Effects of High-Concentrate Diets on Growth Performance, Serum Biochemical Indexes, and Rumen Microbiota in House-Fed Yaks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Diets and Feeding Management
2.3. Sampling and Measurement
2.4. DNA Extraction, PCR, and Sequencing
2.5. Sequencing Data Processing and Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Indexes
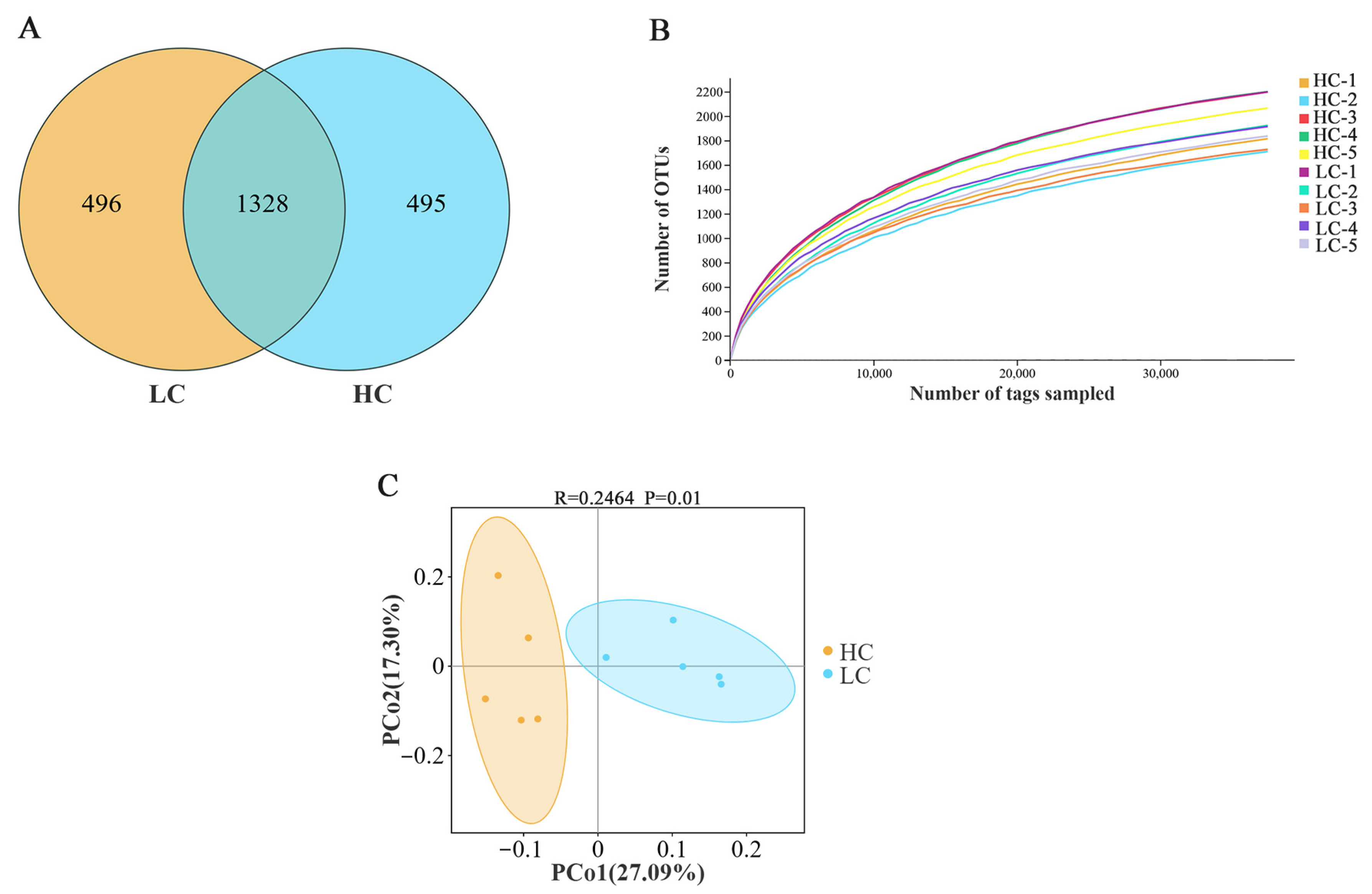
3.3. Richness and Diversity of Rumen Bacteria
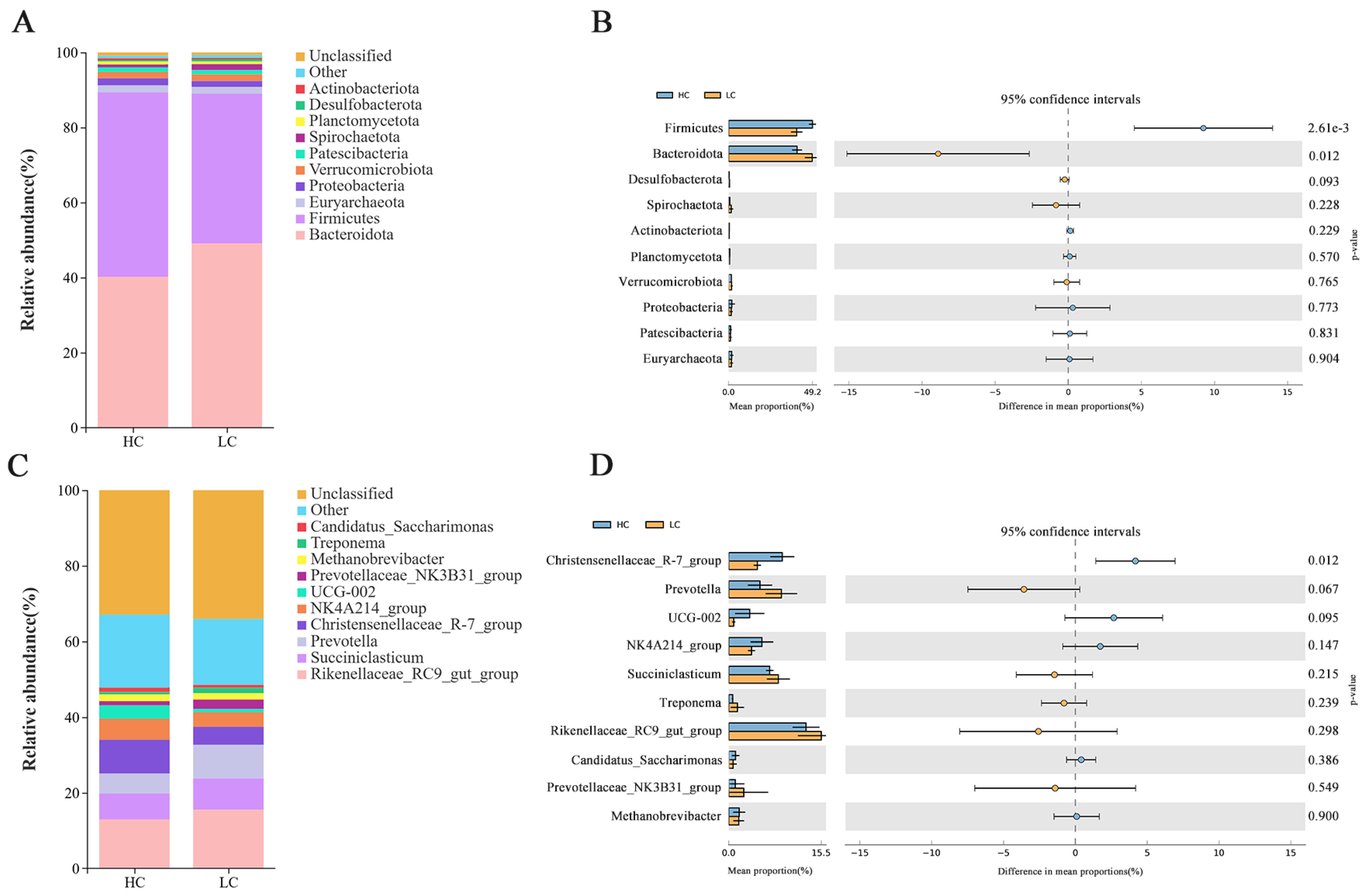
3.4. Structural Composition of Rumen Bacteria
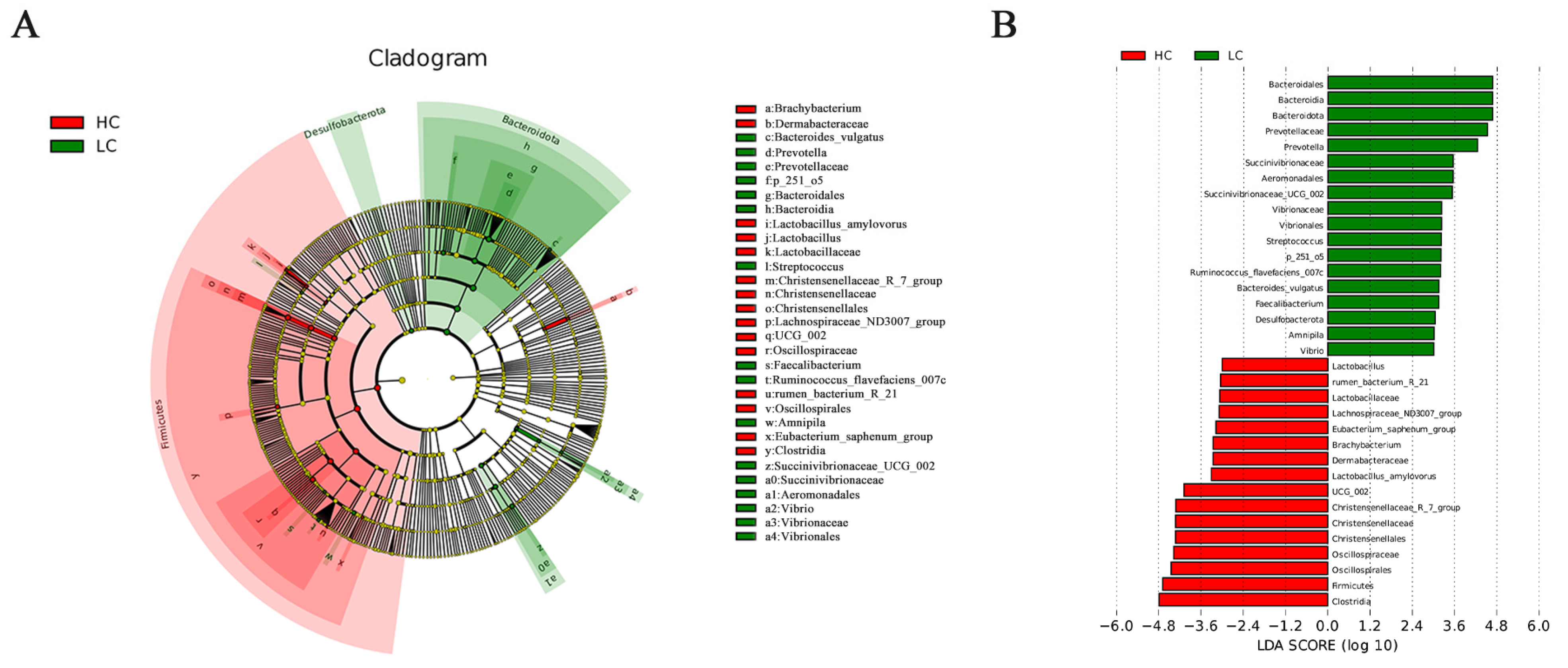
3.5. LEfse Analysis of Rumen Bacteria
3.6. PICRUSt 2 Function Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.L.; Zong, W.L.; Zhao, S.S.; Qie, M.J.; Yang, X.T.; Zhao, Y. Nutrition and edible characteristics, origin traceability and authenticity identification of yak meat and milk: A review. Trends Food Sci. Technol. 2023, 139, 104133. [Google Scholar] [CrossRef]
- He, S.; Yuan, Z.; Dai, S.; Wang, Z.; Zhao, S.; Wang, R.; Li, Q.; Mao, H.; Wu, D. Intensive feeding alters the rumen microbiota and its fermentation parameters in natural grazing yaks. Front. Vet. Sci. 2024, 11, 1365300. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bai, B.; Huang, Y.; Degen, A.; Mi, J.; Xue, Y.; Hao, L. Yaks Are Dependent on Gut Microbiota for Survival in the Environment of the Qinghai Tibet Plateau. Microorganisms 2024, 12, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, J.; Xiong, L.; Bao, P.; Chu, M.; Ma, X.; La, Y.; Liang, C.; Yan, P.; Guo, X. Genetic diversity, phylogeography, and maternal origin of yak (Bos grunniens). BMC Genom. 2024, 25, 481. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Yang, J.; Wang, D.; Niu, J.; Bai, B.; Sun, W.; Ma, S.; Cheng, Y.; Hao, L. A Comparative study of growth performance, blood biochemistry, rumen fermentation, and ruminal and fecal bacterial structure between yaks and cattle raised under high concentrate feeding conditions. Microorganisms 2023, 11, 2399. [Google Scholar] [CrossRef]
