Predicting Tuberculosis Risk in Cattle, Buffaloes, Sheep, and Goats in China Based on Air Pollutants and Meteorological Factors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Data
2.2. Environmental Variables
- (1)
- Nineteen bioclimatic variables (Bio 1–Bio 19) representing the global climate conditions (temperature and precipitation) were downloaded from WorldClim (https://worldclim.org/data/worldclim21.html#, accessed on 21 June 2024).
- (2)
- Six common air pollutants, namely PM2.5, PM10, CO, NO2, SO2, and O3, were downloaded from Zenodo (https://zenodo.org/communities/chap, accessed on 20 May 2024) to obtain raster maps. The Raster Calculator in ArcToolbox (version 2.5) was used to calculate their average raster values.
- (3)
- Distribution density maps of four livestock species (cattle, buffaloes, sheep, and goats) were downloaded from the Food and Agriculture Organization of the United Nations (http://www.fao.org, accessed on 25 June 2024).
2.3. Establishment of the MaxEnt Ecological Niche Model
3. Results
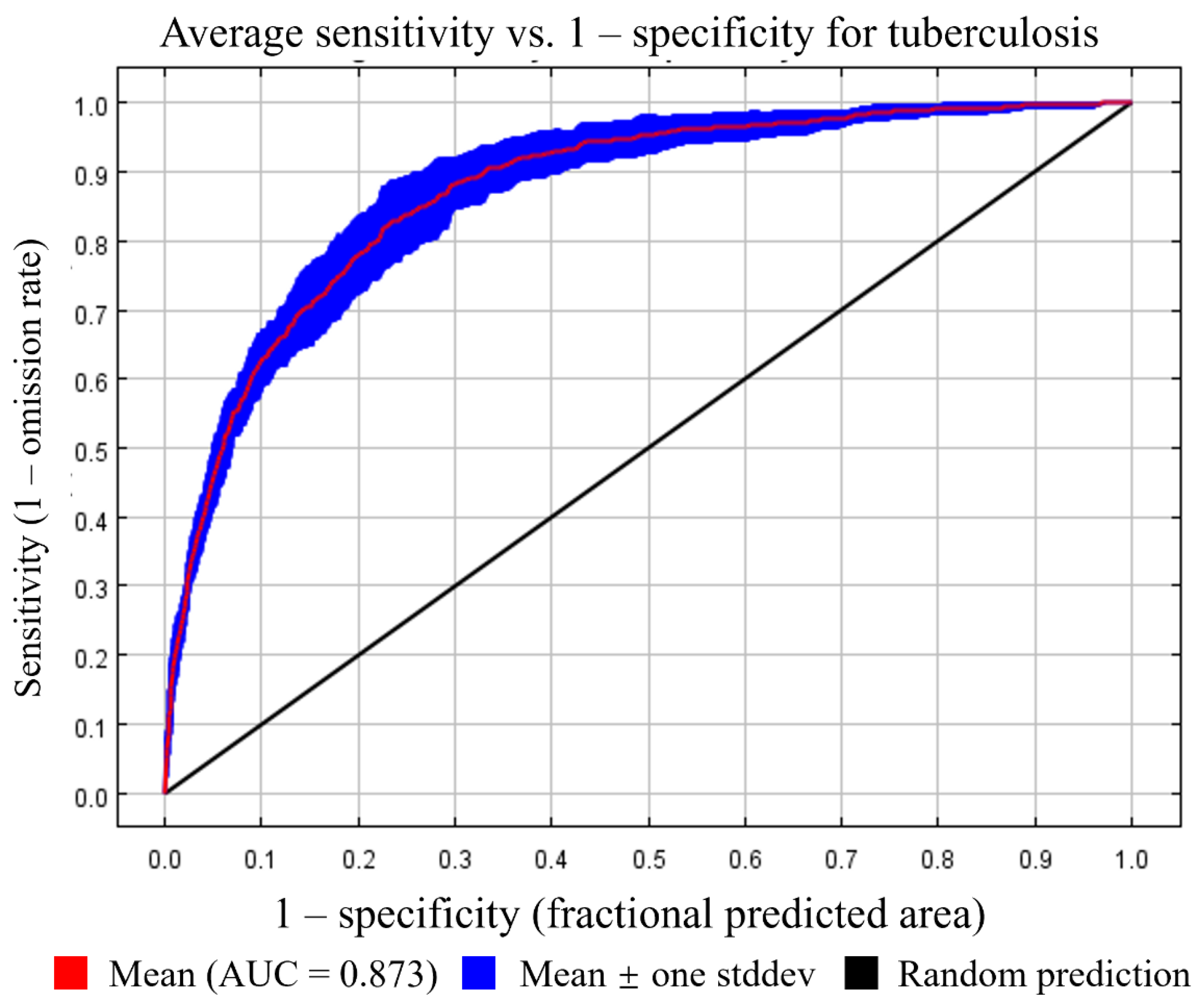
3.1. Variables Included in the Tuberculosis Model
3.2. Potential Risk Areas of Tuberculosis in Domestic Ruminants
4. Discussion
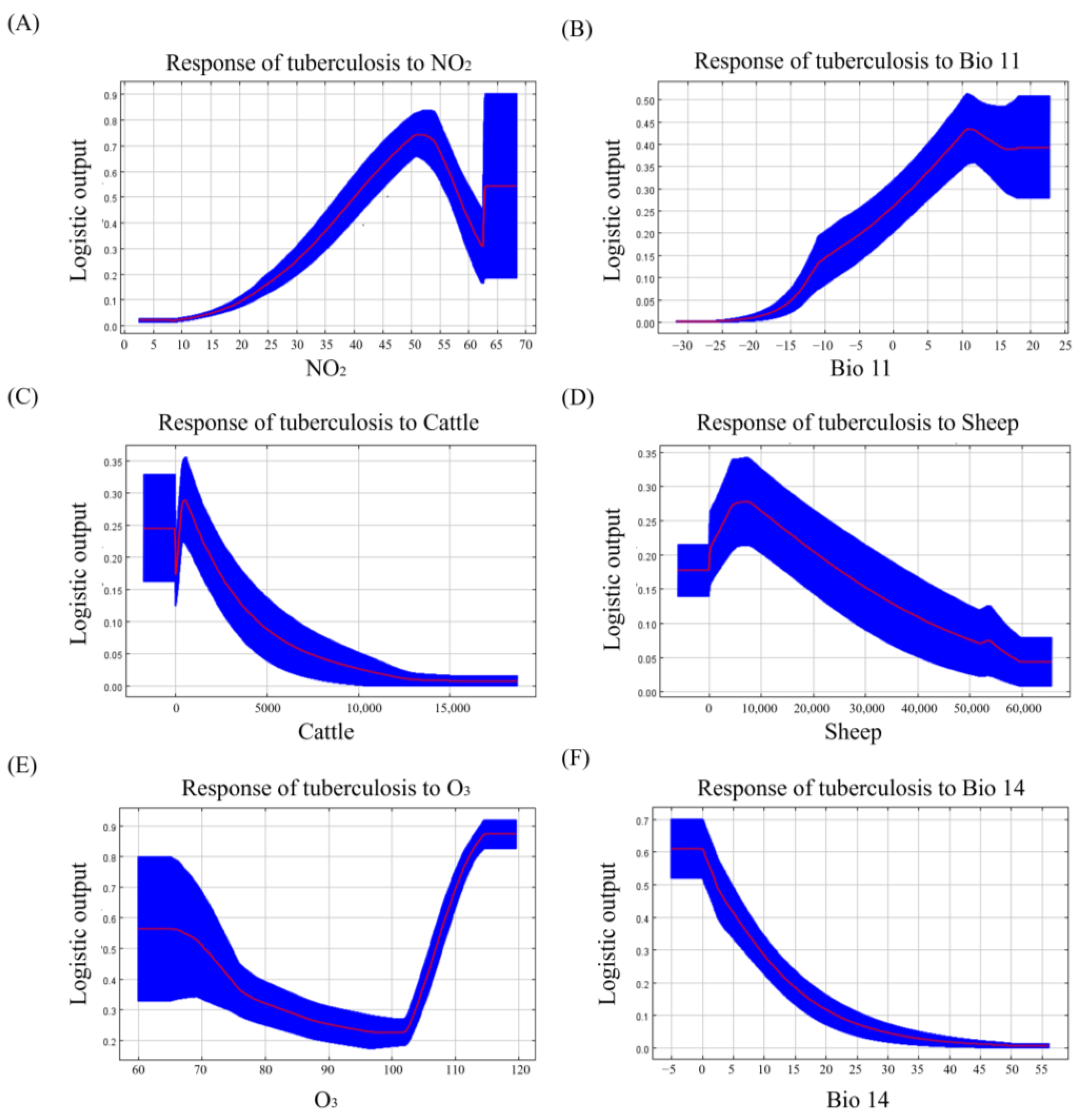
4.1. Analysis of the Important Risk Factors
4.2. Potential High-Risk Distribution of Tuberculosis
4.3. Recommendations for the Prevention and Control of Tuberculosis
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doherty, R.M.; Heal, M.R.; O’Connor, F.M. Climate change impacts on human health over Europe through its effect on air quality. Environ. Health 2017, 16, 118. [Google Scholar] [CrossRef]
- Ni, J.Q.; Erasmus, M.A.; Croney, C.C.; Li, C.M.; Li, Y.S. A critical review of advancement in scientific research on food animal welfare-related air pollution. J. Hazard. Mater. 2021, 408, 124468. [Google Scholar] [CrossRef] [PubMed]
- Uguz, S.; Anderson, G.; Yang, X.; Simsek, E.; Osabutey, A. Cultivation of Scenedesmus dimorphus with air contaminants from a pig confinement building. J. Environ. Manag. 2022, 314, 115129. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Su, X.; Zhao, D.; Zhang, Y.; Cheng, Q.; Zhang, H.; Wang, S.; Xie, M.; Su, H. Exposure to traffic-related air pollution and acute bronchitis in children: Season and age as modifiers. J. Epidemiol. Community Health 2018, 72, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef] [PubMed]
