Artemisia annua Residue Regulates Immunity, Antioxidant Ability, Intestinal Barrier Function, and Microbial Structure in Weaned Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Analysis of the Nutritional Value and Main Active Components of Artemisia annua Residue
2.3. Animal Experimental Details
2.4. Growth Performance
2.5. Sample Collection
2.6. Serum Biochemical Indices
2.7. Serum Immunoglobulin and Cytokine Concentrations
2.8. Serum Antioxidative Levels
2.9. Intestinal Histomorphologic Analysis
2.10. RNA Extraction and Quantitative Real-Time PCR
2.11. Colonic Bacterial 16S rDNA Gene Sequencing and Function Prediction
2.12. Detection of Short-Chain Fatty Acids (SCFAs) in Colonic Contents
2.13. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Indices
3.3. Serum Immune Indexes
3.4. Serum Antioxidant Indices
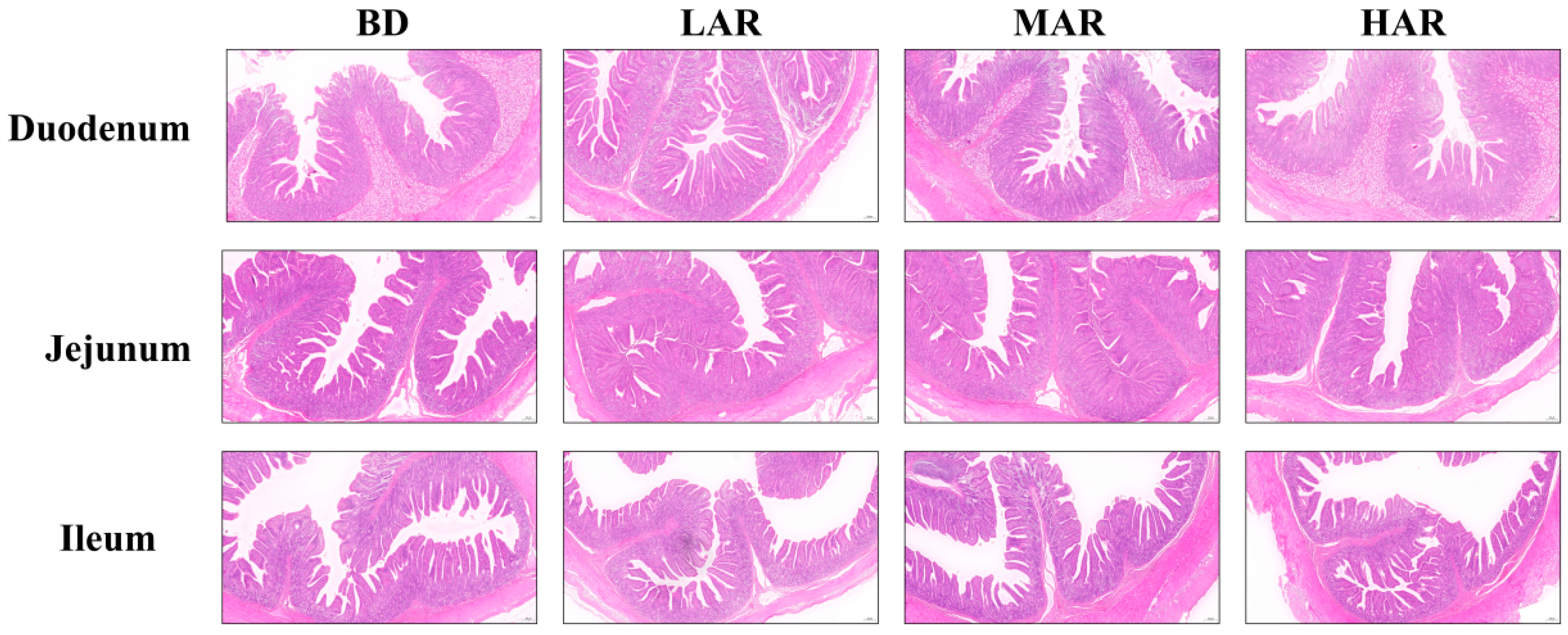
3.5. Intestinal Histomorphologic Analysis
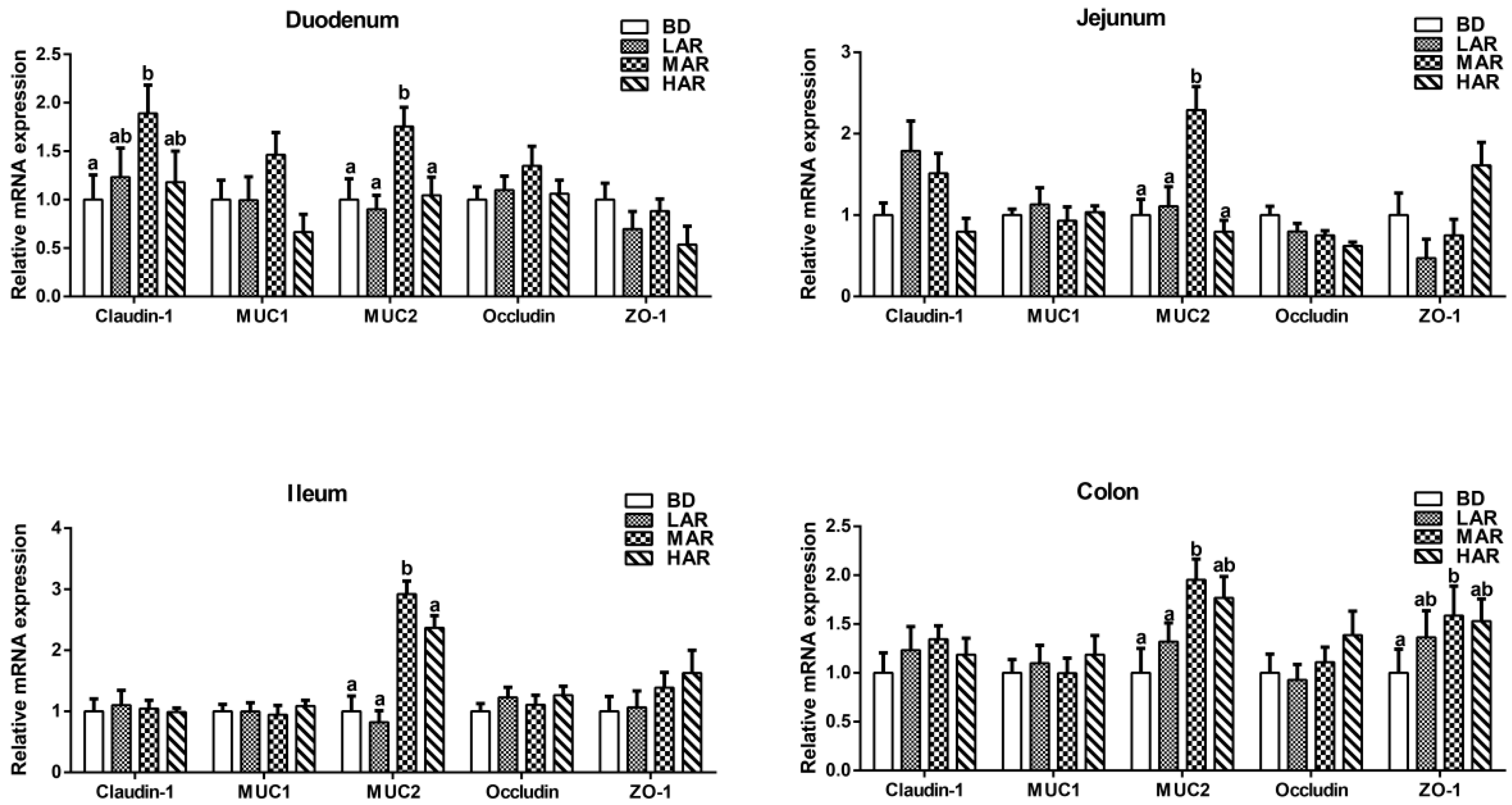
3.6. Expression of Genes Associated with Intestinal Mucosal Barrier
