Study of Variation of ACOX1 Gene Among Different Horse Breeds Maintained in Iran
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
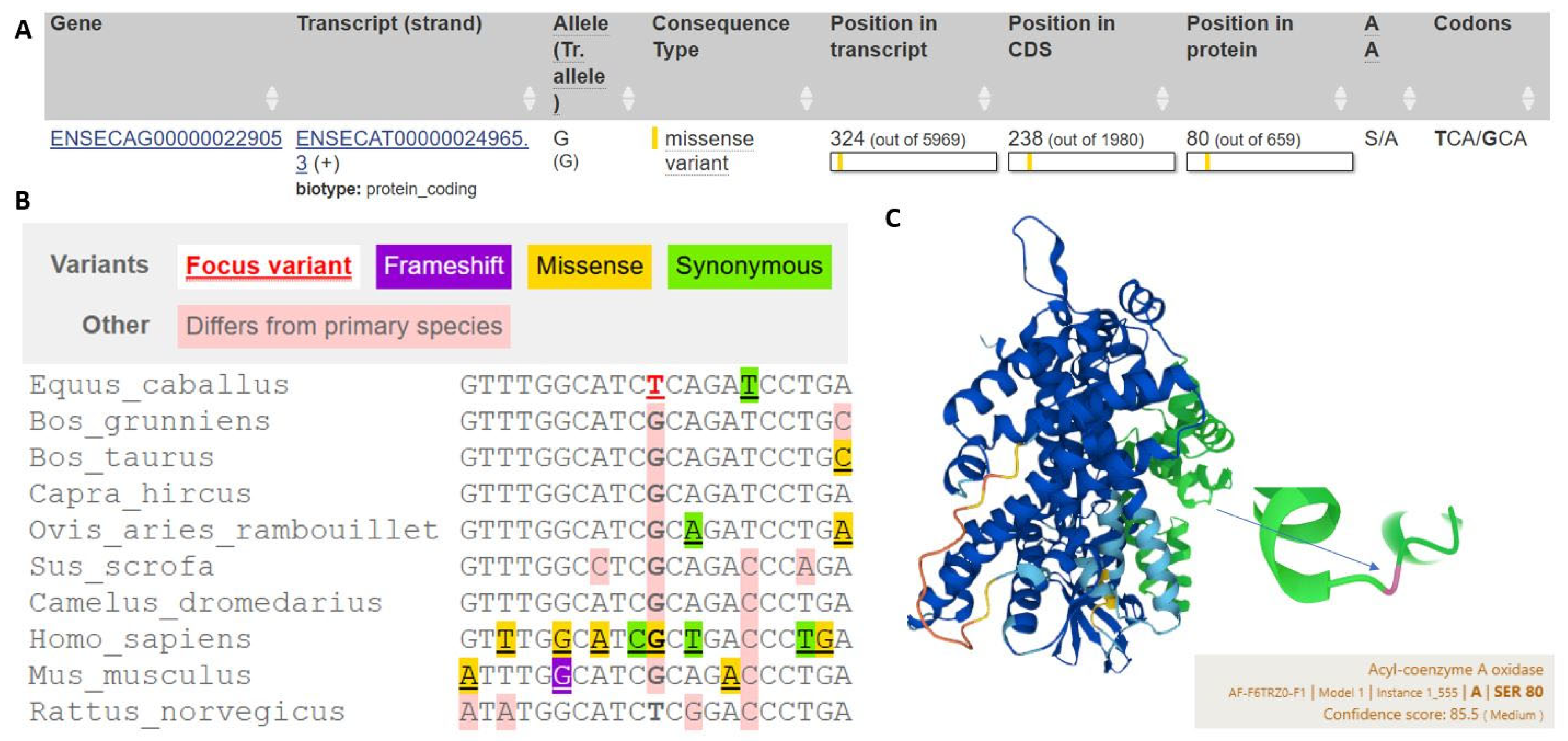
2.2. Identification of ACOX1 Polymorphism and Genotyping
3. Results
3.1. Genotype Distribution
3.2. The Chi-Square Tests for Independence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lönker, N.S.; Fechner, K.; Wahed, A.A.E. Horses as a Crucial Part of One Health. Vet. Sci. 2020, 7, 28. [Google Scholar] [CrossRef]
- Benedetti, B.; Felici, M.; Nanni Costa, L.; Padalino, B. A review of horse welfare literature from 1980 to 2023 with a text mining and topic analysis approach. Ital. J. Anim. Sci. 2023, 22, 1095–1109. [Google Scholar] [CrossRef]
- Endenburg, N. Perceptions and attitudes towards horses in European societies. Equine Vet. J. Suppl. 1999, 31, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.K.; Friend, T.H.; Evans, J.W.; Bushong, D.M. Behavioral assessment of horses in therapeutic riding programs. Appl. Anim. Behav. Sci. 1999, 63, 11–24. [Google Scholar] [CrossRef]
- Hanot, P.; Bayarsaikhan, J.; Guintard, C.; Haruda, A.; Mijiddorj, E.; Schafberg, R.; Taylor, W. Cranial shape diversification in horses: Variation and covariation patterns under the impact of artificial selection. BMC Ecol. Evol. 2021, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Warmuth, V.; Manica, A.; Eriksson, A.; Barker, G.; Bower, M. Autosomal genetic diversity in non-breed horses from eastern Eurasia provides insights into historical population movements. Anim. Genet. 2013, 44, 53–61. [Google Scholar] [CrossRef]
- Nagel, H.J.; Savier, M.M. The Arabian Horse. Nature’s Creation and the Arte of Breeding; Nawal Media: Spoleto, Italy, 2013. [Google Scholar]