- Gulhan, P.Y.; Elverisli, M.F.; Ercelik, M.; Aytekin, F.; Balbay, O.; Arbak, P. Relationship between diagnosis period and internal and external air quality in patients with tuberculosis. Eurasian J. Med. 2020, 52, 77–80. [Google Scholar] [CrossRef]
- Sumpter, C.; Chandramohan, D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop. Med. Int. Health 2013, 18, 101–108. [Google Scholar] [CrossRef]
- Forrellad, M.A.; Klepp, L.I.; Gioffré, A.; Sabio y García, J.; Morbidoni, H.R.; de la Paz Santangelo, M.; Cataldi, A.A.; Bigi, F. Virulence factors of the Mycobacterium tuberculosis complex. Virulence 2013, 4, 3–66. [Google Scholar] [CrossRef] [PubMed]
- Olea-Popelka, F.; Muwonge, A.; Perera, A.; Dean, A.S.; Mumford, E.; Erlacher-Vindel, E.; Forcella, S.; Silk, B.J.; Ditiu, L.; El Idrissi, A.; et al. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis—A call for action. Lancet Infect. Dis. 2017, 17, e21–e25. [Google Scholar] [CrossRef] [PubMed]
- Taye, H.; Alemu, K.; Mihret, A.; Wood, J.L.N.; Shkedy, Z.; Berg, S.; Aseffa, A. Global prevalence of Mycobacterium bovis infections among human tuberculosis cases: Systematic review and meta-analysis. Zoonoses Public Health 2021, 68, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Vayr, F.; Martin-Blondel, G.; Savall, F.; Soulat, J.M.; Deffontaines, G.; Herin, F. Occupational exposure to human Mycobacterium bovis infection: A systematic review. PLoS Neglected Trop. Dis. 2018, 12, e0006208. [Google Scholar] [CrossRef]
- Didkowska, A.; Żmuda, P.; Orłowska, B.; Nowak, M.; Filip-Hutsch, K.; Cuper, K.; Krajewska-Wędzina, M.; Anusz, K. Suspicion of bovine tuberculosis in sheep in the Małopolskie Voivodeship (southern Poland). Pol. J. Vet. Sci. 2022, 25, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Boschiroli, M.L.; Salguero, F.J.; Jones, G.; Bezos, J. Editorial: Tuberculosis in domestic ruminants: Towards eradication of zoonotic tuberculosis. Front. Vet. Sci. 2024, 11, 1453876. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Li, Y.; Song, W.; Yu, C.; Gao, L.; Ran, J.; He, D.; Li, H. Effect of ambient air pollution on tuberculosis risks and mortality in Shandong, China: A multi-city modeling study of the short- and long-term effects of pollutants. Environ. Sci. Pollut. Res. Int. 2021, 28, 27757–27768. [Google Scholar] [CrossRef]
