Scholar] [CrossRef]
- Ito, K. Distribution of an invasive alien mussel, Limnoperna fortunei, in the Naka River system, Ibaraki, Japan. Jpn. J. Conserv. Ecol. 2016, 21, 67–76. [Google Scholar]
- Pastorino, G.; Darrigran, G.; Martín, S.K.; Lunaschi, L. Limnoperna fortunei (Dunker, 1857) (Mytilidae), nuevo bivalvo invasor en aguas del Río de la Plata. Neotropica 1993, 39, 101–102. [Google Scholar]
- Sylvester, F.; Sardiña, P. Relationships of Limnoperna fortunei with Benthic Animals. In Limnoperna Fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel; Boltovskoy, D., Ed.; Springer: Cham, Switzerland, 2015; pp. 191–210. [Google Scholar]
- Boltovskoy, D.; Xu, M.; Nakano, D. Impacts of Limnoperna fortunei on Man-Made Structures and Control Strategies: General Overview. In Limnoperna Fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel; Boltovskoy, D., Ed.; Springer: Cham, Switzerland, 2015; pp. 375–393. [Google Scholar]
- Reaser, J.K.; Burgiel, S.W.; Kirkey, J.; Brantley, K.A.; Veatch, S.D.; Burgos-Rodríguez, J. The early detection of and rapid response (EDRR) to invasive species: A conceptual framework and federal capacities assessment. Biol. Invasions 2020, 22, 1–19. [Google Scholar] [CrossRef]
- Castro, K.L.; Battini, N.; Giachetti, C.B.; Trovant, B.; Abelando, M.; Basso, N.G.; Schwindt, E. Early detection of marine invasive species following the deployment of an artificial reef: Integrating tools to assist the decision-making process. J. Environ. Manag. 2021, 297, 113333. [Google Scholar] [CrossRef]
- Liebhold, A.; Bascompte, J. The Allee effect, stochastic dynamics and the eradication of alien species. Ecol. Lett. 2003, 6, 133–140. [Google Scholar] [CrossRef]
- Mehta, S.V.; Haight, R.G.; Homans, F.R.; Polasky, S.; Venette, R.C. Optimal detection and control strategies for invasive species management. Ecol. Econ. 2007, 61, 237–245. [Google Scholar] [CrossRef]
- Xia, Z.; Zhan, A.; Johhansson, M.L.; Haffner, G.D.; MacIsaac, H.J. Screening marker sensitivity: Optimizing eDNA-based rare species detection. Divers. Distrib. 2021, 27, 1981–1988. [Google Scholar] [CrossRef]
- Martinez, B.; Reaser, J.K.; Dehgan, A.; Zamft, B.; Baisch, D.; McCormick, C.; Giordano, A.J.; Aicher, R.; Selbe, S. Technology innovation: Advancing capacities for the early detection of and rapid response to invasive species. Biol. Invasions 2020, 22, 75–100. [Google Scholar] [CrossRef]
- Yemshanov, D.; Haight, R.G.; Koch, F.H.; Venette, R.C.; Swystun, T.; Fournier, R.E.; Marcotte, M.; Chen, Y.; Turgeon, J.J. Optimizing surveillance strategies for early detection of invasive alien species. Ecol. Econ. 2019, 162, 87–99. [Google Scholar] [CrossRef]
- Larson, E.R.; Graham, B.M.; Achury, R.; Coon, J.J.; Daniels, M.K.; Gambrell, D.K.; Jonasen, K.L.; King, G.D.; LaRacuente, N.; Perrin-Stowe, T.I.N.; et al. From eDNA to citizen science: Emerging tools for the early detection of invasive species. Front. Ecol. Environ. 2020, 18, 194–202. [Google Scholar] [CrossRef]
- Xia, Z.; Johansson, M.L.; Gao, Y.; Zhang, L.; Haffner, G.D.; MacIsaac, H.J.; Zhan, A. Conventional versus real-time quantitative PCR for rare species detection. Ecol. Evol. 2018, 8, 11799–11807. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Gu, J.; Wen, Y.; Cao, X.; Gao, Y.; Li, S.; Haffner, G.D.; MacIsaac, H.J.; Zhan, A. eDNA-based detection reveals invasion risks of a biofouling bivalve in the world’s largest water diversion project. Ecol. Appl. 2024, 34, e2826. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhan, A.; Gao, Y.; Zhang, L.; Haffner, G.D.; MacIsaac, H.J. Early detection of a highly invasive bivalve based on environmental DNA (eDNA). Biol. Invasions 2018, 20, 437–447. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Pilliod, D.S.; Arkle, R.S.; Waits, L.P. Molecular detection of cryptic vertebrates in stream water: A demonstration using Rocky Mountain tailed frogs and Idaho giant salamanders. PLoS ONE 2011, 6, e22746. [Google Scholar] [CrossRef]
