Effects of Concentrate Feed Starch Source Offered Twice a Day on Feed Intake and Milk Production of Cows During the Early Postpartum Period
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Experimental Design and Treatments
2.3. Data and Sample Collection
2.4. Sample Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, M.S. Symposium review: Integrating the control of energy intake and partitioning into ration formulation. J. Dairy Sci. 2023, 106, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.R. Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livest. Prod. Sci. 2003, 83, 211–218. [Google Scholar] [CrossRef]
- Herdt, T.H. Ruminant adaptation to negative energy balance: Influences on the etiology of ketosis and fatty liver. Vet. Clin. North Am. Food Anim. Pract. 2000, 16, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and β-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J. Dairy Sci. 2010, 93, 3595–3601. [Google Scholar] [CrossRef]
- Allen, M.S. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Albornoz, R.I.; Allen, M.S. Highly fermentable starch at different diet starch concentrations decreased feed intake and milk yield of cows in the early postpartum period. J. Dairy Sci. 2018, 101, 8902–8915. [Google Scholar] [CrossRef]
- Allen, M.S. Review: Control of feed intake by hepatic oxidation in ruminant animals: Integration of homeostasis and ho-meorhesis. Animal 2020, 14 (Suppl. S1), s55–s64. [Google Scholar] [CrossRef]
- Santos, J.; Huber, J.; Theurer, C.; Nussio, C.; Nussio, L.; Tarazon, M.; Fish, D. Effects of grain processing and bovine somatotropin on metabolism and ovarian activity of dairy cows during early lactation. J. Dairy Sci. 2000, 83, 1004–1015. [Google Scholar] [CrossRef]
- De Souza, J.; Lock, A. Milk production and nutrient digestibility responses to triglyceride or fatty acid supplements enriched in palmitic acid. J. Dairy Sci. 2019, 102, 4155–4164. [Google Scholar] [CrossRef]
- Albornoz, R.; Sordillo, L.; Contreras, G.; Nelli, R.; Mamedova, L.; Bradford, B.; Allen, M. Diet starch concentration and starch fermentability affect markers of inflammatory response and oxidant status in dairy cows during the early postpartum period. J. Dairy Sci. 2020, 103, 352–367. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th ed.; Australian Government: Canberra, Australia, 2013. [Google Scholar]
- Earle, D.F. A guide to scoring dairy cow condition. J. Agric. 1976, 74, 228–232. [Google Scholar]
- Norbu, N.; Alvarez-Hess, P.S.; Leury, B.J.; Wright, M.M.; Douglas, M.L.; Moate, P.J.; Williams, S.R.O.; Marett, L.C.; Garner, J.B.; Wales, W.J.; et al. Assessment of RumiWatch noseband sensors for the quantification of ingestive behaviors of dairy cows at grazing or fed in stalls. Anim. Feed. Sci. Technol. 2021, 280, 115076. [Google Scholar] [CrossRef]