- Wang, H.; Tian, C.; Wang, W.; Luo, X. Temporal cross-correlations between ambient air pollutants and seasonality of tuberculosis: A time-series analysis. Int. J. Environ. Res. Public Health 2019, 16, 1585. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wu, D.; Yu, C.; Wang, T.; Ji, M.; Wang, X. Impacts of meteorological parameters on the occurrence of air pollution episodes in the Sichuan basin. J. Environ. Sci. 2022, 114, 308–321. [Google Scholar] [CrossRef]
- Wen, W.; Ma, X.; Tang, Y.; Wei, P.; Wang, J.; Guo, C. The impacts of meteorology on source contributions of air pollution in winter in Beijing, 2015–2017 changes. Atmos. Pollut. Res. 2020, 11, 1953–1962. [Google Scholar] [CrossRef]
- Tritscher, T.; Raz, R.; Levi, Y.; Levy, I.; Broday, D.M. Emissions vs. turbulence and atmospheric stability: A study of their relative importance in determining air pollutant concentrations. Sci. Total Environ. 2020, 733, 139300. [Google Scholar]
- Wang, Q.; Li, Y.L.; Yin, Y.L.; Hu, B.; Yu, C.C.; Wang, Z.D.; Li, Y.H.; Xu, C.J.; Wang, Y.B. Association of air pollutants and meteorological factors with tuberculosis: A national multicenter ecological study in China. Int. J. Biometeorol. 2023, 67, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Lu, Y.; Wang, C.; Yang, Z.; Sun, Y.; Zhang, Y.; Tian, M.; Rifhat, R.; Zhang, L. Effects and interaction of meteorological factors on pulmonary tuberculosis in Urumqi, China, 2013–2019. Front. Public Health 2022, 10, 951578. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Emam, M.; Chen, X.; Lu, D.; Zhang, L.; Zheng, Y. An investigation of the effects of meteorological factors on the incidence of tuberculosis. Sci. Rep. 2024, 14, 2088. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y.; Hu, Z.; Jiao, H.; Wang, L. Study on the associations between meteorological factors and the incidence of pulmonary tuberculosis in Xinjiang, China. Atmosphere 2022, 13, 533. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, Z.; Ma, J.; Gao, X.; Xiao, J.; Hayat, M.A.; Wang, H. Modelling the potential distribution of arbovirus vector Aedes aegypti under current and future climate scenarios in Taiwan, China. Pest Manag. Sci. 2019, 75, 3076–3083. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Soares Magalhaes, R.J.; Bi, P.; Ding, F.; Sun, H.; Li, S.; Yin, W.; Wei, L.; Liu, Q.; et al. The role of environmental factors in the spatial distribution of Japanese encephalitis in mainland China. Environ. Int. 2014, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wen, F.; Lu, L.; Nie, C.; Sun, Z.; Liu, R.; Huang, W.; Ye, H. Analysis of spatiotemporal variation in habitat suitability for Oedaleus decorus asiaticus Bei-Bienko on the Mongolian Plateau using maxent and multi-source remote sensing data. Insects 2023, 14, 492. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Li, H.; Ge, M.; Zhang, M. Spatio-temporal distribution of tuberculosis and the effects of environmental factors in China. BMC Infect. Dis. 2022, 22, 565. [Google Scholar] [CrossRef]
- Wang, X.Q.; Li, Y.Q.; Hu, C.Y.; Huang, K.; Ding, K.; Yang, X.J.; Cheng, X.; Zhang, K.D.; Yu, W.J.; Wang, J.; et al. Short-term effect of ambient air pollutant change on the risk of tuberculosis outpatient visits: A time-series study in Fuyang, China. Environ. Sci. Pollut. Res. Int. 2022, 29, 30656–30672. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, M.; Chen, Y.; Ma, L.; Yadikaer, R.; Lu, Y.; Lou, P.; Pu, Y.; Xiang, R.; Rui, B. A study on the relationship between air pollution and pulmonary tuberculosis based on the general additive model in Wulumuqi, China. Int. J. Infect. Dis. 2020, 96, 42–47. [Google Scholar] [CrossRef]
- Zhao, C.N.; Xu, Z.; Wang, P.; Liu, J.; Wang, R.; Pan, H.F.; Bao, F. Associations between air pollutants and acute exacerbation of drug-resistant tuberculosis: Evidence from a prospective cohort study. BMC Infect. Dis. 2024, 24, 121. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Van Den Eeden, S.K.; Garcia, C.; Shan, J.; Baxter, R.; Herring, A.H.; Richardson, D.B.; Van Rie, A.; Emch, M.; Gammon, M.D. Air pollution and pulmonary tuberculosis: A nested case-control study among members of a northern California health plan. Environ. Health Perspect. 