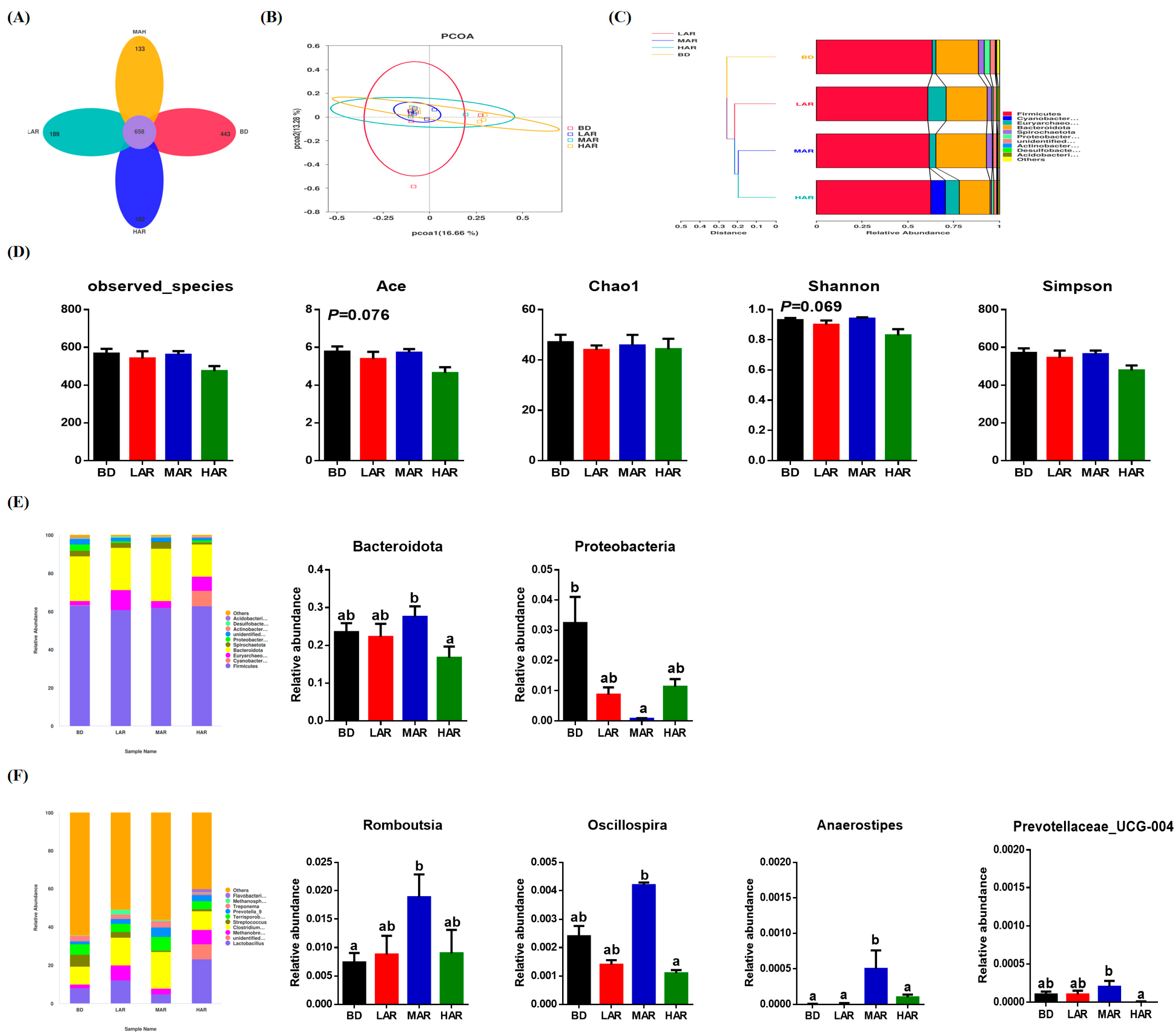
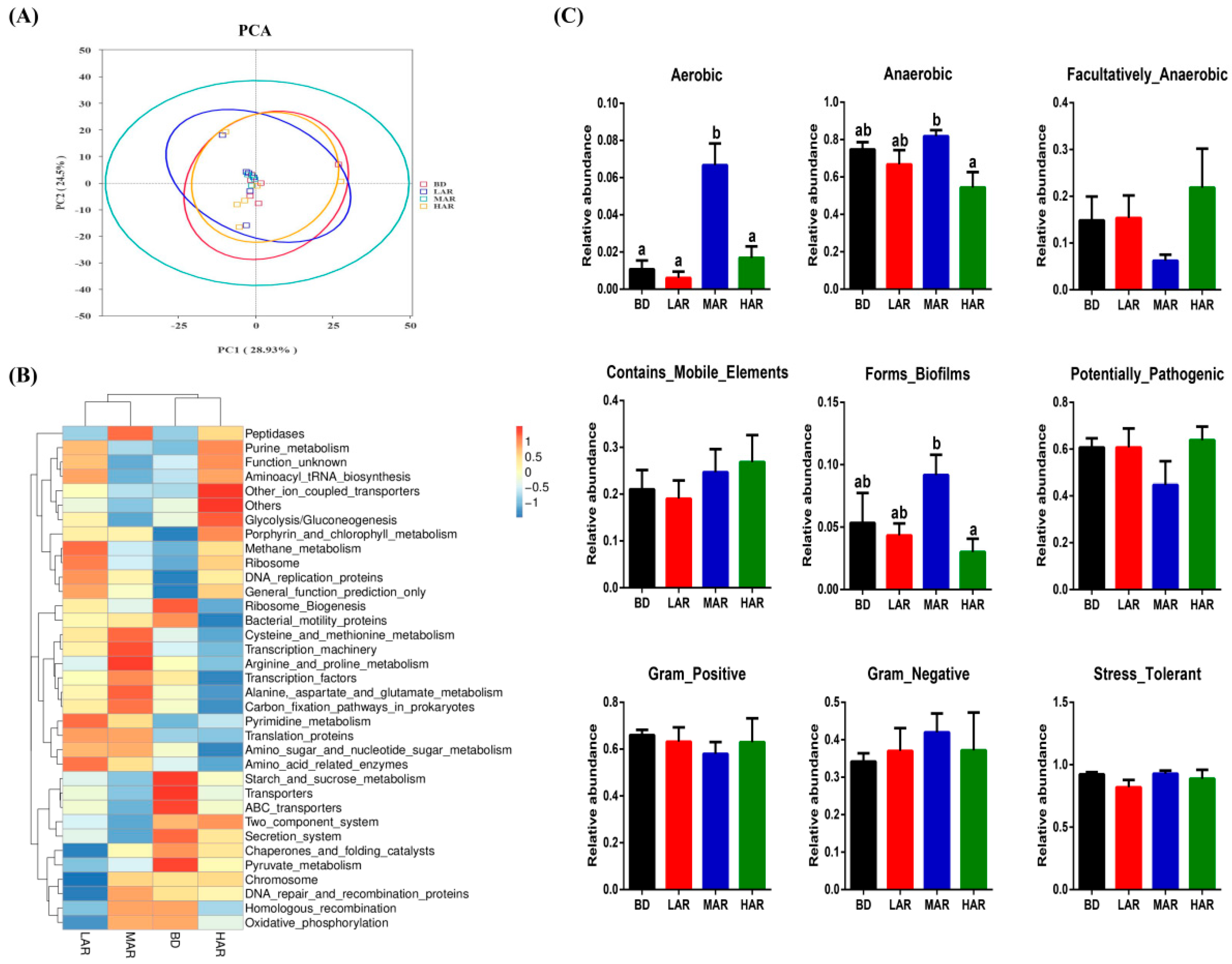
3.7. Colonic Microbiological Compositions and Metabolic Functions
3.8. Colonic SCFA Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Kang, X.; Tan, H.; Cai, H.; Chen, D. Progress in Fermented Unconventional Feed Application in Monogastric Animal Production in China. Fermentation 2023, 9, 947. [Google Scholar] [CrossRef]
- Lian, X.; Shi, M.; Liang, Y.; Lin, Q.; Zhang, L. The Effects of Unconventional Feed Fermentation on Intestinal Oxidative Stress in Animals. Antioxidants 2024, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Leng, X.; Zhao, Y.; Zhao, Y.; Wang, Q. Effects of dietary Artemisia annua supplementation on growth performance, antioxidant capacity, immune function, and gut microbiota of geese. Poult. Sci. 2024, 103, 103594. [Google Scholar] [CrossRef]
