Survival Estimation Using Multistate Cormack–Jolly–Seber Models—The Case of the Bearded Vulture Gypaetus barbatus in Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- -
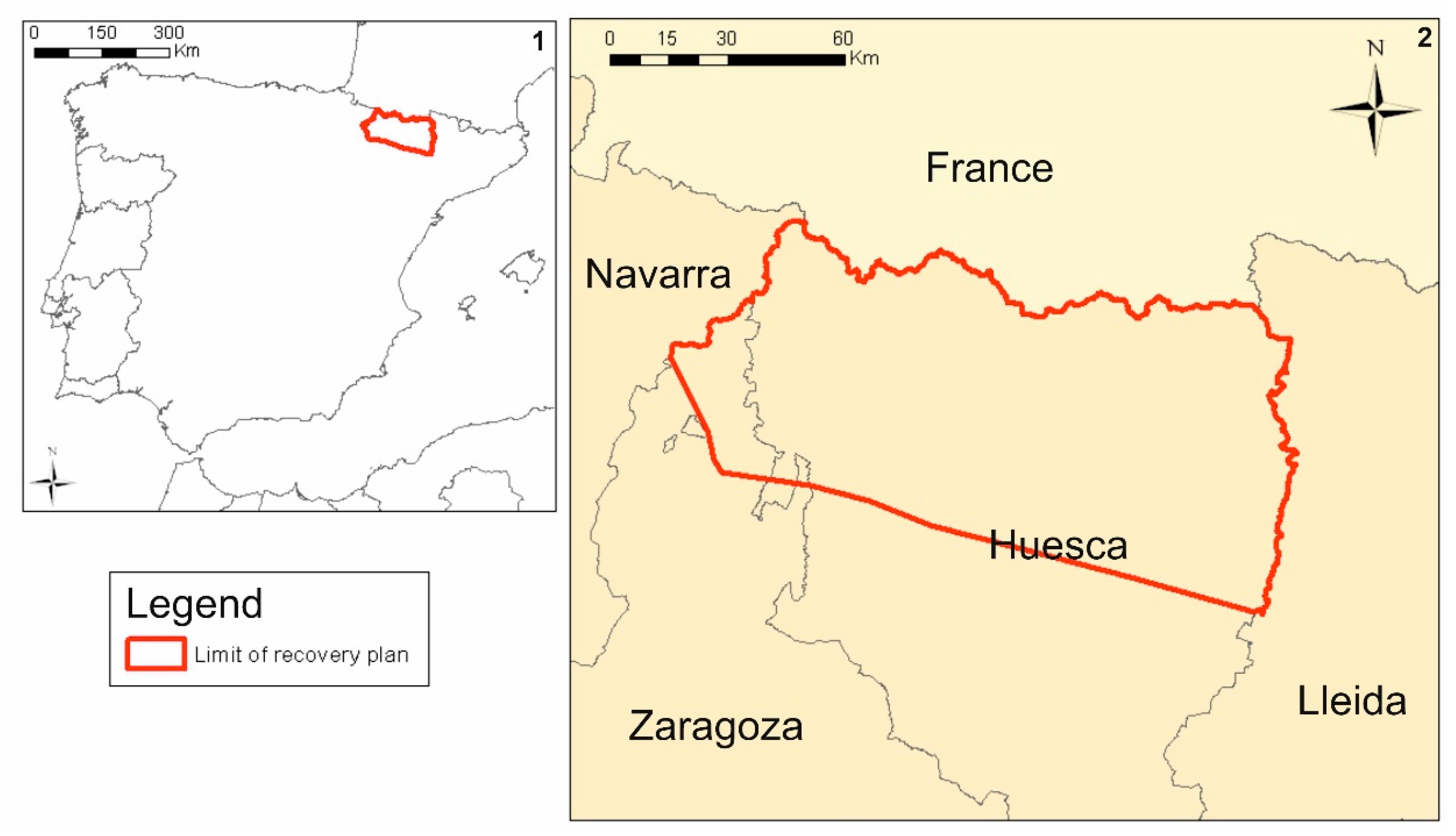
- Study area
- -
- Data collection
- -
- Survival analyses
- -
- Productivity trend analyses
- -
- Number of pairs trend analyses
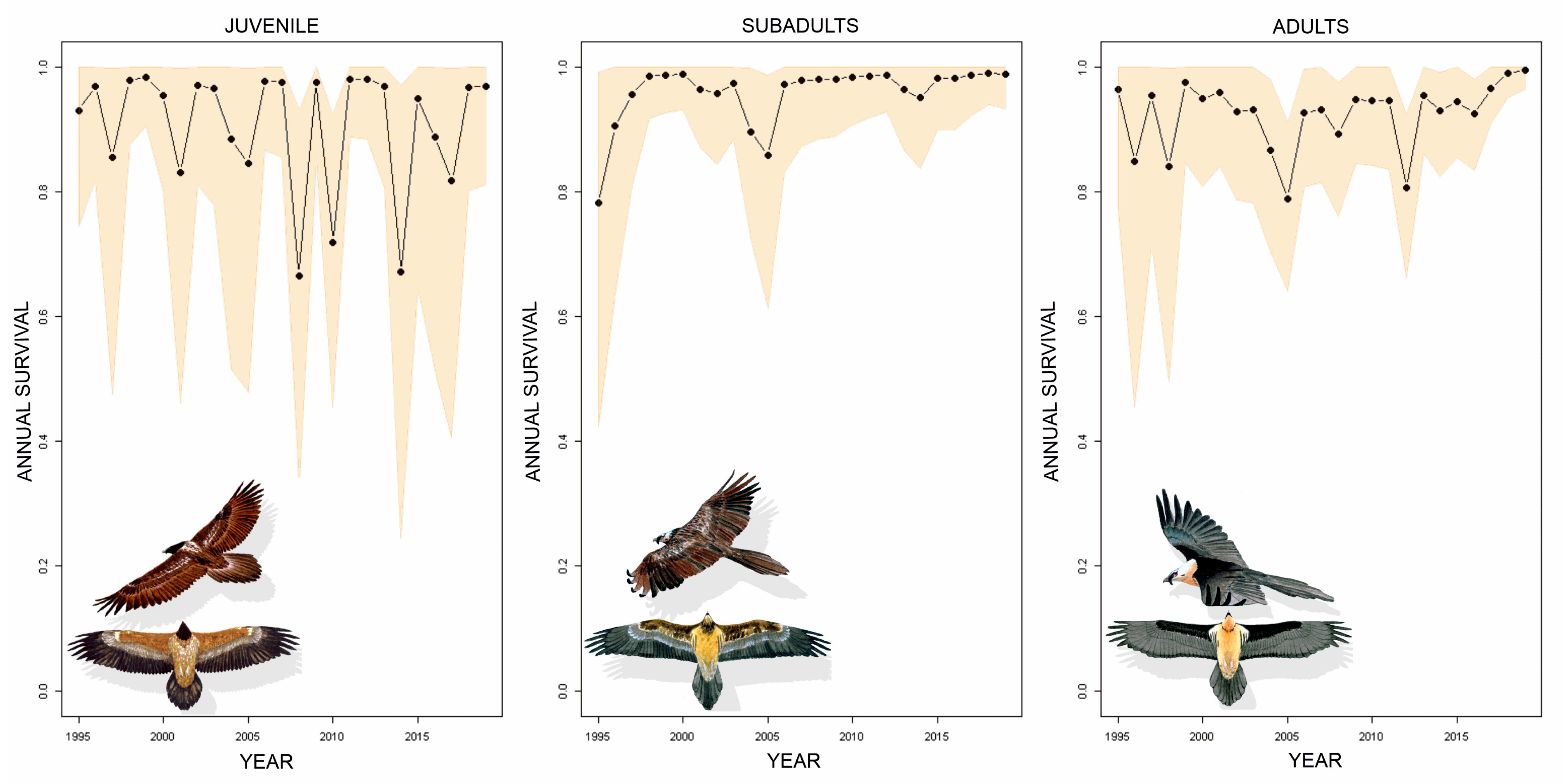
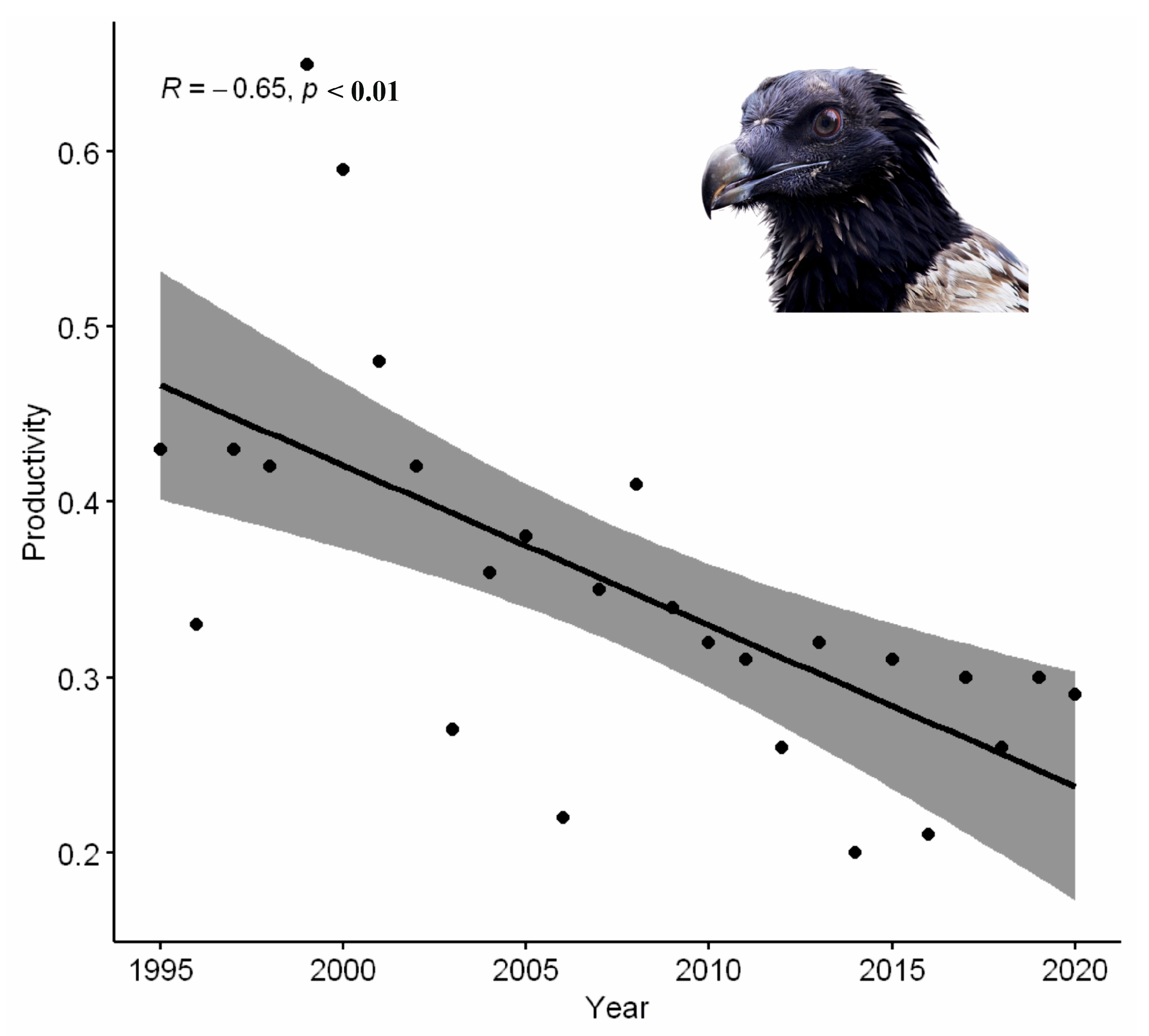
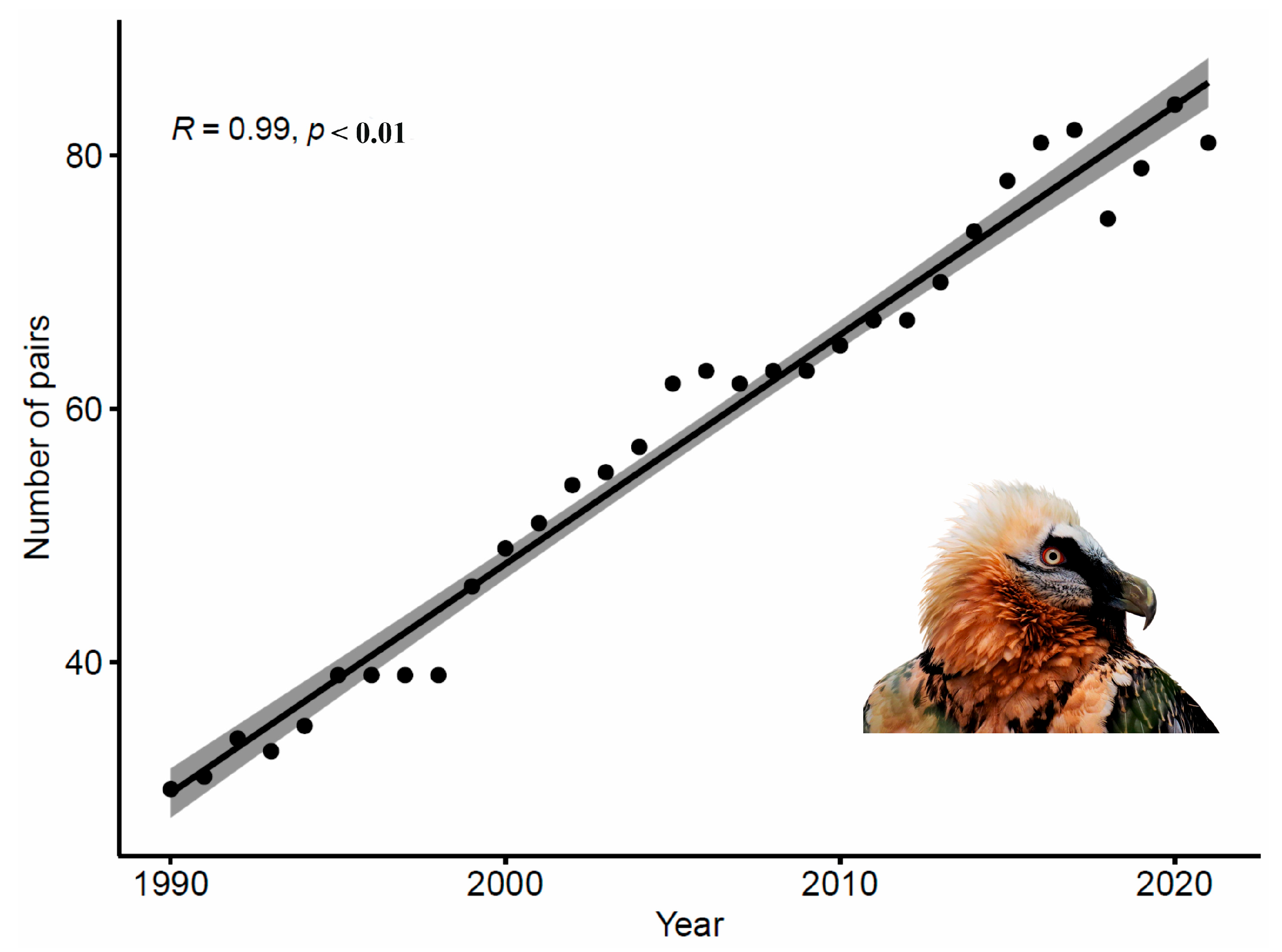
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, N.M.; Scheele, B.C.; Legge, S.; Southwell, D.M.; Carter, O.; Lintermans, M.; Lindenmayer, D.B. How to ensure threatened species monitoring leads to threatened species conservation. Ecol. Manag. Restor. 2018, 19, 222–229. [Google Scholar] [CrossRef]
- Kéry, M.; Schaub, M. Estimation of survival from Capture-Recapture Data Using the Cormack-Jolly-Seber Model. In Bayesian Population Analysis Using WinBUGS. A Hierarchical Perspective; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Likens, G.; Lindenmayer, D. Effective Ecological Monitoring; CSIRO Publishing: Melbourne, Australia, 2018. [Google Scholar]
- Jiménez, J. Modelos Jerárquicos Bayesianos Aplicados al Seguimiento de Fauna. Ph.D. Dissertation, Universidad de Castilla-La Mancha, Ciudad Real, Spain, 2017. [Google Scholar]