2016, 124, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Meca, A.; Díaz, A.; de Miguel Díez, J.; Resino, R.; Resino, S. Environmental Factors Related to Pulmonary Tuberculosis in HIV-Infected Patients in the Combined Antiretroviral Therapy (cART) Era. PLoS ONE 2016, 11, e0165944. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T.W.; Bunn, W.B.; McClellan, R.O.; Hamade, A.K.; Long, C.M.; Valberg, P.A. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: Evidence for NO2 no-effect levels. Crit. Rev. Toxicol. 2009, 39, 743–781. [Google Scholar] [CrossRef]
- Saito, Y.; Azuma, A.; Kudo, S.; Takizawa, H.; Sugawara, I. Long-term inhalation of diesel exhaust affects cytokine expression in murine lung tissues: Comparison between low- and high-dose diesel exhaust exposure. Exp. Lung Res. 2002, 28, 493–506. [Google Scholar] [CrossRef]
- Huang, K.; Ding, K.; Yang, X.J.; Hu, C.Y.; Jiang, W.; Hua, X.G.; Liu, J.; Cao, J.Y.; Zhang, T.; Kan, X.H.; et al. Association between short-term exposure to ambient air pollutants and the risk of tuberculosis outpatient visits: A time-series study in Hefei, China. Environ. Res. 2020, 184, 109343. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.C.; Chiang, C.Y.; Wu, C.F.; Yang, S.L.; Liu, D.P.; Chan, C.C.; Lin, H.H. Ambient air pollution and risk of tuberculosis: A cohort study. Occup. Environ. Med. 2016, 73, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.S.; Kang, S.; Lee, J.Y.; Lee, J.S.; Kim, H.J.; Han, S.K.; Yim, J.J. Impact of outdoor air pollution on the incidence of tuberculosis in the Seoul metropolitan area, South Korea. Korean J. Intern. Med. 2014, 29, 183–190. [Google Scholar] [CrossRef]
- Lu, J.W.; Mao, J.J.; Zhan, R.R.; Li, C.H.; Sun, Y.; Xu, W.Q.; Zhuang, X.; Zhang, B.; Qin, G. Association between long-term exposure to ambient air pollutants and the risk of tuberculosis: A time-series study in Nantong, China. Heliyon 2023, 9, e17347. [Google Scholar] [CrossRef]
- Hua, Q.; Meng, X.; Gong, J.; Qiu, X.; Shang, J.; Xue, T.; Zhu, T. Ozone exposure and cardiovascular disease: A narrative review of epidemiology evidence and underlying mechanisms. Fundam. Res. 2024, in press.