- McMurray, C.H.; Blanchflower, W.J.; Rice, D.A. Automated kinetic method for D-3-hydroxybutyrate in plasma or serum. Clin. Chem. 1984, 30, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Makimura, S.; Suzuki, N. Quantitative Determination of Bovine Serum Haptoglobin and Its Elevation in Some Inflammatory Diseases. Jpn. J. Vet. Sci. 1982, 44, 15–21. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bowers, G.N., Jr.; Hørder, M.; Moss, D.W. Provisional recommendations on IFCC methods for the measurement of catalytic concentrations of enzymes. Clin. Chem. 1977, 23, 887–899. [Google Scholar] [CrossRef]
- McGinlay, J.M.; Payne, R.B. Serum albumin by dye-binding: Bromocresol green or bromocresol purple? The case for conservatism. Ann. Clin. Biochem. 1988, 25 Pt 4, 417–421. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970; pp. 33–61. [Google Scholar]
- VSN International. GenStat release 22; VSN International: Hemel Hempstead, UK, 2022. [Google Scholar]
- Albornoz, R.; Harvatine, K.; Allen, M. Diet starch concentration and starch fermentability affect energy intake and energy balance of cows in the early postpartum period. J. Dairy Sci. 2019, 102, 5161–5171. [Google Scholar] [CrossRef]
- Gomez, L.; Posada, S.L.; Olivera-Angel, M. Starch in ruminant diets: A review. Rev. Colomb. Cienc. Pecu. 2016, 29, 77–90. [Google Scholar] [CrossRef][Green Version]
- Allen, M.S. Drives and limits to feed intake in ruminants. Anim. Prod. Sci. 2014, 54, 1513–1524. [Google Scholar] [CrossRef]
- Dillon, J.L.; Anderson, J.R. The Analysis of Response in Crop and Livestock Production; Pergamon Press: Oxford, UK, 1990. [Google Scholar]
- Mertens, D. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Mertens, D. NDF and DMI-Has Anything Changed. In Proceedings of the Cornell Nutrition Conference for Feed Manufacturers, East Syracuse, Ithaca, NY, USA, 19–21 October 2010; pp. 160–174. [Google Scholar]
- Rockwell, R.; Allen, M. Chromium propionate supplementation during the peripartum period interacts with starch source fed postpartum: Production responses during the immediate postpartum and carryover periods. J. Dairy Sci. 2016, 99, 4453–4463. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, P.; Ylioja, C.M.; Allen, M.S. Feed intake is related to changes in plasma non-esterified fatty acid concentration and hepatic acetyl CoA content following feeding in lactating dairy cows. J. Dairy Sci. 2015, 98, 6839–6847. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.J.; Albright, J.L. Feeding behavior and management factors during the transition period in dairy cattle. J. Anim. Sci. 1995, 73, 2791–2803. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.; Ribeiro, C.V.D.M.; Harvatine, K.J. Meta-analysis of rumination behavior and its relationship with milk and milk fat production, rumen pH, and total-tract digestibility in lactating dairy cows. J. Dairy Sci. 2022, 105, 188–200. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Suthar, V.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef]