- Turner, C.R.; Barnes, M.A.; Xu, C.C.Y.; Jones, S.E.; Jerde, C.L.; Lodge, D.M. Particle size distribution and optimal capture of aqueous macrobial eDNA. Methods Ecol. Evol. 2014, 5, 676–684. [Google Scholar] [CrossRef]
- Kennedy, K.; Hall, M.W.; Lynch, M.D.J.; Moreno-Hagelsieb, G.; Neufeld, J.D. Evaluating bias of Illumina-based bacterial 16S rRNA gene profiles. Appl. Environ. Microbiol. 2014, 80, 5717–5722. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Meyer, F.; Schriml, L.; Joint, I.R.; Mühling, M.; Field, D. Metagenomes and metatranscriptomes from the L4 long-term coastal monitoring station in the Western English Channel. Stand. Genom. Sci. 2010, 3, 183–193. [Google Scholar] [CrossRef]
- Zhan, A.; He, S.; Brown, E.A.; Chain, F.J.J.; Therriault, T.W.; Abbott, C.L.; Heath, D.D.; Cristescu, M.E.; MacIsaac, H.J. Reproducibility of pyrosequencing data for biodiversity assessment in complex communities. Methods Ecol. Evol. 2014, 5, 881–890. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, Y.; Zhao, J.; Yao, M. To the north: eDNA tracing of the seasonal and spatial dynamics of fish assemblages along the world’s largest water diversion project. J. Environ. Manag. 2023, 331, 117217. [Google Scholar] [CrossRef]
- Thibault, I.; Bernatchez, L.; Dodson, J.J. The contribution of newly established populations to the dynamics of range expansion in a one-dimensional fluvial-estuarine system: Rainbow trout (Oncorhynchus mykiss) in Eastern Quebec. Divers. Distrib. 2009, 15, 1060–1072. [Google Scholar] [CrossRef]
- Xia, Z.; Barker, J.R.; Zhan, A.; Haffner, G.D.; MacIsaac, H.J. Golden mussel (Limnoperna fortunei) survival during winter at the northern invasion front implies a potential high latitude distribution. Divers. Distrib. 2021, 27, 1422–1434. [Google Scholar] [CrossRef]
- Xu, M.; Darrigran, G.; Wang, Z.; Zhao, N.; Lin, C.; Pan, B. Experimental study on control of Limnoperna fortune biofouling in water transfer tunnels. J. Hydro-Environ. Res. 2015, 9, 248–258. [Google Scholar] [CrossRef]
- Dlugosch, K.M.; Parker, I.M. Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Mol. Ecol. 2008, 17, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Zhan, A.; Perepelizin, P.V.; Ghabooli, S.; Paolucci, E.; Sylvester, F.; Sardiña, P.; Cristescu, M.E.; MacIsaac, H.J. Scale-dependent post-establishment spread and genetic diversity in an invading mollusc in South America. Divers. Distrib. 2012, 18, 1042–1055. [Google Scholar] [CrossRef]
- Roman, J.; Darling, J.A. Paradox lost: Genetic diversity and the success of aquatic invasions. Trends Ecol. Evol. 2007, 22, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 81–102. [Google Scholar] [CrossRef]
- Johnson, L.E.; Carlton, J.T. Post-establishment spread in large-scale invasions: Dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology 1996, 77, 1686–1690. [Google Scholar] [CrossRef]
- Sylvester, F.; Boltovskoy, D.; Cataldo, D. Fast response of freshwater consumers to a new trophic resource: Predation on the recently introduced Asian bivalve Limnoperna fortunei in the lower Parana river, South America. Austral Ecol. 2007, 32, 403–415. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Sardina, P.; Sylvester, F.; Perepelizin, P.V.; Zhan, A.; Ghabooli, S.; Cristescu, M.E.; Oliveira, M.D.; MacIsaac, H.J. Morphological and genetic variability in an alien invasive mussel across an environmental gradient in South America. Limnol. Oceanogr. 2014, 59, 400–412. [Google Scholar] [CrossRef]
- Crooks, J.A. Lag times and exotic species: The ecology and management of biological invasions in slow-motion. Ecoscience 2005, 12, 316–329. [Google Scholar] [CrossRef]
- Daehler, C.C. Short lag times for invasive tropical plants: Evidence from experimental plantings in Hawai’i. PLoS ONE 2009, 4, e4462. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.D.; Hamilton, S.K.; Jacobi, C.M. Forecasting the expansion of the invasive golden mussel Limnoperna fortunei in Brazilian and North American rivers based on its occurrence in the Paraguay River and Pantanal wetland of Brazil. Biol. Invasions 2010, 5, 5973. [Google Scholar] [CrossRef]