- Niu, Y.; He, J.T.; Zhao, Y.W.; Gan, Z.D.; Shen, M.M.; Zhang, L.L.; Wang, T. Dietary enzymatically treated Artemisia annua L. supplementation improved growth performance and intestinal antioxidant capacity of weaned piglets. Livest. Sci. 2020, 232, 103937. [Google Scholar] [CrossRef]
- Coroian, M.; Pop, L.M.; Popa, V.; Friss, Z.; Oprea, O.; Kalmár, Z.; Pintea, A.; Borșan, S.D.; Mircean, V.; Lobonțiu, I.; et al. Efficacy of Artemisia annua against Coccidiosis in Broiler Chickens: A Field Trial. Microorganisms 2022, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Zheljazkov, V.D.; Gonzalez, J.M. Artemisinin concentration and antioxidant capacity of Artemisia annua distillation byproduct. Ind. Crop. Prod. 2013, 41, 294–298. [Google Scholar] [CrossRef]
- Ding, X.; Pan, H.; Shi, P.; Zhao, S.; Bao, S.; Zhong, S.; Dai, C.; Chen, J.; Gong, L.; Zhang, D.; et al. A comparative analysis of chloroplast genomes revealed the chloroplast heteroplasmy of Artemisia annua. Front. Pharmacol. 2024, 15, 1466578. [Google Scholar] [CrossRef] [PubMed]
- GB/T 6435-2014; Determination of Moisture in Feeds. Standards Press of China: Beijing, China, 2014.