- Dai, D.; Pang, K.; Liu, S.; Wang, X.; Yang, Y.; Chai, S.; Wang, S. Effects of concentrate supplementation on growth performance, rumen fermentation, and bacterial community composition in grazing yaks during the warm season. Animals 2022, 12, 1398. [Google Scholar] [CrossRef]
- Chen, G.; Song, S.; Wang, B.; Zhang, Z.; Peng, Z.; Guo, C.; Zhong, J.; Wang, Y. Effects of forage: Concentrate ratio on growth performance, ruminal fermentation and blood metabolites in housing-feeding yaks. Asian-Australas. J. Anim. Sci. 2015, 28, 1736–1741. [Google Scholar] [CrossRef]
- Yi, S.; Dai, D.; Wu, H.; Chai, S.; Liu, S.; Meng, Q.; Zhou, Z. Dietary concentrate-to-forage ratio affects rumen bacterial community composition and metabolome of yaks. Front. Nutr. 2022, 9, 927206. [Google Scholar] [CrossRef]
- Gao, Z.; Raza, S.H.A.; Ma, B.; Wang, Z.; Mubarak Alwutayd, K.; Al Abdulmonem, W.; Mesfer Alharbi, Y.; Aljohani, A.S.; Hou, S.; Gui, L. Effects of dietary forage-to-concentrate ratio on fat deposition, fatty acid composition, oxidative stability and mRNA expression of sirtuins genes of subcutaneous fat in sheep (Ovis aries). J. Appl. Anim. Res. 2023, 51, 382–387. [Google Scholar] [CrossRef]
- Pang, K.; Dai, D.; Yang, Y.; Wang, X.; Liu, S.; Huang, W.; Xue, B.; Chai, S.; Wang, S. Effects of high concentrate rations on ruminal fermentation and microbiota of yaks. Front. Microbiol. 2022, 13, 957152. [Google Scholar] [CrossRef]
- Feng, W.; Limin, W.; Xinli, Z.; Hailong, L.; Ruiping, S.; Zhe, C.; Lili, H.; Liang, F.; Quanwei, L. Effects of Dietary Concentrate to Forage Ratios on Production Performance and Serum Biochemical Indicators in Post-fattening Hainan Yellow Cattle. Anim. Husb. Feed. Sci. 2020, 12, 17–20. [Google Scholar] [CrossRef]
