4. Discussion
In this study, we explored the effects of adding L. paracasei as a probiotic feed supplement to broilers using formulations with different concentrations of a single-strain product (L. paracasei LK01).
Several studies have demonstrated that lactobacilli species as dietary supplements have an enhancing effect on broiler growth performance [
6,
19,
33]. However, the growth effect varies due to many factors such as the source of the strain, the vitality and concentration of the bacteria used, the administration method, and experimental conditions [
19,
34,
35]. A previous study found that when broilers are fed a mixture of
Lactobacillus plantarum and
Lactobacillus rhamnosus, their ADG begins to be significantly higher than that of the control group after 2 weeks [
36]. Kalavathy et al. [
37] found that dietary supplementation with 1% of a 12-strain lactic acid bacteria mixture increased the ADG of broilers from 1 to 42 days of age compared to the control group. Peng et al. [
38] showed that supplementing the broiler diet with 2 × 10
9 CFU/kg
Lactobacillus plantarum B1 can significantly increase ADG from 1 to 42 days compared to the control group. Another study found that the body weight of 1-day-old male broiler chickens fed a probiotic combination of 2 × 10
6 CFU/g brewer’s yeast and 1 × 10
7 CFU/g fermented lactobacillus was higher than that of the control group on the 21st day [
39]. This is similar to our research results, which found that all experimental groups increased the body weight of broilers from 0 to 21 days, and that 10
6 CFU/kg group had the best growth effect. In addition, the FCR of the 10
6, 10
7, and 10
8 CFU/kg groups was significantly reduced during the period from 28 to 35 days.
Serum biochemical indices are important indicators of nutritional metabolism and stress status of the body. In the process of hepatotoxicity, damaged hepatocytes release the liver-specific enzymes ALT and AST into the bloodstream, which then leads to elevation of these two enzymes in the serum [
40]. Yilmaz et al. [
41] found that sustained chronic heat stress increased serum TG and TC as well as ALT and AST activities and decreased TP and ALB levels. Dietary supplementation of broiler chickens with a variety of
Lactobacillus strains of synbiotics was found to significantly reduce serum AST activity [
42]. Another study found that dietary supplementation with a mixture of
Bacillus licheniformis and
Bacillus coagulans significantly reduced serum ALT and AST activities and UA levels [
43]. This is similar to our findings, which showed that serum AST levels were significantly reduced in groups 10
6, 10
8, and 10
10 compared to the CON group; serum ALT levels were also significantly reduced in group 10
8. In addition, UA levels of uric acid were lower than those in the CON group in all experimental groups, and the difference was significant in groups 10
7, 10
8, and 10
9, whereas UA is a nitrogenous excretion product of protein metabolism in poultry, and its serum level is also a direct response to metabolic stress in the kidney [
44,
45]. Therefore, our results indicate that dietary supplementation with
L. paracasei LK01 may have a certain effect on improving the liver function of broilers and may reduce serum non-protein nitrogen (UA) to reduce the stress on the kidneys in poultry.
In addition, we found that except for the 10
10 CFU/kg group, the TC levels of all experimental groups were significantly lower than those of the CON group, and the TG levels of the 10
6 and 10
7 groups were significantly lower than those of the CON group. This is similar to the results of several other studies, which found that supplementing the diet of broilers with a 0.1% lactic acid bacteria mixture significantly reduced serum TC and TG levels [
46]. Shokryazdan et al. [
47] found that dietary supplementation of broilers with 0.5 or 1 g/kg of a mixture of Lactobacillus salivarius both resulted in significantly lower serum TC and TG concentrations in broilers. Elleithy et al. [
43] found that supplementing the diet of broiler chickens with a mixture of
Bacillus licheniformis and
Bacillus coagulans significantly reduced serum TC levels. Another study showed that dietary supplementation of broiler chickens with
Bacillus amyloliquefaciens significantly reduced serum TG and TC levels [
48]. In addition, our results showed that the ALP and TP levels in all experimental groups were slightly higher than those in the control group, but the difference was not significant. ALP is not only a biomarker of the hepatobiliary system; it is also involved in bone formation [
40]. In the event of tissue damage, TP can be used as a raw material to repair and maintain the body’s metabolism [
49]. The levels of TC and TG in the blood serum are often used as important indicators of the body’s lipid metabolism [
50]. Therefore, we hypothesized that dietary supplementation of broiler chickens with
L. paracasei LK01 would help to enhance lipid metabolism, promote bone growth and metabolism, and thereby increase body weight.