- GB/T 6438-2007; Determination of Ash in Feeds. Standards Press of China: Beijing, China, 2007.
- GB/T 6433-2006; Determination of Crude Fat in Feeds. Standards Press of China: Beijing, China, 2006.
- GB/T 6432-2018; Determination of Crude Protein in Feeds-Kjeldahl Method. Standards Press of China: Beijing, China, 2018.
- GB/T 20805-2006; Determination of Acid Detergent Lignin in Feeds. Standards Press of China: Beijing, China, 2006.
- GB/T 6434-2022; Determination of Crude Fibre in Feeds. Standards Press of China: Beijing, China, 2022.
- GB/T 20806-2022; Determination of Neutral Detergent Fibre in Feeds. Standards Press of China: Beijing, China, 2022.
- NY/T 1459-2022; Determination of Acid Detergent Fibre in Feeds. Standards Press of China: Beijing, China, 2022.
- Zhou, H.; Wang, C.; Ye, J.; Chen, H.; Tao, R. Effects of dietary supplementation of fermented Ginkgo biloba L. residues on growth performance, nutrient digestibility, serum biochemical parameters and immune function in weaned piglets. Anim. Sci. J. 2015, 86, 790–799. [Google Scholar] [CrossRef]
- Liu, H.; Bai, M.; Tan, B.; Xu, K.; Yu, R.; Huang, R.; Yin, Y. Influence of supplemented coated-cysteamine on morphology, apoptosis and oxidative stress status of gastrointestinal tract. BMC Vet. Res. 2019, 15, 328. [Google Scholar] [CrossRef]
- Bai, M.; Wang, L.; Liu, H.; Xu, K.; Deng, J.; Huang, R.; Yin, Y. Imbalanced dietary methionine-to-sulfur amino acid ratio can affect amino acid profiles, antioxidant capacity, and intestinal morphology of piglets. Anim. Nutr. 2020, 6, 447–456. [Google Scholar] [CrossRef]
- Bai, M.; Liu, H.; Zhang, Y.; Wang, S.; Shao, Y.; Xiong, X.; Hu, X.; Yu, R.; Lan, W.; Cui, Y.; et al. Peppermint extract improves egg production and quality, increases antioxidant capacity, and alters cecal microbiota in late-phase laying hens. Front. Microbiol. 2023, 14, 1252785. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.; Liu, D.; Lin, C.-H. Functional prediction of microbial communities in sediment microbial fuel cells. Bioengineering 2023, 10, 199. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Hou, L.; Sun, L.; Gao, J.; Gao, K.; Yang, X.; Jiang, Z.; Wang, L. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int. Immunopharmacol. 2022, 105, 108520. [Google Scholar] [CrossRef]
- Gao, J.; Yin, J.; Xu, K.; Li, T.; Yin, Y. What is the impact of diet on nutritional diarrhea associated with gut microbiota in weaning piglets: A System Review. BioMed Res. Int. 2019, 2019, 6916189. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, L.; Cui, C.; Zhao, H.; Zhang, Y.; Tian, Z.; Guan, W.; Chen, F. Maternal supplementation with Artemisia annua L. ameliorates intestinal inflammation via inhibiting the TLR4/NF-κB and MAPK pathways and improves the oxidative stability of offspring. Food Funct. 2022, 13, 9311–9323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Heng, J.; Kim, S.W.; Chen, F.; Deng, Z.; Zhang, S.; Guan, W. Dietary enzymatically-treated Artemisia annua L. supplementation could alleviate oxidative injury and improve reproductive performance of sows reared under high ambient temperature. J. Therm. Biol. 2020, 94, 102751. [Google Scholar] [CrossRef]
- Xiong, L.; Zhang, W.; Zhao, H.; Tian, Z.; Ren, M.; Chen, F.; Guan, W.; Zhang, S. Dietary supplementation of enzymatically treated Artemisia annua L. improves lactation performance, alleviates inflammatory response of sows reared under heat stress, and promotes gut development in preweaning offspring. Front. Vet. Sci. 2022, 9, 843673. [Google Scholar] [CrossRef]
- Guo, S.; Ma, J.; Xing, Y.; Xu, Y.; Jin, X.; Yan, S.; Shi, L.; Zhang, L.; Shi, B. Effects of Artemisia annua L. water extract on growth performance and intestinal related indicators in broilers. J. Poult. Sci. 2023, 60, 2023024. [Google Scholar] [CrossRef]
- Zong, E.; Yan, S.; Wang, M.; Yin, L.; Wang, Q.; Yin, J.; Li, J.; Li, Y.; Ding, X.; Huang, P.; et al. The effects of dietary supplementation with hyodeoxycholic acid on the differentiation and function of enteroendocrine cells and the serum biochemical indices in weaned piglets. J. Anim. Sci. 2019, 97, 1796–1805. [Google Scholar] [CrossRef]