| Health Event | Treatment Period (1–23 d Postpartum) | Carryover Period (24–72 d Postpartum) | ||
|---|---|---|---|---|
| CRN 1 | WHT 1 | CRN 1 | WHT 1 | |
| Mastitis | 4 | 1 | 1 | 2 |
| Metritis | 3 | 1 | 1 | 0 |
| Ketosis | 0 | 5 | 0 | 0 |
| Lameness | 0 | 1 | 0 | 1 |
| Udder edema | 0 | 0 | 1 | 0 |
| Ingredients (% DM) | Concentrate Diets 1 | |
|---|---|---|
| CRN | WHT | |
| Wheat grain | - | 67.9 |
| Corn grain | 65.6 | - |
| Canola meal (solvent extracted) | 21.1 | 18.2 |
| Milled almond hulls | 8.6 | 9.3 |
| Mineral-vitamin mix 2 | 3 | 3 |
| Limestone | 0.9 | 0.9 |
| AcidBuf 3 | 0.5 | 0.5 |
| Magnesium Oxide | 0.2 | 0.2 |
| Elitox 4 | 0.12 | 0.12 |
| Nutrient Parameter | Starch Source | Carryover Concentrate | Perennial Ryegrass Silage | |
|---|---|---|---|---|
| CRN 1 | WHT 1 | |||
| % DM | 92.6 | 93.4 | 90.5 | 88.5 |
| Neutral detergent fiber (% DM) | 14.4 | 14.4 | 11.7 | 56.2 |
| Acid detergent fiber (% DM) | 8.8 | 8.5 | 4.8 | 39.8 |
| Crude protein (% DM) | 16.3 | 18.5 | 14.2 | 16.6 |
| Starch (% DM) | 48 | 48.1 | 60.5 | 1.4 |
| Ash (% DM) | 7.3 | 8.7 | 9.3 | 10.7 |
| In vitro starch digestibility, 7 h (% DM) | 58.8 | 65.8 | 56.6 | Not tested |
| Metabolizable energy (MJ/kg DM) | 13.0 | 12.6 | 12.5 | 9.3 |
| Starch Source | SED | p-Value | ||
|---|---|---|---|---|
| CRN 1 | WHT 1 | |||
| Feed intake | ||||
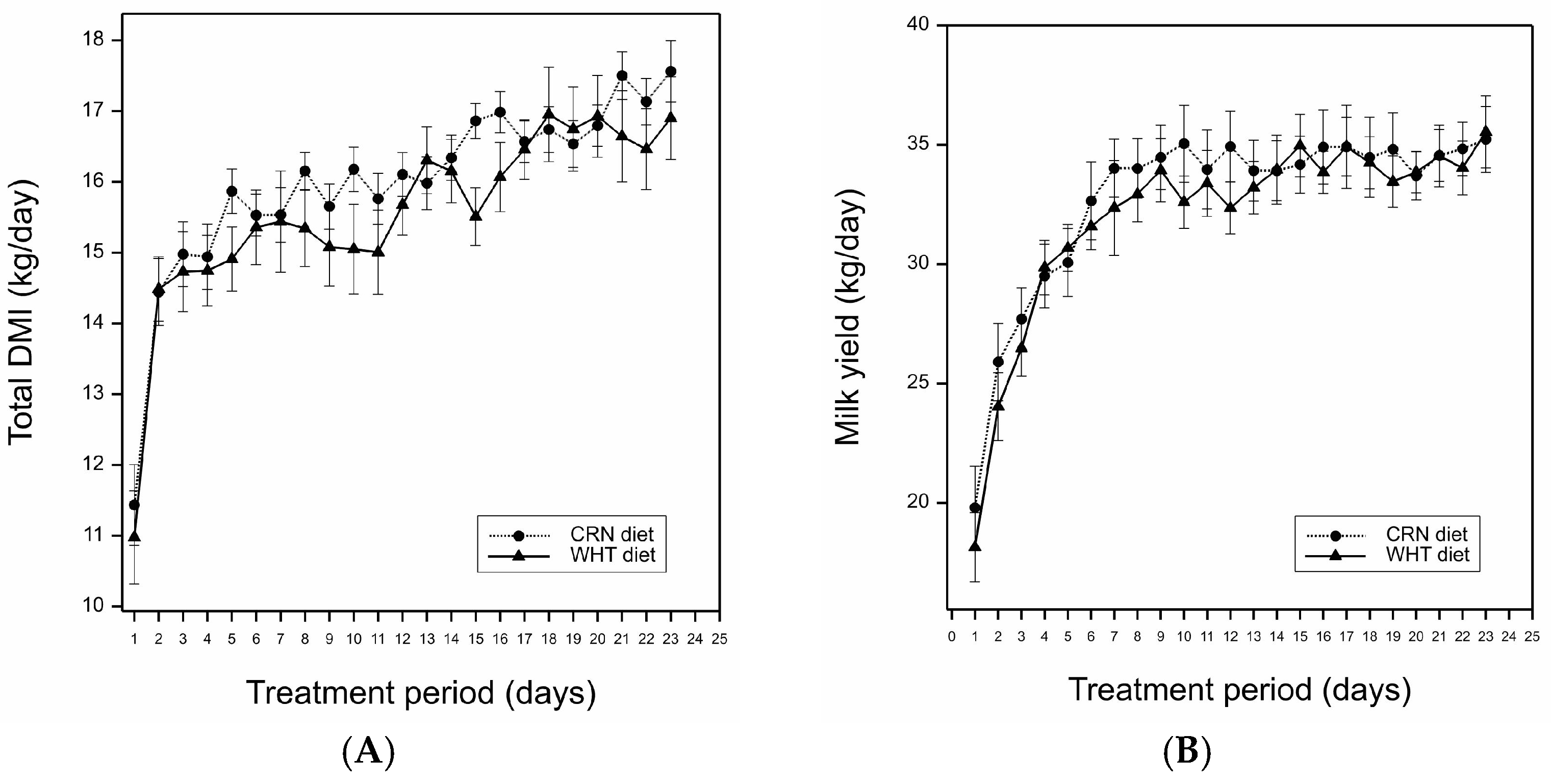
| Total DMI 2 (kg/d) | 16.0 | 15.6 | 0.393 | 0.28 |
| Concentrate DMI 2 (kg/d) | 7.92 | 7.88 | 0.035 | 0.21 |
| Silage DMI 2 (kg/d) | 8.06 | 7.67 | 0.373 | 0.31 |
| Neutral detergent fiber intake (kg/d) | 5.63 | 5.45 | 0.208 | 0.40 |
| Acid detergent fiber intake (kg/d) | 3.88 | 3.73 | 0.147 | 0.32 |
| Starch intake (kg/d) | 3.92 | 3.90 | 0.020 | 0.38 |
| Consumed diet nutrient composition | ||||
| Neutral detergent fiber (% DM) | 35.2 | 34.5 | 0.005 | 0.16 |
| Acid detergent fiber (% of DM) | 24.3 | 23.6 | 0.004 | 0.08 |
| Starch (% DM) | 24.8 | 25.6 | 0.006 | 0.14 |
| Body energy reserves | ||||
| Average post-calving BW 3 (kg) | 666 | 693 | 11.3 | 0.03 |
| Δ BW 3 at 3 weeks (Week 3–Week 1, kg) | −45.4 | −55.7 | 6.6 | 0.13 |
| Average post-calving BCS 3 (1–8 scale, units) | 4.63 | 4.66 | 0.055 | 0.62 |
| Δ BCS 3 (Week 3–Week 1, units) | −0.31 | −0.35 | 0.051 | 0.42 |
| Feeding Behavior | ||||
| Rumination time (min/d) | 81.2 | 67.0 | 6.29 | 0.03 |
| Eating time (min/d) | 269 | 250 | 14.0 | 0.20 |
| Idling time (min/d) | 66 | 94 | 9.75 | 0.01 |
| Milk | ||||
| Milk yield (kg/d) | 32.7 | 31.9 | 1.56 | 0.60 |
| ECM 4 (kg/d) | 38.9 | 39.4 | 1.88 | 0.79 |
| FCM 4 (kg/d) | 39.6 | 40.1 | 2.03 | 0.80 |
| Fat (kg/d) | 1.57 | 1.62 | 0.091 | 0.57 |
| Protein (kg/d) | 1.03 | 1.04 | 0.045 | 0.83 |
| Lactose (kg/d) | 1.61 | 1.58 | 0.075 | 0.65 |