- Jiang, Y.; Dai, P.; Dai, Q.; Ma, J.; Wang, Z.; Hu, R.; Zou, H.; Peng, Q.; Wang, L.; Xue, B. Effects of the higher concentrate ratio on the production performance, ruminal fermentation, and morphological structure in male cattle-yaks. Vet. Med. Sci. 2022, 8, 771–780. [Google Scholar] [CrossRef]
- Grilli, D.J.; Fliegerová, K.; Kopečný, J.; Lama, S.P.; Egea, V.; Sohaefer, N.; Pereyra, C.; Ruiz, M.S.; Sosa, M.A.; Arenas, G.N. Analysis of the rumen bacterial diversity of goats during shift from forage to concentrate diet. Anaerobe 2016, 42, 17–26. [Google Scholar] [CrossRef]
- Cantalapiedra-Hijar, G.; Yáñez-Ruiz, D.; Martín-García, A.; Molina-Alcaide, E. Effects of forage: Concentrate ratio and forage type on apparent digestibility, ruminal fermentation, and microbial growth in goats. J. Anim. Sci. 2009, 87, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhang, Y.; Wang, L. The effects of different concentrate-to-forage ratio diets on rumen bacterial microbiota and the structures of Holstein cows during the feeding cycle. Animals 2020, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q.; Wang, L.; Liu, Y.; Yan, T. Effects of a High-Concentrate Diet on the Blood Parameters and Liver Transcriptome of Goats. Animals 2023, 13, 1559. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shah, A.M.; Wang, Z.; Fan, X. Potential protective effects of thiamine supplementation on the ruminal epithelium damage during subacute ruminal acidosis. Anim. Sci. J. 2021, 92, e13579. [Google Scholar] [CrossRef]
- Mao, J.; Wang, L.; Wang, Z.; Xue, B.; Peng, Q.; Hu, R.; Xiao, J. High concentrate diets altered the structure and function of rumen microbiome in goats. Front. Microbiol. 2024, 15, 1416883. [Google Scholar] [CrossRef]
- Meng, M.; Li, X.; Wang, Z.; Huo, R.; Ma, N.; Chang, G.; Shen, X. A high-concentrate diet induces inflammatory injury via regulating Ca2+/CaMKKβ-mediated autophagy in mammary gland tissue of dairy cows. Front. Immunol. 2023, 14, 1186170. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Song, J.; Ma, Y.; Zhang, H.; Wang, L.; Zhang, Y.; Zhang, G. Fermented Total Mixed Ration Alters Rumen Fermentation Parameters and Microbiota in Dairy Cows. Animals 2023, 13, 1062. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Li, R.; Teng, Z.; Lang, C.; Zhou, H.; Zhong, W.; Ban, Z.; Yan, X.; Yang, H.; Farouk, M.H.; Lou, Y. Effect of different forage-to-concentrate ratios on ruminal bacterial structure and real-time methane production in sheep. PLoS ONE 2019, 14, e0214777. [Google Scholar] [CrossRef]
- Musco, N.; Tudisco, R.; Grossi, M.; Mastellone, V.; Morittu, V.; Pero, M.; Wanapat, M.; Trinchese, G.; Cavaliere, G.; Mollica, M. Effect of a high forage: Concentrate ratio on milk yield, blood parameters and oxidative status in lactating cows. Anim. Prod. Sci. 2020, 60, 1531–1538. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, Z.; Liu, Y.; Wang, C.; Peng, Z.; Zhong, J.; Zhang, M.; Wang, H.J.A. Effect of Methionine Analogues on Growth Performance, Serum Biochemical Parameters, Serum Free Amino Acids and Rumen Fermentation of Yaks. Animals 2022, 12, 3175. [Google Scholar] [CrossRef]
- Abonyi, F.O.; Machebe, N.S.; Ezea, M.S.; Eze, J.I.; Omeke, B.C.; Marire, B.N. Effects of substituting soya bean meal (SBM) with blood meal (BM) on biochemical profile of pregnant pigs. Trop. Anim. Health Prod. 2013, 45, 957–963. [Google Scholar] [CrossRef]
- Khan, S.; Shahzadi, F.; Hayat, S.U.; Khan, F.; Khan, A.H.; Iqbal, M.; Majid, H.A.; Afridi, R.J. Effect of feeding different levels of forages and concentrate ration on production performance, serum biochemical and hematological profile in Ghaljo sheep (Ovis aries). Pure Appl. Biol. 2023, 12, 1034–1043. [Google Scholar] [CrossRef]