- Zhan, A.; MacIsaac, H.J. Rare biosphere exploration using high-throughput sequencing: Research progress and perspectives. Conserv. Genet. 2015, 16, 513–522. [Google Scholar] [CrossRef]
- Xiong, W.; Li, H.; Zhan, A. Early detection of invasive species in marine ecosystems using high-throughput sequencing: Technical challenges and possible solutions. Mar. Biol. 2016, 163, 139. [Google Scholar] [CrossRef]
- Harvey, C.T.; Qureshi, S.A.; MacIsaac, H.J. Detection of a colonizing, aquatic, non-indigenous species. Divers. Distrib. 2009, 15, 429–437. [Google Scholar] [CrossRef]
- Harrison, J.B.; Sunday, J.M.; Rogers, S.M. Predicting the fate of eDNA in the environment and implications for studying biodiversity. Proc. Royal Soc. B 2019, 286, 20191409. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Chen, X.; Zhang, J.; Wang, S.; Zhu, J.; Fu, X. Establishment risk of invasive golden mussel in a water diversion project: An assessment framework. Environ. Sci. Ecotechnology 2024, 17, 100305. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Cheng, J.; Zhan, A. Effectiveness and mechanisms of recoverable magnetic nanoparticles on mitigating golden mussel biofouling. Environ. Sci. Technol. 2021, 55, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Gao, Y.; Xia, Z.; Zhan, A. Chemical oxidants affect byssus adhesion in the highly invasive fouling mussel Limnoperna fortunei. Sci. Total Environ. 2019, 646, 1367–1375. [Google Scholar] [CrossRef] [PubMed]

| Site (Coordinate) | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|
| Shahe Dam, Wenyu River (40°00′16″ N, 116°16′55″ E) | N | E | E | E, C (1 ind./m2) |
| Qijiahuozi, Tucheng River (39°58′55″ N, 116°23′23″ E) | N | N | E, C (1 ind./m2) | E |
| Beiguan Bridge, Xiaozhong River (39°56′13″ N, 116°40′10″ E) | N | N | E | E, C (6 ind./m2) |
| Gaobeidian Dam, Tonghui River (39°54′51″ N, 116°32′8″ E) | E | N | E | E, C (2 ind./m2) |
| Longtan Dam, Nanhucheng River (39°53′13″ N, 116°27′06″ E) | N | N | E, C (1 ind./m2) | E |
| Songlin Dam, Beihucheng River (39°57′21″ N, 116°23′01″ E) | N | E, C (1 ind./m2) | E, C (1 ind./m2) | E, C (4 ind./m2) |
| Luodao Village, Yongyin Channel (39°55′27″ N, 116°18′28″ E) | N | N | E, C (3 ind./m2) | E |
| Erre Dam, Yongyin Channel (39°54′18″ N, 116°21′21″ E) | N | E, C (1 ind./m2) | E, C (6 ind./m2) | E |
| Longbei Village, Jingmi Channel (40°01′20″ N, 116°16′27″ E) | N | N | E, C (1 ind./m2) | N |
| Bayi Lake (39°55′11″ N, 116°19′31″ E) | N | N | N | E, C (4 ind./m2) |
| West Yuyuan Lake (39°55′24″ N, 116°19′07″ E) | N | N | E, C (30 ind./m2) | E, C (5 ind./m2) |
| East Yuyuan Lake (39°55′25″ N, 116°19′48″ E) | N | N | E, C (7 ind./m2) | E, C (7 ind./m2) |
| Zizhuyuan Lake (39°56′49″ N, 116°19′30″ E) | N | N | N | E, C (4 ind./m2) |
| Beijing Zoo Ponds (39°56′50″ N, 116°20′14″ E) | N | N | E, C (2 ind./m2) | E, C (1 ind./m2) |
| Gaobeidian wastewater drainage (39°53′56″ N, 116°31′51″ E) | N | N | N | E, C (2 ind./m2) |
| Huairou wastewater drainage (40°17′03″ N, 116°37′10″ E) | N | N | N | E, C (1 ind./m2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Li, S.; Zhan, A. eDNA-Based Early Detection Illustrates Rapid Spread of the Non-Native Golden Mussel Introduced into Beijing via Water Diversion. Animals 2024, 14, 399. https://doi.org/10.3390/ani14030399
Guo W, Li S, Zhan A. eDNA-Based Early Detection Illustrates Rapid Spread of the Non-Native Golden Mussel Introduced into Beijing via Water Diversion. Animals. 2024; 14(3):399. https://doi.org/10.3390/ani14030399
Chicago/Turabian StyleGuo, Wei, Shiguo Li, and Aibin Zhan. 2024. "eDNA-Based Early Detection Illustrates Rapid Spread of the Non-Native Golden Mussel Introduced into Beijing via Water Diversion" Animals 14, no. 3: 399. https://doi.org/10.3390/ani14030399
APA StyleGuo, W., Li, S., & Zhan, A. (2024). eDNA-Based Early Detection Illustrates Rapid Spread of the Non-Native Golden Mussel Introduced into Beijing via Water Diversion. Animals, 14(3), 399. https://doi.org/10.3390/ani14030399