- Ricard, A.; Robert, C.; Blouin, C.; Baste, F.; Torquet, G.; Morgenthaler, C.; Rivière, J.; Mach, N.; Mata, X.; Schibler, L.; et al. Endurance Exercise Ability in the Horse: A Trait with Complex Polygenic Determinism. Front. Genet. 2017, 8, 89. [Google Scholar] [CrossRef]
- Rovere, G.; Ducro, B.J.; van Arendonk, J.A.; Norberg, E.; Madsen, P. Analysis of competition performance in dressage and show jumping of Dutch Warmblood horses. J. Anim. Breed Genet. 2016, 133, 503–512. [Google Scholar] [CrossRef]
- Cassidy, R. The Sport of Kings: Kinship, Class and Thoroughbred Breeding in Newmarket; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Gaffney, B.; Cunningham, E.P. Estimation of genetic trend in racing performance of Thoroughbred horses. Nature 1988, 332, 722–724. [Google Scholar] [CrossRef]
- Katz, L.M.; Bayly, W.M.; Hines, M.T.; Sides, R.H. Differences in the ventilatory responses of horses and ponies to exercise of varying intensities. Equine Vet. J. Suppl. 1999, 31, 49–51. [Google Scholar] [CrossRef]
- Pösö, A.R.; Essén-Gustavsson, B.; Persson, S.G. Metabolic response to standardised exercise test in standardbred trotters with red cell hypervolaemia. Equine Vet. J. 1993, 25, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-D.; Park, J.; Ko, J.; Kim, B.C.; Kim, H.-S.; Ahn, K.; Do, K.-T.; Choi, H.; Kim, H.-M.; Song, S.; et al. Whole transcriptome analyses of six Thoroughbred horses before and after exercise using RNA-Seq. BMC Genom. 2012, 13, 473. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Mianji, G.; Nejati-Javaremi, A.; Farhadi, A. Genetic diversity, parentage verification and genetic bottlenecks evaluation in Iranian Turkmen horse breed. Genetika 2015, 51, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Pluta, M.; Bańka, K.; Ciesla, A.; Rogala, Ł. The state of breeding and use of Caspian horses in Europe and around the world. Acta Sci. Pol. Zootech. 2021, 19, 79–84. [Google Scholar] [CrossRef]