- Zarantonello, A.; Revel, M.; Grunenwald, A.; Roumenina, L.T. C3-dependent effector functions of complement. Immunol. Rev. 2023, 313, 120–138. [Google Scholar] [CrossRef]
- Hansen, A.L.; Reily, C.; Novak, J.; Renfrow, M.B. Immunoglobulin A glycosylation and its role in disease. Exp. Suppl. 2021, 112, 433–477. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Cheng, K.; Zheng, X.C.; Ahmad, H.; Zhang, L.L.; Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hu, Y.; Wang, L.; Ao, C. Ethanol extract of Artemisia Annua prevents LPS-Induced inflammation and blood–milk barrier disruption in bovine mammary epithelial cells. Animals 2022, 12, 1228. [Google Scholar] [CrossRef]
- Wang, D.; Kuang, Y.; Lv, Q.; Xie, W.; Xu, X.; Zhu, H.; Zhang, Y.; Cong, X.; Cheng, S.; Liu, Y. Selenium-enriched Cardamine violifolia protects against sepsis-induced intestinal injury by regulating mitochondrial fusion in weaned pigs. Sci. China Life Sci. 2023, 66, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Yu, B.; He, J.; Tian, G.; Luo, Y.; Mao, X.; Zhang, K.; Che, L.; Chen, D. Protective effects of dietary arginine supplementation against oxidative stress in weaned piglets. Br. J. Nutr. 2013, 109, 2253–2260. [Google Scholar] [CrossRef]
- Xu, J.; Xu, C.; Chen, X.; Cai, X.; Yang, S.; Sheng, Y.; Wang, T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 2014, 30, 584–589. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Artemisia annua L.: Essential oil and acetone extract composition and antioxidant capacity. Ind. Crop. Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, K.; Zhang, L.; Wang, T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017, 69, 184–190. [Google Scholar] [CrossRef]
- Wan, X.L.; Song, Z.H.; Niu, Y.; Cheng, K.; Zhang, J.F.; Ahmad, H.; Zhang, L.L.; Wang, T. Evaluation of enzymatically treated Artemisia annua L. on growth performance, meat quality, and oxidative stability of breast and thigh muscles in broilers. Poult. Sci. 2017, 96, 844–850. [Google Scholar] [CrossRef]
- Wu, M.; Yu, Z.; Li, X.; Zhang, X.; Wang, S.; Yang, S.; Hu, L.; Liu, L. Paeonol for the treatment of atherosclerotic Cardiovascular disease: A pharmacological and mechanistic overview. Front. Cardiovasc. Med. 2021, 8, 690116. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Chen, Z.; Yang, X.D.; Deng, R.R.; Shi, L.X.; Yao, L.Y.; Xiang, D.X. Piperazine ferulate prevents high-glucose-induced filtration barrier injury of glomerular endothelial cells. Exp. Ther. Med. 2021, 22, 1175. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, N.; Ji, Y.; Tso, P.; Wu, Z. Weaning stress in piglets alters the expression of intestinal proteins involved in fat absorption. J. Nutr. 2022, 152, 2387–2395. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Z.; Tang, W.; Zhou, J.; Guo, L.; Gong, C.; Liu, G.; Wan, D.; Yin, Y. Dietary iron regulates intestinal goblet cell function and alleviates Salmonella typhimurium invasion in mice. Sci. China. Life Sci. 2023, 66, 2006–2019. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Wang, H.; Liang, S.; Li, X.; Yang, X.; Long, F.; Yang, X. Effects of encapsulated essential oils and organic acids on laying performance, egg quality, intestinal morphology, barrier function, and microflora count of hens during the early laying period. Poult. Sci. 2019, 98, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Ajuwon, K.M. Mechanism of endocytic regulation of intestinal tight junction remodeling during nutrient starvation in jejunal IPEC-J2 cells. FASEB J. 2021, 35, e21356. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. EBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Wang, X.; Huang, J.; Zhao, D.; Tan, Y.; Zhang, Z.; Zhang, Z.; Zhu, L.; Wu, B.; et al. Anti-Alzheimers molecular mechanism of icariin: Insights from gut microbiota, metabolomics, and network pharmacology. J. Transl. Med. 2023, 21, 277. [Google Scholar] [CrossRef]