Interleukins (IL-1β, IL-2, IL-6) are cytokines that leukocytes interact with during the immune response and have the role of transmitting information, activating and regulating immune cells [
51]. TNF-α is an innate immune-associated cytokine with pro-inflammatory properties that are critical for host defense, induction of inflammation, and triggering of apoptosis [
52]. In vitro studies have shown that
L. paracasei can relieve lipopolysaccharide (LPS)-induced cell inflammation by reducing the expression of pro-inflammatory cytokines IL-1β, IL-2, and TNF-α and increasing the expression of the anti-inflammatory cytokine interleukin-10 (IL-10) [
24,
25]. In vivo studies have found that supplementing mice with
L. paracasei PS23 significantly increases serum IL-10 levels and exhibits lower serum corticosterone levels [
53]. In addition, Kang et al. [
54] found that supplementing the diet of broilers with 10
7 CFU/g of
L. paracasei XLK401 significantly reduced the level of the pro-inflammatory factor IL-6 in the serum. Xiao et al. [
35] found that drinking water supplemented with 2 × 10
8 CFU/L
Lactobacillus plantarum HJZW08 significantly reduced serum IL-2, IL-1β, IL-6, and TNF-α levels in broiler chickens. Our results showed that IL-1β levels decreased significantly in all experimental groups except 10
10 group; TNF-α levels decreased significantly in the 10
7, 10
8, 10
9, and 10
10 groups.
In addition, we found that serum immunoglobulin (IgA, IgM, and IgG) levels were higher in the experimental group than in the control group, but the differences in IgA and IgG were not significant, whereas serum IgM levels were significantly higher in the 10
6 and 10
7 groups. The results of Zhang et al. [
2] in
Bacillus coagulans are almost the same as ours; his study found that feeding Bacillus coagulans to broiler chickens significantly reduced serum pro-inflammatory factors (IL-1β, IL-6, and TNF-α) levels, increased anti-inflammatory factor (IL-10) concentrations, and significantly elevated serum immunoglobulin (IgA, IgM, and IgY) levels. In addition, several studies have shown that probiotics can promote the production of relevant immunoglobulins (IgG, IgA, and IgM), thus enhancing the immunity of chickens [
55,
56,
57]. Therefore, we hypothesized that supplementation with
L. paracasei LK01 could likewise reduce inflammation by decreasing inflammatory gene expression, or increasing the production of anti-inflammatory factors, as with other probiotics, and could improve the immune function of the body by stimulating B-lymphocytes to enhance the production of immunoglobulins (IgG, IgA, and IgM).
T-SOD scavenges free radicals and protects cells from damage; MDA is a marker of oxidative stress and reflects the degree of lipid peroxidation in the body [
55,
56,
57,
58]. Studies have shown that sustained chronic heat stress increases the levels of MDA in blood and tissues and increases the activity of antioxidant enzymes [
41]. In contrast, Liu et al. [
59] found that the addition of 5 × 10
8 cfu/kg of
Bacillus subtilis HC6 to broiler diets significantly increased serum levels of T-AOC and T-SOD. T-AOC represents the total antioxidant level composed of various antioxidant substances and antioxidant enzymes, etc., and the higher its value, the higher the antioxidant capacity of broilers. In addition, in a study of the antioxidant effect of
Lactobacillus plantarum on broilers with necrotic enteritis, it was found that the MDA content of the serum in the experimental group was reduced and the T-SOD activity was increased [
60]. In addition, Chen et al. found that adding aflatoxin to the feed increased the MDA content and decreased the T-SOD activity in the livers of broiler chickens, while adding
Lactobacillus salivarius increased the activity of antioxidant enzymes and decreased the MDA content [
61]. Our results showed that the MDA level of the experimental group was significantly lower than that of the control group; in addition, the T-AOC and T-SOD levels of the experimental group were higher than those of the control group, but the difference was not significant. This suggests that dietary supplementation with