- Firouz, L. The Caspian Miniature Horse of Iran; International Caspian Stud Book: Lancashire, UK, 1972. [Google Scholar]
- Yousefi, N.; Mehrabani, H.; Nejati Javaremi, A.; Maloufi, F. A novel approach to establish breed type and standards for an equine breed: Persian Kurdish horse. J. Agric. Sci. Technol. 2020, 22, 1219–1233. [Google Scholar]
- Myćka, G.; Musiał, A.D.; Stefaniuk-Szmukier, M.; Piórkowska, K.; Ropka-Molik, K. Variability of ACOX1 gene polymorphisms across different horse breeds with regard to selection pressure. Animals 2020, 10, 2225. [Google Scholar] [CrossRef]
- Al Abri, M.; Holl, H.; Kalla, S.; Sutter, N.; Brooks, S. Whole genome detection of sequence and structural polymorphism in six diverse horses. PLoS ONE 2020, 15, e0230899. [Google Scholar] [CrossRef]
- Głażewska, I. Speculations on the origin of the Arabian horse breed. Livest. Sci. 2010, 129, 49–55. [Google Scholar] [CrossRef]
- Varanasi, U.; Chu, R.; Chu, S.; Espinosa, R.; LeBeau, M.M.; Reddy, J.K. Isolation of the human peroxisomal acyl-CoA oxidase gene: Organization, promoter analysis, and chromosomal localization. Proc. Natl. Acad. Sci. USA 1994, 91, 3107–3111. [Google Scholar] [CrossRef]
- Poirier, Y.; Antonenkov, V.D.; Glumoff, T.; Hiltunen, J.K. Peroxisomal β-oxidation—A metabolic pathway with multiple functions. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 1413–1426. [Google Scholar] [CrossRef]
- Barish, G.D.; Narkar, V.A.; Evans, R.M. PPAR delta: A dagger in the heart of the metabolic syndrome. J. Clin. Investig. 2006, 116, 590–597. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, H.I.; Vluggens, A.; Andreoletti, P.; Ragot, K.; Mandard, S.; Kersten, S.; Waterham, H.R.; Lizard, G.; Wanders, R.J.A.; Reddy, J.K.; et al. The inflammatory response in acyl-CoA oxidase 1 deficiency (pseudoneonatal adrenoleukodystrophy). Endocrinology 2012, 153, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Detilleux, J.; Cello, C.; Amory, H.; Marcillaud-Pitel, C.; Richard, E.; Van Galen, G.; Van Loon, G.; Lefère, L.; Votion, D.