- Su, Y.N.; Wang, M.J.; Yang, J.P.; Wu, X.L.; Xia, M.; Bao, M.H.; Ding, Y.B.; Feng, Q.; Fu, L.J. Effects of Yulin Tong Bu formula on modulating gut microbiota and fecal metabolite interactions in mice with polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1122709. [Google Scholar] [CrossRef]
- Spragge, F.; Bakkeren, E.; Jahn, M.T.; Araujo, E.B.N.; Pearson, C.F.; Wang, X.; Pankhurst, L.; Cunrath, O.; Foster, K.R. Microbiome diversity protects against pathogens by nutrient blocking. Science 2023, 382, eadj3502. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhu, S.; Ye, J.; Xu, Q.; Yi, T.; Wu, C.; Wang, B.; Luo, K.; Gao, W. Effects of dietary enzymatically treated Artemisia annua L. in low fish meal diet on growth, antioxidation, metabolism and intestinal health of Micropterus salmoides. Aquacult. Rep. 2023, 33, 101843. [Google Scholar] [CrossRef]
- He, G.; Sun, H.; Liao, R.; Wei, Y.; Zhang, T.; Chen, Y.; Lin, S. Effects of herbal extracts (Foeniculum vulgare and Artemisia annua) on growth, liver antioxidant capacity, intestinal morphology and microorganism of juvenile largemouth bass, Micropterus salmoides. Aquacult. Rep. 2022, 23, 101081. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Ali, M.; D’Souza, M.; Hughes, P.D.; Sulakhe, D.; Wang, A.Z.; Xie, B.; Yeasin, R.; Msall, M.E.; Andrews, B.; et al. Bacteroidota and Lachnospiraceae integration into the gut microbiome at key time points in early life are linked to infant neurodevelopment. Gut. Microbes 2021, 13, 1997560. [Google Scholar] [CrossRef]
- Zheng, X.; Nie, K.; Xu, Y.; Zhang, H.; Xie, F.; Xu, L.; Zhang, Z.; Ding, Y.; Yin, Z.; Zhang, X. Fecal microbial structure and metabolic profile in post-weaning diarrheic piglets. Genes 2023, 14, 1166. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A gentral, enigmatic component of the human gut microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Ricaboni, D.; Mailhe, M.; Khelaifia, S.; Raoult, D.; Million, M. Romboutsia timonensis, a new species isolated from human gut. New Microbes. New Infect. 2016, 12, 6–7. [Google Scholar] [CrossRef]
- Chen, X.; Su, X.; Li, J.; Yang, Y.; Wang, P.; Yan, F.; Yao, J.; Wu, S. Real-time monitoring of ruminal microbiota reveals their roles in dairy goats during subacute ruminal acidosis. npj Biofilms Microbiomes 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Arunasri, K.; Mohan, S.V. Chapter 2.3—Biofilms: Microbial Life on the Electrode Surface. In Microbial Electrochemical Technology (MET); Elsevier: Amsterdam, The Netherlands, 2019; pp. 295–313. [Google Scholar] [CrossRef]
- Arapovic, L.; Huang, Y.; Manell, E.; Verbeek, E.; Keeling, L.; Sun, L.; Landberg, R.; Lundh, T.; Lindberg, J.E.; Dicksved, J. Age rather than supplementation with Oat β-Glucan influences development of the intestinal microbiota and SCFA concentrations in suckling piglets. Animals 2023, 13, 1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, J.; Song, X.; Yang, M.; Wang, H.; Wu, Y. Feeding dietary fermentable fiber improved fecal microbial composition and increased acetic acid production in a nursery pig model. J. Anim. Sci. 2023, 101, skad260. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef]
| Items | Calculation Based on Sample Weight | Calculation Based on Dry Matter |
|---|---|---|
| Nutritional values 1 | ||
| Moisture (%) | 6.19 | |
| Dry matter (%) | 93.81 | |
| Crude ash (%) | 13.15 | 14.01 |
| Fat (%) | 1.51 | 1.61 |
| Crude protein (%) | 18.44 | 19.65 |
| Acid detergent lignin (%) | 4.50 | 4.80 |
| Crude fiber (%) | 13.02 | 13.88 |
| Acid detergent fiber (%) | 26.50 | 28.25 |
| Neutral detergent fiber (%) | 55.04 | 58.66 |
| Main active components 2 | ||
| Retention Time, Molecular weight | Relative content (air-dry basis, %) | |
| Alcohol extract of Artemisia annua L. residue | ||
| Rosmarinic acid | 22.54, 359.077 | 13.71 |
| Chrysosplenetin B | 23.957, 373.0928 | 3.61 |
| Scopoletin | 14.115, 191.0349 | 3.52 |
| Homovanillic acid | 18.766, 181.0506 | 2.35 |
| Water extract of Artemisia annua L. residue | ||
| Quinic acid | 1.453, 150.053 | 4.86 |
| Cryptochlorogenic acid | 10.495, 354.095 | 3.55 |
| 3-O-Caffeoylquinic acid methyl ester | 12.242, 368.109 | 5.16 |
| Artemisinin | 18.777, 282.146 | 12.33 |