- Jankowiak, L.; Wysocki, D.; Greño, J. Survival and Site Fidelity of Urban Blackbirds Turdus merula—Comparison of Cormack-Jolly-Seber and Barker Models. Acta Ornithol. 2016, 51, 189–197. [Google Scholar] [CrossRef]
- Cormack, R.M. Estimates of survival from the sighting of marked animals. Biometrika 1964, 62, 142–149. [Google Scholar]
- Jolly, G.M. Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrika 1965, 52, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Seber, G.A. A Note on the Multiple-Recapture Census. Biometrika 1965, 52, 149–159. [Google Scholar] [CrossRef]
- Bonner, S.; Schwarz, C. An extension of the Cormack-Jolly-Seber model for continuous covariates with application to Microtus pennsylvanicus. Biometrics 2006, 62, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Pollock, K.H. The use of auxiliary variables in capture-recapture modelling: An overview. J. Appl. Stat. 2002, 29, 85–102. [Google Scholar] [CrossRef]
- Margalida, A.; Martínez, J. El Quebrantahuesos en España: Población Reproductora en 2018 y Método de Censo; Instituto de Investigación de Recursos Cinegéticos (CSIC-UCLM-JCCM): Ciudad Real, Spain, 2020. [Google Scholar]
- Ferguson-Lees, J.; Christie, D.A. Raptors of the World; Christopher Helm: London, UK, 2001. [Google Scholar]
- Orta, J.; De Juana, E.; Marks, J.S.; Sharpe, C.J.; García, E.F. Bearded vulture (Gypaetus barbatus). In Birds of the World; Cornell Lab of Ornithology: New York, NY, USA, 2020. [Google Scholar]