-M. Acylcarnitines profile best predicts survival in horses with atypical myopathy. PLoS ONE 2017, 12, e0182761. [Google Scholar] [CrossRef] [PubMed]
- Fontanel, M.; Todd, E.; Drabbe, A.; Ropka-Molik, K.; Stefaniuk-Szmukier, M.; Myćka, G.; Velie, B.D. Variation in the SLC16A1 and the ACOX1 genes is associated with gallop racing performance in Arabian horses. J. Equine Vet. Sci. 2020, 93, 103202. [Google Scholar] [CrossRef]
- Parker, K.L.; Gillingham, M.P.; Hanley, T.A.; Robbins, C.T. Seasonal patterns in body mass, body composition, and water transfer rates of free-ranging and captive black-tailed deer (Odocoileus hemionus sitkensis) in Alaska. Can. J. Zool. 1993, 71, 1397–1404. [Google Scholar] [CrossRef]
- Gu, J.; Orr, N.; Park, S.D.; Katz, L.M.; Sulimova, G.; MacHugh, D.E.; Hill, E.W. A genome scan for positive selection in Thoroughbred horses. PLoS ONE 2009, 4, e5767. [Google Scholar] [CrossRef]


| TT | GG | TG | Number of Horses | G * | T * | HWE | ||
|---|---|---|---|---|---|---|---|---|
| Arabian | 6 (7%) | 63 (70%) | 21 (23%) | 90 | 0.82 | 0.18 | 0.03 | |
| Caspian | 2 (10%) | 11 (52%) | 8 (38% | 21 | 0.71 | 0.29 | 0.75 | |
| KWPN | 10 (20%) | 31 (62%) | 9 (18%) | 50 | 0.71 | 0.29 | 0.000069 | |
| HORSE BREEDS | Kurdish | 1 (2%) | 63 (86%) | 9 (12%) | 73 | 0.92 | 0.08 | 0.32 |
| Thoroughbred | 8 (23%) | 16 (46%) | 11 (31%) | 35 | 0.61 | 0.39 | 0.046 | |
| Turkmen | 13 (24%) | 21 (38%) | 21 (38%) | 55 | 0.57 | 0.43 | 0.102 | |
| TOTAL | 40 (12.34%) | 205 (63.27%) | 79 (24.38%) | 324 | 0.75 | 0.25 | 0.000000 |
| Arabian | Caspian | KWPN | Kurdish | Thoroughbred | Turkmen | |
|---|---|---|---|---|---|---|