| Ingredients | Content (%) | Calculated Nutrient Levels | Content |
|---|---|---|---|
| Corn (4.52% crude protein) | 48.97 | Digestible energy (MJ/kg) | 14.75 |
| Soybean meal (43% crude protein) | 13.40 | Crude protein (%) | 17.10 |
| Puffing corn powder | 10.00 | Calcium (%) | 0.43 |
| Extruded soybean | 10.00 | Total phosphorus (%) | 0.63 |
| Soybean oil | 4.00 | Available phosphorus (%) | 0.36 |
| Fish meal | 6.00 | Lysine (%) | 1.22 |
| Whey powder | 4.00 | Methionine (%) | 0.38 |
| Monocalcium phosphate | 0.60 | Methionine + cysteine (%) | 0.66 |
| Antioxidants | 0.20 | Analyzed nutrient levels 2 | |
| Limestone | 0.70 | Gross energy (MJ/kg) | 16.32 |
| Salt | 0.60 | Crude protein | 17.60 |
| Lysine (98%) | 0.30 | Calcium | 0.48 |
| Methionine | 0.15 | Total phosphorous | 0.66 |
| Threonine | 0.08 | Lysine | 1.28 |
| Premix 1 | 1.00 | Methionine | 0.32 |
| Methionine + cysteine | 0.64 | ||
| Total | 100.00 |
| Items 2 | Groups 1 | p Values | |||||
|---|---|---|---|---|---|---|---|
| BD | LAR | MAR | HAR | AR | Linear | Quadratic | |
| Initial body weight, kg | 7.48 ± 0.27 | 7.56 ± 0.59 | 7.76 ± 0.46 | 7.32 ± 0.64 | 0.988 | 0.835 | 0.914 |
| Final body weight, kg | 12.36 ± 0.43 | 13.40 ± 0.80 | 15.04 ± 1.05 | 12.08 ± 1.03 | 0.188 | 0.557 | 0.501 |
| ADG, g/d | 212.17 ± 27.22 a | 253.91 ± 58.14 ab | 316.52 ± 54.17 b | 206.96 ± 64.90 ac | 0.017 | 0.647 | 0.827 |
| ADFI, g/d | 588.36 ± 101.93 a | 648.54 ± 91.91 a | 730.30 ± 90.55 b | 719.42 ± 96.88 b | 0.014 | 0.019 | 0.064 |
| F/G ratio | 2.80 ± 0.36 | 2.70 ± 0.64 | 2.30 ± 0.43 | 3.90 ± 1.90 | 0.123 | 0.190 | 0.427 |
| Items 2 | Groups 1 | p Values | |||||
|---|---|---|---|---|---|---|---|
| BD | LAR | MAR | HAR | AR | Linear | Quadratic | |
| TP, g/L | 54.29 ± 1.10 a | 60.88 ± 2.52 bc | 61.88 ± 1.55 c | 55.68 ± 2.40 ab | 0.026 | 0.509 | 0.149 |
| ALB, g/L | 31.30 ± 1.90 | 35.56 ± 1.53 | 35.36 ± 1.73 | 34.39 ± 2.69 | 0.426 | 0.431 | 0.293 |
| GLU, mmol/L | 5.43 ± 0.21 | 6.11 ± 0.18 | 6.04 ± 0.27 | 5.74 ± 0.28 | 0.183 | 0.648 | 0.123 |
| TG, mmol/L | 0.64 ± 0.07 | 0.71 ± 0.06 | 0.80 ± 0.08 | 0.83 ± 0.11 | 0.324 | 0.080 | 0.179 |
| CHOL, mmol/L | 2.96 ± 0.13 | 3.29 ± 0.19 | 3.31 ± 0.18 | 3.30 ± 0.20 | 0.431 | 0.241 | 0.275 |
| LDL-C, mmol/L | 1.72 ± 0.09 | 1.97 ± 0.12 | 1.83 ± 0.17 | 1.87 ± 0.15 | 0.631 | 0.676 | 0.737 |
| HDL-C, mmol/L | 1.34 ± 0.05 | 1.44 ± 0.11 | 1.51 ± 0.13 | 1.53 ± 0.09 | 0.502 | 0.160 | 0.302 |
| ALT, U/L | 82.93 ± 6.99 | 93.84 ± 8.62 | 111.3 ± 12.59 | 93.93 ± 8.16 | 0.216 | 0.413 | 0.136 |
| AST, U/L | 43.63 ± 4.04 | 52 ± 4.88 | 53.75 ± 5.13 | 56.25 ± 4.40 | 0.264 | 0.077 | 0.143 |
| ALP, U/L | 274.75 ± 29.48 | 274.75 ± 19.69 | 306.38 ± 28.46 | 300.75 ± 29.59 | 0.766 | 0.402 | 0.662 |
| UN, nmol/mL | 4.24 ± 0.45 | 4.53 ± 0.64 | 4.4 ± 0.49 | 3.45 ± 0.45 | 0.461 | 0.203 | 0.270 |
| C3, g/L | 0.02 ± 0 a | 0.03 ± 0 b | 0.04 ± 0 b | 0.03 ± 0 b | 0.011 | 0.097 | 0.033 |
| C4, g/L | 0.03 ± 0 | 0.04 ± 0 | 0.03 ± 0 | 0.03 ± 0 | 0.600 | 0.969 | 0.602 |
| Items 2 | Groups 1 | p Values | |||||
|---|---|---|---|---|---|---|---|
| BD | LAR | MAR | HAR | AR | Linear | Quadratic | |
| IgA, µg/mL | 38.29 ± 1.47 a | 45.58 ± 1.26 b | 49.56 ± 1.34 b | 44.59 ± 2.69 b | 0.001 | 0.094 | 0.012 |
| IgG, µg/mL | 438.3 ± 27.48 | 438.55 ± 20.9 | 430.67 ± 15.88 | 442.43 ± 25.67 | 0.987 | 0.072 | 0.158 |
| IgM, µg/mL | 51.63 ± 2.47 | 51.93 ± 2.86 | 54.91 ± 2.23 | 52.66 ± 1.61 | 0.752 | 0.821 | 0.928 |
| IL-1β, ng/mL | 55.94 ± 3.22 | 51.9 ± 1.99 | 54.37 ± 3.03 | 54.4 ± 1.85 | 0.745 | 0.769 | 0.953 |
| IL-6, ng/mL | 868.25 ± 85.92 b | 797.4 ± 62.95 ab | 622.91 ± 19.67 a | 638.31 ± 20.55 a | 0.032 | 0.010 | 0.018 |
| TNF-α, pg/mL | 480.24 ± 31.28 | 449.94 ± 22.7 | 441.34 ± 13.06 | 453.5 ± 31.28 | 0.736 | 0.531 | 0.526 |
| Items 2 | Groups 1 | p Values | |||||
|---|---|---|---|---|---|---|---|
| BD | LAR | MAR | HAR | AR | Linear | Quadratic | |
| T-AOC, U/mL | 0.54 ± 0.02 | 0.56 ± 0.04 | 0.47 ± 0.04 | 0.51 ± 0.04 | 0.295 | 0.382 | 0.499 |
| GSH-Px, U/mL | 241.68 ± 22.87 | 246.13 ± 27.93 | 228.43 ± 23.84 | 218.47 ± 8.4 | 0.820 | 0.374 | 0.676 |
| CAT, U/mL | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.01 ± 0.002 | 0.004 ± 0 | 0.333 | 0.114 | 0.126 |