- Gil, J.A.; Báguena, G.; Díez, O. El Quebrantahuesos y sus Montañas: Biología y Conservación; Gobierno de Aragón: Zaragoza, Spain, 2019. [Google Scholar]
- Hiraldo, F.; Delibes, M.; Calderón, J. El quebrantahuesos (Gypaetus barbatus) (L.). In Sistemática, Taxonomía, Biología, Distribución y Protección; Monografías 22; Ministerio de Agricultura, ICONA: Madrid, Spain, 1979. [Google Scholar]
- Gil, J.A. Pasado, Presente y Futuro del Quebrantahuesos (Gypaetus barbatus) en el Maestrazgo (Teruel); Archivo de la Comarca de Andorra-Sierra de Arcos: Ejulve, Spain, 2020. [Google Scholar]
- Lozano, P.J.; Jauregui, M. Utilización de los sistemas de información geográfica para la modelización de la conectividad biogeográfica: El ejemplo del quebrantahuesos (Gypaetus barbatus) en el Pirineo Occidental. Lurralde Investig. Espac. 2021, 44, 257–290. [Google Scholar] [CrossRef]
- Pătru-Stupariu, I.; Hossu, C.A.; Grădinaru, S.R.; Nita, A.; Stupariu, M.S.; Huzui-Stoiculescu, A.; Gavrilidis, A.A. A Review of Changes in Mountain Land Use and Ecosystem Services: From Theory to Practice. Land 2020, 9, 336. [Google Scholar] [CrossRef]
- Muñoz, E.; Martín, D.; Tenza, A.; Casasús, I.; Bernués, A.; Villalba, D. Exploración preliminar del impacto del cambio climático sobre los sistemas ganaderos de montaña en el Pirineo aragonés. In Proceedings of the XIX Jornadas Sobre Producción Animal, Virtual, 1–2 June 2021; AIDA: Zaragoza, Spain, 2021; Volume 11. [Google Scholar]