- Dai, Q.; Ma, J.; Cao, G.; Hu, R.; Zhu, Y.; Li, G.; Zou, H.; Wang, Z.; Peng, Q.; Xue, B. Comparative study of growth performance, nutrient digestibility, and ruminal and fecal bacterial community between yaks and cattle-yaks raised by stall-feeding. AMB Express 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Diao, Q.; Wang, H.; Tu, Y.; Tao, X.; Zhang, N. Effects of weaning age on growth, nutrient digestibility and metabolism, and serum parameters in Hu lambs. Anim. Nutr. 2015, 1, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zheng, M.; Ren, A.; Mao, H.; Long, D.; Yang, L. Effects of High-Concentrate-Induced SARA on Antioxidant Capacity, Immune Levels and Rumen Microbiota and Function in Goats. Animals 2024, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Abd Ellah, M.R.; Hamed, M.I.; Ibrahim, D.R.; Rateb, H.Z. Serum biochemical and haematological reference intervals for water buffalo (Bubalus bubalis) heifers. J. S. Afr. Vet. Assoc. 2014, 85, 1–7. [Google Scholar] [CrossRef]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef]
- Sha, Y.; Hu, J.; Shi, B.; Dingkao, R.; Wang, J.; Li, S.; Zhang, W.; Luo, Y.; Liu, X. Characteristics and Functions of the Rumen Microbial Community of Cattle-Yak at Different Ages. Biomed. Res. Int. 2020, 2020, 3482692. [Google Scholar] [CrossRef]
- Chen, M.; Xie, W.; Zhou, S.; Ma, N.; Wang, Y.; Huang, J.; Shen, X.; Chang, G. A high-concentrate diet induces colonic inflammation and barrier damage in Hu sheep. J. Dairy Sci. 2023, 106, 9644–9662. [Google Scholar] [CrossRef]
- Perez, H.G.; Stevenson, C.K.; Lourenco, J.M.; Callaway, T.R. Understanding Rumen Microbiology: An Overview. Encyclopedia 2024, 4, 148–157. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Wang, Y.; Li, S.; Cao, Z.; Ji, S.; He, Y.; Zhang, H. Effect of dietary forage to concentrate ratios on dynamic profile changes and interactions of ruminal microbiota and metabolites in Holstein heifers. Front. Microbiol. 2017, 8, 2206. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Xu, S.; Ma, L.; Han, X.; Wang, X.; Zhang, X.; Hu, L.; Zhao, N.; Chen, Y. Effect of dietary concentrate to forage ratio on growth performance, rumen fermentation and bacterial diversity of Tibetan sheep under barn feeding on the Qinghai-Tibetan plateau. PeerJ 2019, 7, e7462. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.; Sun, L.; Wang, Y.; Li, J.; Ge, G.; Jia, Y.; Du, S. Effects of different forage proportions in fermented total mixed ration on muscle fatty acid profile and rumen microbiota in lambs. Front. Microbiol. 2023, 14, 1197059. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Li, S.; Tun, H.; Derakhshani, H.; Moossavi, S.; Plaizier, J. Effects of grain feeding on microbiota in the digestive tract of cattle. Anim. Front. 2016, 6, 13–19. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, R.; Wang, D.; Zhu, W. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing. Anaerobe 2013, 24, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Huasai, S.; Chen, A. Effects of dietary forage to concentrate ratio on nutrient digestibility, ruminal fermentation and rumen bacterial composition in Angus cows. Sci. Rep. 2021, 11, 17023. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, C.; Diao, Q.; Tu, Y. Effect of Dietary and Age Changes on Ruminal Microbial Diversity in Holstein Calves. Microorganisms 2023, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Jang, M.; Kim, H.; Kwak, W.; Park, W.; Hwang, J.Y.; Lee, C.-K.; Jang, G.W.; Park, M.N.; Kim, H.-C. Comparative transcriptome analysis of adipose tissues reveals that ECM-receptor interaction is involved in the depot-specific adipogenesis in cattle. PLoS ONE 2013, 8, e66267. [Google Scholar] [CrossRef]

| Items | Group 2 | |
|---|---|---|
| LC | HC | |
| Diet composition | ||
| Alfalfa hay | 30.00 | 20.00 |
| Corn straw | 30.00 | 20.00 |
| Concentrate 1 | 40.00 | 60.00 |
| Total | 100.00 | 100.00 |
| Nutrient levels | ||
| Dry matter | 91.30 | 90.20 |
| Crude protein | 13.08 | 13.02 |
| Ether extract | 1.70 | 2.10 |
| Neutral detergent fiber | 45.20 | 37.10 |
| Acid detergent fiber | 26.90 | 19.30 |