| SOD, U/mL | 8.07 ± 0.77 a | 11.67 ± 0.38 b | 11.96 ± 0.49 b | 11.96 ± 0.83 b | <0.001 | 0.003 | <0.001 |
| MAD, nmol/mL | 24.59 ± 0.47 | 24.25 ± 0.36 | 23.77 ± 0.21 | 24.02 ± 0.29 | 0.396 | 0.343 | 0.258 |
| Items | Groups 1 | p Values | |||||
|---|---|---|---|---|---|---|---|
| BD | LAR | MAR | HAR | AR | Linear | Quadratic | |
| Duodenum | |||||||
| Villus height, μm | 389.89 ± 12.80 a | 542.6 ± 17.05 d | 449.67 ± 12.73 b | 503.37 ± 13.76 c | <0.001 | <0.001 | <0.001 |
| Crypt depth, μm | 241.42 ± 8.45 a | 296.3 ± 12.97 c | 259.46 ± 8.53 ab | 285.37 ± 12 bc | 0.001 | 0.023 | 0.046 |
| Villus height/crypt depth | 1.7 ± 0.05a | 2.03 ± 0.10 b | 1.88 ± 0.07 ab | 1.99 ± 0.10 b | 0.016 | 0.034 | 0.048 |
| Jejunum | |||||||
| Villus height, μm | 382.23 ± 9.92 a | 370.53 ± 8.47 a | 427.7 ± 10.01 b | 462.66 ± 11.52 c | <0.001 | <0.001 | <0.001 |
| Crypt depth, μm | 189.56 ± 6.53 a | 227.3 ± 6.84 b | 215.66 ± 6.34 b | 250.5 ± 8.77 c | <0.001 | <0.001 | <0.001 |
| Villus height/crypt depth | 2.26 ± 0.11 b | 1.7 ± 0.08 a | 2.06 ± 0.08 b | 2 ± 0.09 ab | 0.001 | 0.267 | 0.136 |
| Ileum | |||||||
| Villus height, μm | 445.47 ± 6.74 | 428.99 ± 5.85 | 407.14 ± 9.17 | 392.36 ± 10.39 | 0.265 | 0.126 | 0.214 |
| Crypt depth, μm | 265.39 ± 5.79 b | 252.84 ± 5.99 b | 214.42 ± 7.72 a | 230.77 ± 6.51 a | <0.001 | <0.001 | <0.001 |
| Villus height/crypt depth | 1.76 ± 0.05 a | 1.79 ± 0.05 a | 2.07 ± 0.12 b | 1.84 ± 0.05 a | 0.032 | 0.323 | 0.009 |
| Items (Y) | Correlation, p-Value | Optimum Addition Amount of Artemisia annua Residue (X, %) |
|---|---|---|
| Jejunum | ||
| Occludin mRNA expression | Linear model: Y = 0.942 − 0.077 X, p = 0.019 Quadratic model: Y = 0.021 X2 − 0.163 X + 0.983, p = 0.049 | 3.88 |
| Ileum | ||
| MUC2 mRNA expression | Linear model: Y = 0.721 + 0.637 X, p = 0.005 Quadratic model: Y = 0.077 X2 − 0.320 X + 0.872, p = 0.019 | 2.08 |
| Colon | ||
| MUC2 mRNA expression | Linear model: Y = 0.732 + 0.426 X, p = 0.016 Quadratic model: Y = 0.046 X2 − 0.350 X + 0.776, p = 0.026 | 3.80 |
| Items | Groups 1 | p Values | |||||
|---|---|---|---|---|---|---|---|
| BD | LAR | MAR | HAR | AR | Linear | Quadratic | |
| Acetic acid, µg/g | 1954.47 ± 101.530 a | 2042.85 ± 92.29 a | 2493.15 ± 140.96 b | 2017.79 ± 138.51 a | 0.005 | 0.524 | 0.013 |
| Propionic acid, µg/g | 935.81 ± 64.82 | 964.80 ± 61.46 | 1031.80 ± 64.52 | 992.85 ± 103.38 | 0.810 | 0.532 | 0.656 |
| Butyric acid, µg/g | 677.71 ± 53.52 a | 728.14 ± 57.33 ab | 924.67 ± 57.95 b | 715.08 ± 94.63 ab | 0.039 | 0.549 | 0.046 |
| Valeric acid, µg/g | 129.58 ± 15.01 | 182.81 ± 29.67 | 161.95 ± 23.60 | 130.31 ± 16.66 | 0.251 | 0.831 | 0.497 |
| Isobutyric acid, µg/g | 55.22 ± 3.06 | 58.84 ± 5.99 | 54.32 ± 4.98 | 55.23 ± 7.14 | 0.938 | 0.864 | 0.981 |
| Isopentanoic acid, µg/g | 126.31 ± 11.32 | 147.28 ± 14.69 | 149.33 ± 10.51 | 126.19 ± 17.58 | 0.466 | 0.658 | 0.719 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Bai, M.; Xing, Y.; Liu, J.; Xu, K.; Xiong, X.; Liu, H.; Yin, Y. Artemisia annua Residue Regulates Immunity, Antioxidant Ability, Intestinal Barrier Function, and Microbial Structure in Weaned Piglets. Animals 2024, 14, 3569. https://doi.org/10.3390/ani14243569
Hu J, Bai M, Xing Y, Liu J, Xu K, Xiong X, Liu H, Yin Y. Artemisia annua Residue Regulates Immunity, Antioxidant Ability, Intestinal Barrier Function, and Microbial Structure in Weaned Piglets. Animals. 2024; 14(24):3569. https://doi.org/10.3390/ani14243569
Chicago/Turabian StyleHu, Jinjie, Miaomiao Bai, Yueyao Xing, Junhong Liu, Kang Xu, Xia Xiong, Hongnan Liu, and Yulong Yin. 2024. "Artemisia annua Residue Regulates Immunity, Antioxidant Ability, Intestinal Barrier Function, and Microbial Structure in Weaned Piglets" Animals 14, no. 24: 3569. https://doi.org/10.3390/ani14243569
APA StyleHu, J., Bai, M., Xing, Y., Liu, J., Xu, K., Xiong, X., Liu, H., & Yin, Y. (2024). Artemisia annua Residue Regulates Immunity, Antioxidant Ability, Intestinal Barrier Function, and Microbial Structure in Weaned Piglets. Animals, 14(24), 3569. https://doi.org/10.3390/ani14243569