- Estévez-Moreno, L.X.; Miranda-de la Lama, G.C.; Villarroel, M.; García, L.; Abecia, J.A.; Santolaria, P.; María, G.A. Revisiting cattle temperament in beef cow-calf systems: Insights from farmers’ perceptions about an autochthonous breed. Animals 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Reiné, R.J. ¿Por qué investigar los pastos del Pirineo aragonés? Lucas Mallada Rev. Cienc. 2017, 19, 9–22. [Google Scholar]
- Margalida, A.; Jiménez, J.; Martínez, J.M.; Sesé, J.A.; García-Ferré, D.; Llamas, A.; Razin, M.; Colomer, M.; Arroyo, B. An assessment of population size and demographic drivers of the Bearded vulture using integrated population models. Ecol. Monogr. 2020, 90, e01414. [Google Scholar] [CrossRef]
- Allaoui, I.; Cherkaoui, S. New breeding record of Lammergeier (Gypaetus barbatus barbatus) in Morocco and proposals for its conservation. Go-South Bull. 2018, 15, 137–140. [Google Scholar]
- Krüger, S.C. Bearded vulture Gypaetus barbatus. In The Eskom Red Data Book of Birds of South Africa, Lesotho and Swaziland; Taylor, M.R., Peacock, F., Wanless, R.W., Eds.; BirdLife South Africa: Johannesburg, South Africa, 2015; pp. 55–57. [Google Scholar]