| SCC 4 [1000/mL] (log10 SCC) | 95.5 (4.98) | 134.9 (5.13) | (0.461) | 0.75 |
| Fat (%) | 4.86 | 5.13 | 0.188 | 0.17 |
| Protein (%) | 3.20 | 3.32 | 0.100 | 0.24 |
| Lactose (%) | 4.94 | 4.94 | 0.043 | 1.00 |
| Metabolic indicators | ||||
| BHB 5 [mmol/l] (log10 BHB) | 0.36 (−0.44) | 0.40 (−0.39) | (0.142) | 0.69 |
| NEFA 5 (mmol/L) | 1.05 | 1.22 | 0.137 | 0.23 |
| Glucose (mmol/L) | 3.39 | 3.54 | 0.090 | 0.10 |
| Triglycerides (mmol/L) | 0.12 | 0.13 | 0.007 | 0.58 |
| Stress indicators | ||||
| Haptoglobin [g/L] (log10 haptoglobin) | 0.022 (−1.64) | 0.016 (−1.80) | (0.300) | 0.58 |
| Bilirubin (mmol/L) | 6.37 | 5.67 | 0.421 | 0.11 |
| Albumin (g/L) | 34.5 | 35.0 | 0.731 | 0.47 |
| Starch Source | SED | p-Value | ||
|---|---|---|---|---|
| CRN 1 | WHT 1 | |||
| Body energy reserves | ||||
| Average BW 2 (kg) | 606 | 614 | 15.9 | 0.62 |
| Average Δ BW 2 (Week 10–Week 3, kg) | −6.66 | −19.66 | 9.79 | 0.20 |
| Average BCS 2 (1–8 scale, units) | 4.41 | 4.35 | 0.066 | 0.31 |
| Average Δ BCS 2 (Week 10–Week 3, units) | −0.17 | −0.23 | 0.056 | 0.27 |
| Milk | ||||
| Milk yield (kg/d) | 38.8 | 38.5 | 1.50 | 0.87 |
| ECM 3 (kg/d) | 40.8 | 40.7 | 1.55 | 0.91 |
| FCM 3 (kg/d) | 40.6 | 40.4 | 1.47 | 0.89 |
| Fat (kg/d) | 1.47 | 1.46 | 0.061 | 0.92 |
| Protein (kg/d) | 1.20 | 1.19 | 0.056 | 0.98 |
| Lactose (kg/d) | 1.96 | 1.96 | 0.076 | 0.96 |
| SCC 3 [1000/mL] (log10 SCC) | 39.8 (4.60) | 15.5 (4.19) | (0.432) | 0.36 |
| Fat (%) | 3.80 | 3.81 | 0.135 | 0.93 |
| Protein (%) | 3.09 | 3.09 | 0.066 | 0.96 |
| Lactose (%) | 5.05 | 5.08 | 0.046 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albornoz, R.I.; Russo, V.M.; Ho, C.K.M.; Giri, K.; Allen, M.S.; Lock, A.L.; Wales, W.J.; Knight, M.I. Effects of Concentrate Feed Starch Source Offered Twice a Day on Feed Intake and Milk Production of Cows During the Early Postpartum Period. Animals 2024, 14, 3622. https://doi.org/10.3390/ani14243622
Albornoz RI, Russo VM, Ho CKM, Giri K, Allen MS, Lock AL, Wales WJ, Knight MI. Effects of Concentrate Feed Starch Source Offered Twice a Day on Feed Intake and Milk Production of Cows During the Early Postpartum Period. Animals. 2024; 14(24):3622. https://doi.org/10.3390/ani14243622
Chicago/Turabian StyleAlbornoz, Rodrigo I., Victoria M. Russo, Christie K. M. Ho, Khageswor Giri, Michael S. Allen, Adam L. Lock, William J. Wales, and Matthew I. Knight. 2024. "Effects of Concentrate Feed Starch Source Offered Twice a Day on Feed Intake and Milk Production of Cows During the Early Postpartum Period" Animals 14, no. 24: 3622. https://doi.org/10.3390/ani14243622
APA StyleAlbornoz, R. I., Russo, V. M., Ho, C. K. M., Giri, K., Allen, M. S., Lock, A. L., Wales, W. J., & Knight, M. I. (2024). Effects of Concentrate Feed Starch Source Offered Twice a Day on Feed Intake and Milk Production of Cows During the Early Postpartum Period. Animals, 14(24), 3622. https://doi.org/10.3390/ani14243622