- Prudêncio, C.V.; Mantovani, H.C.; Cecon, P.R.; Prieto, M.; Vanetti, M.C.D. Temperature and pH influence the susceptibility of Salmonella Typhimurium to nisin combined with EDTA. Food Control 2016, 61, 248–253. [Google Scholar] [CrossRef]
- Alene, K.A.; Viney, K.; Gray, D.J.; McBryde, E.S.; Wagnew, M.; Clements, A.C. Mapping tuberculosis treatment outcomes in Ethiopia. BMC Infect. Dis. 2019, 19, 474. [Google Scholar] [CrossRef]
- Bonell, A.; Contamin, L.; Thai, P.Q.; Thuy, H.T.T.; van Doorn, H.R.; White, R.; Nadjm, B.; Choisy, M. Does sunlight drive seasonality of TB in Vietnam? A retrospective environmental ecological study of tuberculosis seasonality in Vietnam from 2010 to 2015. BMC Infect Dis 2020, 20, 184. [Google Scholar] [CrossRef]
- Onozuka, D.; Hagihara, A. The association of extreme temperatures and the incidence of tuberculosis in Japan. Int. J. Biometeorol. 2015, 59, 1107–1114. [Google Scholar] [CrossRef]
- Fares, A. Seasonality of tuberculosis. J. Glob. Infect. Dis. 2011, 3, 46–55. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Dimitroulopoulou, C.; Thornes, J.; Lai, K.M.; Taylor, J.; Myers, I.; Heaviside, C.; Mavrogianni, A.; Shrubsole, C.; Chalabi, Z.; et al. Impact of climate change on the domestic indoor environment and associated health risks in the UK. Environ. Int. 2015, 85, 299–313. [Google Scholar] [CrossRef]
- Wang, X.; Yin, S.; Li, Y.; Wang, W.; Du, M.; Guo, W.; Xue, M.; Wu, J.; Liang, D.; Wang, R.; et al. Spatiotemporal epidemiology of, and factors associated with, the tuberculosis prevalence in northern China, 2010–2014. BMC Infect. Dis. 2019, 19, 365. [Google Scholar] [CrossRef]
- Xiao, Y.; He, L.; Chen, Y.; Wang, Q.; Meng, Q.; Chang, W.; Xiong, L.; Yu, Z. The influence of meteorological factors on tuberculosis incidence in Southwest China from 2006 to 2015. Sci. Rep. 2018, 8, 10053. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Liu, P.; Mu, F.; Xie, Z.; Lu, R.; Qi, Y.; Wang, B.; Jin, C. Characteristics of airborne opportunistic pathogenic bacteria during autumn and winter in Xi’an, China. Sci. Total Environ. 2019, 672, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Norvihoho, L.K.; Zhou, Z.F.; Chen, B.; Wu, W.T. Investigation on the evaporation and dispersion of human respiratory droplets with COVID-19 virus. Int. J. Multiph. Flow 2022, 147, 103904. [Google Scholar] [CrossRef]
- Wells, W.F. On air-borne infection: Study II. Droplets and droplet nuclei. Am. J. Epidemiol. 1934, 20, 611–618. [Google Scholar] [CrossRef]
- Xu, M.; Li, Y.; Liu, B.; Chen, R.; Sheng, L.; Yan, S.; Chen, H.; Hou, J.; Yuan, L.; Ke, L.; et al. Temperature and humidity associated with increases in tuberculosis notifications: A time-series study in Hong Kong. Epidemiol. Infect. 2021, 149, e8. [Google Scholar] [CrossRef]
- Conlan, A.J.; McKinley, T.J.; Karolemeas, K.; Pollock, E.B.; Goodchild, A.V.; Mitchell, A.P.; Birch, C.P.; Clifton-Hadley, R.S.; Wood, J.L. Estimating the hidden burden of bovine tuberculosis in Great Britain. PLoS Comput. Biol. 2012, 8, e1002730. [Google Scholar] [CrossRef] [PubMed]
- Tulu, B.; Zewede, A.; Belay, M.; Zeleke, M.; Girma, M.; Tegegn, M.; Ibrahim, F.; Jolliffe, D.A.; Abebe, M.; Balcha, T.T.; et al. Epidemiology of bovine tuberculosis and its zoonotic implication in Addis Ababa Milkshed, Central Ethiopia. Front. Vet. Sci. 2021, 8, 595511. [Google Scholar] [CrossRef] [PubMed]

| Classification | Variable | Description | Unit | Included |
|---|---|---|---|---|
| Bioclimatic variables | Bio 1 | Annual mean temperature | °C | N |