- Anders, A.; Dearborn, D.; Faaborg, J.; Thompson, F. Juvenile Survival in a Population of Neotropical Migrant Birds. Conserv. Biol. 1997, 11, 698–707. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Valero-Garcés, B.L. Historical geomorphic processes and human activities in the Central Spanish Pyrenees. Mt. Res. Dev. 1998, 18, 309–320. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://www.R-project.org/ (accessed on 1 September 2023).
- Plummer, M. JAGS Version 4.0 User Manual; Lyon, France. 2015. Available online: http://sourceforge.net/projects/mcmc-jags/ (accessed on 1 September 2023).
- Du, H.; Ke, Z.; Jiang, G.; Huang, S. The Performances of Gelman-Rubin and Geweke’s Convergence Diagnostics of Monte Carlo Markov Chains in Bayesian Analysis. J. Behav. Data Sci. 2022, 2, 47–72. [Google Scholar] [CrossRef]
- Kellner, K.; Meredith, M.; Kellner, M.K. Package ‘jagsUI’. A Wrapper Around ‘rjags’ to Streamline ‘JAGS’ Analyses, R Package Version; 2019, Volume 1. Available online: http://CRAN.R-project.org/package=jagsUI (accessed on 1 September 2023).
- Brooks, S.; Gelman, A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar]
- Spearman, C. The Proof and Measurement of Association Between Two Things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Sergio, F.; Tavecchia, G.; Blas, J.; Tanferna, A.; Hiraldo, F. Demographic modeling to fine-tune conservation targets: Importance of pre-adults for the decline of an endangered raptor. Ecol. Appl. 2021, 31, e02266. [Google Scholar] [CrossRef] [PubMed]