| Calcium | 0.96 | 0.84 |
| Phosphorus | 0.34 | 0.38 |
| ME, MJ/kg 3 | 9.45 | 9.50 |
| Concentrate–forage | 40:60 | 60:40 |
| Items 1 | Experimental Stage | Group 2 | p-Value | |
|---|---|---|---|---|
| LC | HC | |||
| BW (kg) | 0 d | 151.86 ± 14.73 | 151.61 ± 15.43 | 0.973 |
| 30 d | 173.98 ± 14.85 | 186.32 ± 14.53 | 0.115 | |
| 60 d | 195.63 ± 19.52 | 205.54 ± 14.72 | 0.270 | |
| 90 d | 202.59 ± 17.83 | 216.34 ± 15.73 | 0.124 | |
| ADFI (kg/d) | 1–30 d | 5.24 ± 0.77 | 5.65 ± 0.75 * | 0.004 |
| 31–60 d | 5.96 ± 0.56 | 6.08 ± 0.54 | 0.238 | |
| 61–90 d | 5.76 ± 0.60 | 5.70 ± 0.61 | 0.631 | |
| 1–90 d | 5.62 ± 0.75 | 5.84 ± 0.64 * | 0.005 | |
| ADG (kg/d) | 1–30 d | 0.74 ± 0.25 | 1.22 ± 0.07 * | <0.001 |
| 31–60 d | 0.72 ± 0.23 | 0.64 ± 0.19 | 0.459 | |
| 61–90 d | 0.23 ± 0.19 | 0.36 ± 0.22 | 0.230 | |
| 1–90 d | 0.56 ± 0.13 | 0.72 ± 0.12 * | 0.026 | |
| Items 1 | Experimental Stage | Group 2 | p-Value | |
|---|---|---|---|---|
| LC | HC | |||
| TP (g/L) | 30 d | 69.01 ± 7.58 | 64.96 ± 4.61 | 0.218 |
| 60 d | 64.64 ± 4.16 | 67.00 ± 4.20 | 0.279 | |
| 90 d | 61.92 ± 5.90 | 66.69 ± 5.18 | 0.108 | |
| ALB (g/L) | 30 d | 33.14 ± 4.19 | 32.76 ± 2.24 | 0.822 |
| 60 d | 32.05 ± 1.95 | 35.31 ± 2.98 * | 0.021 | |
| 90 d | 31.19 ± 2.90 | 35.25 ± 0.38 * | 0.009 | |
| GLB (g/L) | 30 d | 35.86 ± 6.95 | 32.20 ± 3.68 | 0.209 |
| 60 d | 32.59 ± 4.77 | 31.69 ± 4.51 | 0.702 | |
| 90 d | 30.72 ± 5.06 | 31.44 ± 4.50 | 0.768 | |
| CREA (umol/L) | 30 d | 97.66 ± 15.84 | 93.73 ± 6.43 | 0.551 |
| 60 d | 101.29 ± 9.99 | 112.60 ± 20.62 | 0.184 | |
| 90 d | 106.90 ± 15.27 | 117.16 ± 18.10 | 0.241 | |
| GLU (mmol/L) | 30 d | 4.95 ± 0.36 | 5.34 ± 0.45 | 0.070 |
| 60 d | 4.11 ± 0.27 | 4.20 ± 0.31 | 0.572 | |
| 90 d | 4.17 ± 0.47 | 4.20 ± 0.30 | 0.894 | |
| AST (U/L) | 30 d | 100.74 ± 22.18 | 96.77 ± 15.05 | 0.681 |
| 60 d | 99.06 ± 22.03 | 98.63 ± 14.43 | 0.964 | |
| 90 d | 87.27 ± 18.06 | 82.07 ± 13.45 | 0.543 | |