| Bio 2 | Mean diurnal range | °C | Y | |
| Bio 3 | Isothermality | % | N | |
| Bio 4 | Temperature seasonality | °C | N | |
| Bio 5 | Max temperature of the warmest month | °C | Y | |
| Bio 6 | Min temperature of the coldest month | °C | N | |
| Bio 7 | Annual temperature range | °C | N | |
| Bio 8 | Mean temperature of the wettest quarter | °C | N | |
| Bio 9 | Mean temperature of the driest quarter | °C | N | |
| Bio 10 | Mean temperature of the warmest quarter | °C | N | |
| Bio 11 | Mean temperature of the coldest quarter | °C | Y | |
| Bio 12 | Annual precipitation | mm | N | |
| Bio 13 | Precipitation of the wettest month | mm | N | |
| Bio 14 | Precipitation of the driest month | mm | Y | |
| Bio 15 | Precipitation seasonality | / | Y | |
| Bio 16 | Precipitation of the wettest quarter | mm | N | |
| Bio 17 | Precipitation of the driest quarter | mm | N | |
| Bio 18 | Precipitation of the warmest quarter | mm | N | |
| Bio 19 | Precipitation of the coldest quarter | mm | N | |
| Air pollutants | PM2.5 | Level of particulate matter 2.5 | μg/m3 | N |
| PM10 | Level of particulate matter 10 | μg/m3 | Y | |
| SO2 | Level of sulfur dioxide | μg/m3 | Y | |
| NO2 | Level of nitrogen dioxide | μg/m3 | Y | |
| CO | Level of carbon monoxide | μg/m3 | Y | |
| O3 | Level of ozone | μg/m3 | Y | |
| Host distribution density | Cattle | Cattle distribution density | head/km2 | Y |
| Buffalo | Buffalo distribution density | head/km2 | Y | |
| Sheep | Sheep distribution density | head/km2 | Y | |
| Goat | Goat distribution density | head/km2 | Y |
| Variables | Percent Contribution (%) |
|---|---|
| Level of nitrogen dioxide (NO2) Mean temperature of the coldest quarter (Bio 11) Cattle distribution density (Cattle) | 34.1 |
| 16.1 | |
| 9.6 | |
| Sheep distribution density (Sheep) Level of ozone (O3) Precipitation of the driest month (Bio 14) Precipitation seasonality (Bio 15) | 7.1 |
| 5.1 | |
| 4.9 | |
| 4.8 | |
| Max temperature of the warmest month (Bio 5) Goat distribution density (Goat) | 3.9 |
| 3.7 | |
| Level of sulfur dioxide (SO2) | 2.6 |
| Level of particulate matter 10 (PM10) | 2.5 |
| Isothermality (Bio 3) | 2.4 |
| Level of carbon monoxide (CO) | 2.4 |
| Buffalo distribution density (Buffalo) | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Li, S.; Li, H.; Pan, H.; Li, S.; Yang, Y.; Jiao, Y.; Lan, F.; Chen, S.; Chen, Q.; et al. Predicting Tuberculosis Risk in Cattle, Buffaloes, Sheep, and Goats in China Based on Air Pollutants and Meteorological Factors. Animals 2024, 14, 3704. https://doi.org/10.3390/ani14243704
Xu L, Li S, Li H, Pan H, Li S, Yang Y, Jiao Y, Lan F, Chen S, Chen Q, et al. Predicting Tuberculosis Risk in Cattle, Buffaloes, Sheep, and Goats in China Based on Air Pollutants and Meteorological Factors. Animals. 2024; 14(24):3704. https://doi.org/10.3390/ani14243704
Chicago/Turabian StyleXu, Le, Suya Li, Hong Li, Haoju Pan, Shiyuan Li, Yingxue Yang, Yuqing Jiao, Feng Lan, Si Chen, Qiaoling Chen, and et al. 2024. "Predicting Tuberculosis Risk in Cattle, Buffaloes, Sheep, and Goats in China Based on Air Pollutants and Meteorological Factors" Animals 14, no. 24: 3704. https://doi.org/10.3390/ani14243704
APA StyleXu, L., Li, S., Li, H., Pan, H., Li, S., Yang, Y., Jiao, Y., Lan, F., Chen, S., Chen, Q., Du, L., Man, C., Wang, F., & Gao, H. (2024). Predicting Tuberculosis Risk in Cattle, Buffaloes, Sheep, and Goats in China Based on Air Pollutants and Meteorological Factors. Animals, 14(24), 3704. https://doi.org/10.3390/ani14243704