- Margalida, A.; Pérez-García, J.M.; Afonso, I.; Moreno-Opo, R. Spatial and temporal movements in Pyrenean bearded vultures (Gypaetus barbatus): Integrating movement ecology into conservation practice. Sci. Rep. 2016, 6, 35746. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, I.; Farfán, M.Á.; Gil, J.A.; Muñoz, A.R. Survival Estimation Using Multistate Cormack–Jolly–Seber Models—The Case of the Bearded Vulture Gypaetus barbatus in Spain. Animals 2024, 14, 403. https://doi.org/10.3390/ani14030403
Navarro I, Farfán MÁ, Gil JA, Muñoz AR. Survival Estimation Using Multistate Cormack–Jolly–Seber Models—The Case of the Bearded Vulture Gypaetus barbatus in Spain. Animals. 2024; 14(3):403. https://doi.org/10.3390/ani14030403
Chicago/Turabian StyleNavarro, Inmaculada, Miguel Ángel Farfán, Juan Antonio Gil, and Antonio Román Muñoz. 2024. "Survival Estimation Using Multistate Cormack–Jolly–Seber Models—The Case of the Bearded Vulture Gypaetus barbatus in Spain" Animals 14, no. 3: 403. https://doi.org/10.3390/ani14030403
APA StyleNavarro, I., Farfán, M. Á., Gil, J. A., & Muñoz, A. R. (2024). Survival Estimation Using Multistate Cormack–Jolly–Seber Models—The Case of the Bearded Vulture Gypaetus barbatus in Spain. Animals, 14(3), 403. https://doi.org/10.3390/ani14030403