L. paracasei LK01 may be similarly characterized to enhance antioxidant function in broilers.
The intestinal villi are located in the finger-like projections of the small intestinal epithelium and lamina propria that bulge into the intestinal lumen, which not only have the role of nutrient absorption, but also the villus oscillation pushes pathogenic microorganisms out of the way and filters out the harmful factors effectively [
62]. Therefore, maintaining the integrity of the small intestinal epithelium is crucial for nutrient digestion and absorption, in which villus height and crypt depth are key factors [
63,
64]. A previous study found that dietary supplementation of broiler chickens with
Bacillus coagulans and
Bacillus licheniformis significantly elevated duodenal, jejunal, and intestinal villus lengths and significantly decreased duodenal crypt depth [
43]. Another study found that dietary supplementation of broiler chickens with
Bacillus subtilis HC6 increased jejunal and ileal villus heights and increased ileal V/C values [
59]. Song et al. [
65] showed that dietary supplementation with a mixture of
Bacillus licheniformis,
Bacillus subtilis, and
Lactobacillus plantarum increased jejunal villus height and improved partial intestinal barrier function in broilers. In addition, Gyawali et al. [
20] found that dietary supplementation of broiler chickens with a novel
L. paracasei capsule significantly elevated villus height in all intestinal sections and enhanced V/C values. These studies showed the positive effects of probiotics on the broiler intestine, namely, an increase in villus height and V/C value and a decrease in crypt depth. In our study, we found no significant changes in villus height in all intestinal segments of broilers, but an improvement in crypt depth in some experimental groups compared to the control group was observed. In addition, group 10
6 significantly increased the V/C value of the jejunum and ileum. This means that
L. paracasei LK01 added to the diet can have a positive effect on the digestion and absorption of nutrients by maintaining the morphological health of the intestines, which is reflected to some extent in the lower FCR during the 28–35 d period.
Lipase, amylase, and protease, respectively, break down triglycerides, starch, and proteins; they promote the digestion and absorption of nutrients such as lipids, carbohydrates, and amino acids [
66,
67]. Therefore, the magnitude of activity of digestive enzymes is also one of the key factors affecting the growth and development of the organism. A previous study found that supplementing broiler diets with
Lactobacillus johnsonii BS15 significantly increased protease and lipase activity in the small intestine at 21 days and lipase activity in the ileum at 42 days [
68]. Jin et al. [
69] found that a diet containing a mixture of 12 lactic acid bacteria significantly increased the level of amylase in the small intestine of chickens, but did not affect the activity of proteases and lipases in the small intestine. In addition, Wang et al. [
70] found that supplementing broiler diets with
Bacillus coagulans significantly increased the activity of protease and amylase in the duodenum. In our study, group 10
6 significantly increased the protease activity of the duodenum, jejunum, and ileum, while group 10
10 significantly increased the protease activity of the duodenum and jejunum. This suggests that dietary supplementation with