| Arabian | ---- | |||||
| Caspian | ns | ----- | ||||
| KWPN | ns | ns | ---- | |||
| Kurdish | 0.0356 | 0.0028 | 0.0007 | ---- | ||
| Thoroughbred | 0.0117 | ns | ns | <0.0001 | ---- | |
| Turkmen | 0.0003 | ns | 0.0319 | <0.0001 | ns | ---- |
| Genotypes | Sex | |||
| Mare Count | Stallion Count | |||
| GG | Arabian | 35 | 28 | |
| Caspian | 7 | 4 | ||
| KWPN | 14 | 17 | ||
| Kurdish | 21 | 42 | ||
| Thoroughbred | 4 | 12 | ||
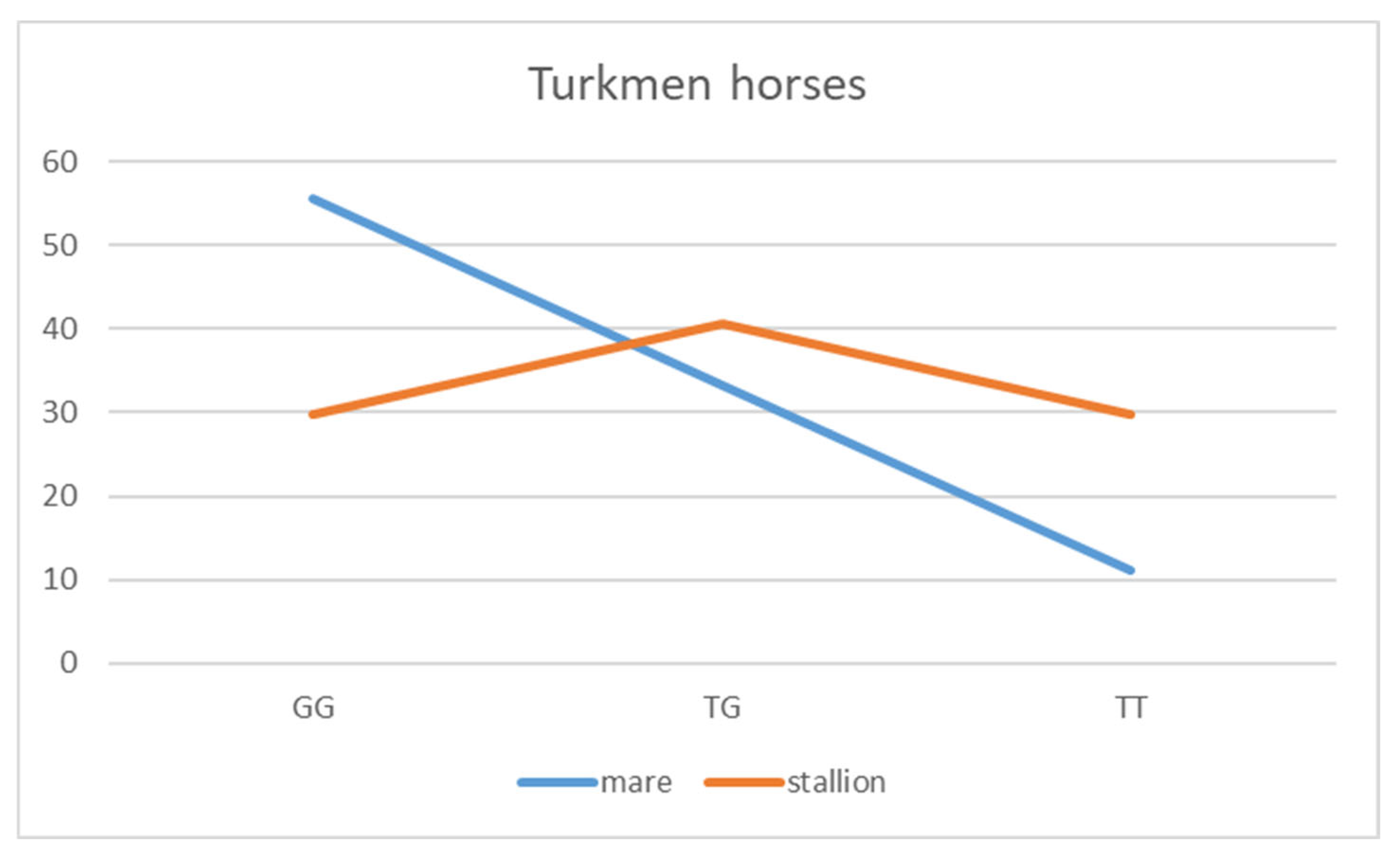
| Turkmen | 10 (55.5) | 11 (29.7) | ||
| TG | Arabian | 12 | 9 | |
| Caspian | 4 | 4 | ||
| KWPN | 6 | 3 | ||
| Kurdish | 4 | 5 | ||
| Thoroughbred | 4 | 7 | ||
| Turkmen | 6 (33.3) | 15 (40.6) | ||
| TT | Arabian | 3 | 3 | |
| Caspian | 1 | 1 | ||
| KWPN | 4 | 6 | ||
| Kurdish | 0 | 1 | ||
| Thoroughbred | 3 | 5 | ||
| Turkmen | 2 (11.1) | 11 (29.7) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boozarjomehri Amnieh, S.; Hassanpour, A.; Moghaddam, S.; Sakhaee, F.; Ropka-Molik, K. Study of Variation of ACOX1 Gene Among Different Horse Breeds Maintained in Iran. Animals 2024, 14, 3566. https://doi.org/10.3390/ani14243566
Boozarjomehri Amnieh S, Hassanpour A, Moghaddam S, Sakhaee F, Ropka-Molik K. Study of Variation of ACOX1 Gene Among Different Horse Breeds Maintained in Iran. Animals. 2024; 14(24):3566. https://doi.org/10.3390/ani14243566
Chicago/Turabian StyleBoozarjomehri Amnieh, Shayan, Ali Hassanpour, Sina Moghaddam, Fatemeh Sakhaee, and Katarzyna Ropka-Molik. 2024. "Study of Variation of ACOX1 Gene Among Different Horse Breeds Maintained in Iran" Animals 14, no. 24: 3566. https://doi.org/10.3390/ani14243566
APA StyleBoozarjomehri Amnieh, S., Hassanpour, A., Moghaddam, S., Sakhaee, F., & Ropka-Molik, K. (2024). Study of Variation of ACOX1 Gene Among Different Horse Breeds Maintained in Iran. Animals, 14(24), 3566. https://doi.org/10.3390/ani14243566