| ALT (U/L) | 30 d | 34.67 ± 14.39 | 36.33 ± 0.79 | 0.804 |
| 60 d | 36.35 ± 3.01 | 31.11 ± 10.32 | 0.219 | |
| 90 d | 29.21 ± 10.17 | 30.95 ± 7.86 | 0.708 | |
| ALP (U/L) | 30 d | 161.19 ± 28.67 | 176.74 ± 16.79 | 0.231 |
| 60 d | 153.32 ± 34.32 | 182.16 ± 67.64 | 0.300 | |
| 90 d | 131.24 ± 27.41 | 157.73 ± 66.89 | 0.318 | |
| LDH (U/L) | 30 d | 1017.40 ± 135.52 | 970.06 ± 151.37 | 0.520 |
| 60 d | 953.50 ± 141.00 | 1022.33 ± 167.00 | 0.388 | |
| 90 d | 818.85 ± 82.20 | 874.96 ± 100.02 | 0.261 | |
| Items | Group 1 | p-Value | |
|---|---|---|---|
| LC | HC | ||
| Shannon | 7.76 ± 0.39 | 7.96 ± 0.39 | 0.492 |
| Chao1 | 2332.36 ± 139.11 | 2397.05 ± 128.47 | 0.514 |
| ACE | 2455.60 ± 144.67 | 2538.95 ± 145.74 | 0.440 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wang, X.; Ding, Z.; Kang, Y.; Guo, S.; Cao, M.; Hu, L.; Xiong, L.; Pei, J.; Guo, X. Effects of High-Concentrate Diets on Growth Performance, Serum Biochemical Indexes, and Rumen Microbiota in House-Fed Yaks. Animals 2024, 14, 3594. https://doi.org/10.3390/ani14243594
Zhang B, Wang X, Ding Z, Kang Y, Guo S, Cao M, Hu L, Xiong L, Pei J, Guo X. Effects of High-Concentrate Diets on Growth Performance, Serum Biochemical Indexes, and Rumen Microbiota in House-Fed Yaks. Animals. 2024; 14(24):3594. https://doi.org/10.3390/ani14243594
Chicago/Turabian StyleZhang, Ben, Xingdong Wang, Ziqiang Ding, Yandong Kang, Shaoke Guo, Mengli Cao, Liyan Hu, Lin Xiong, Jie Pei, and Xian Guo. 2024. "Effects of High-Concentrate Diets on Growth Performance, Serum Biochemical Indexes, and Rumen Microbiota in House-Fed Yaks" Animals 14, no. 24: 3594. https://doi.org/10.3390/ani14243594
APA StyleZhang, B., Wang, X., Ding, Z., Kang, Y., Guo, S., Cao, M., Hu, L., Xiong, L., Pei, J., & Guo, X. (2024). Effects of High-Concentrate Diets on Growth Performance, Serum Biochemical Indexes, and Rumen Microbiota in House-Fed Yaks. Animals, 14(24), 3594. https://doi.org/10.3390/ani14243594