L. paracasei LK01 has the potential to promote protease secretion in the small intestine of broilers.
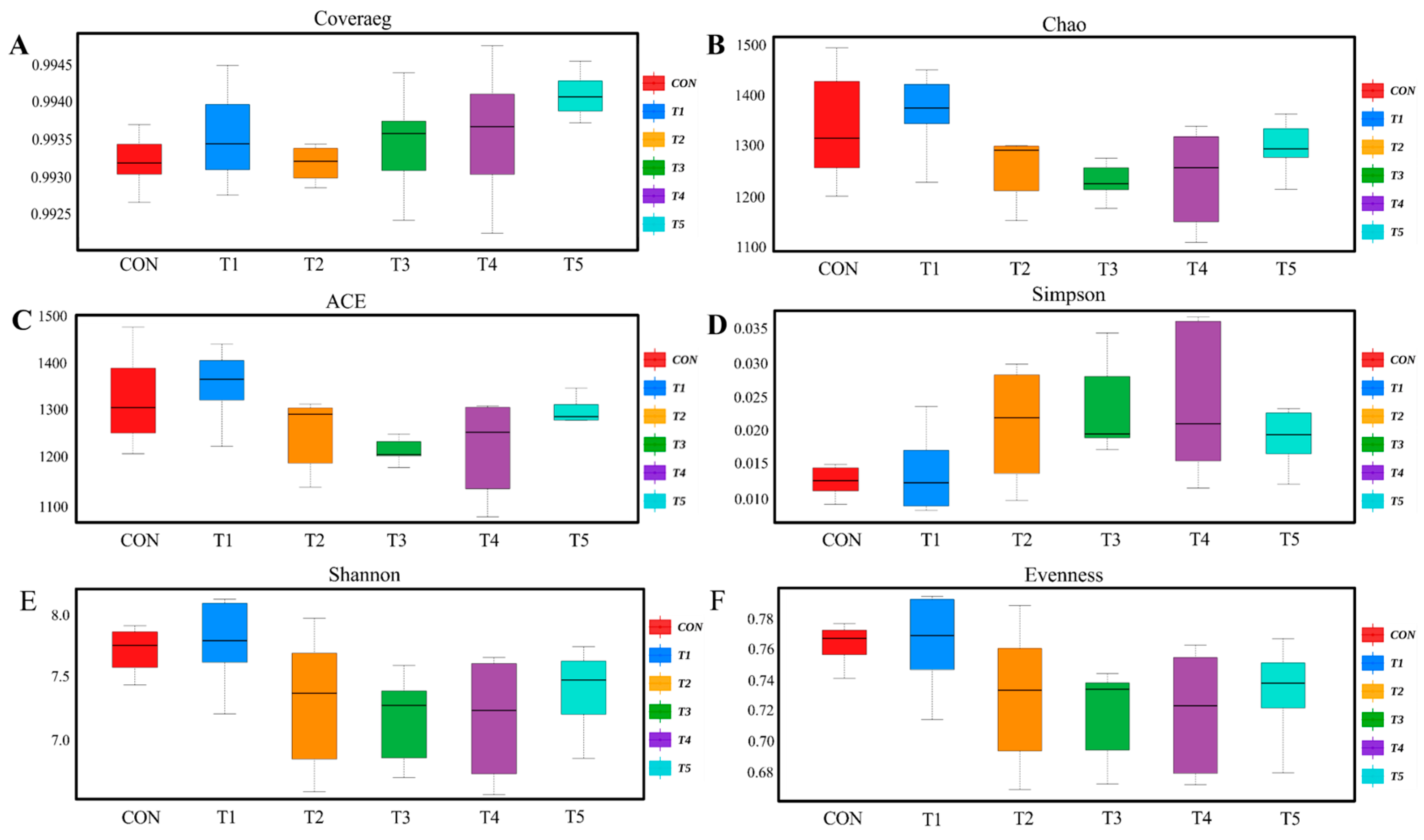
The cecum is the most microbiologically diverse region of the gastrointestinal tract and possesses a complex, diverse, and stable microbial community. And the gut microbiota is not only an important barrier against invasive substances, but also regulates symbiotic homeostasis and normal physiological processes [
71]. Therefore, the balance of intestinal microorganisms is of great importance in the production of livestock and poultry, and it is directly related to their health and production performance [
72]. Previous studies have found that supplementing broiler diets with
Bacillus subtilis had no effect on the α diversity index of the cecal flora, but significantly increased the β diversity index of the cecal microbiota [
59,
73]. Zhang et al. [
2] showed the same results on
Bacillus coagulans, where no significant changes in Shannon and Simpson indices were found in the experimental group, but the β diversity in the scatter plots of the principal component analysis and principal coordinate analysis showed a significant separation between the experimental group and the control group. Another study showed that adding 500 ppm encapsulated
L. paracasei microcapsules to the diet of broilers had no effect on the α diversity index of the cecal flora but changed the structure and aggregation of the cecal flora [
20]. Our research results are similar to this.
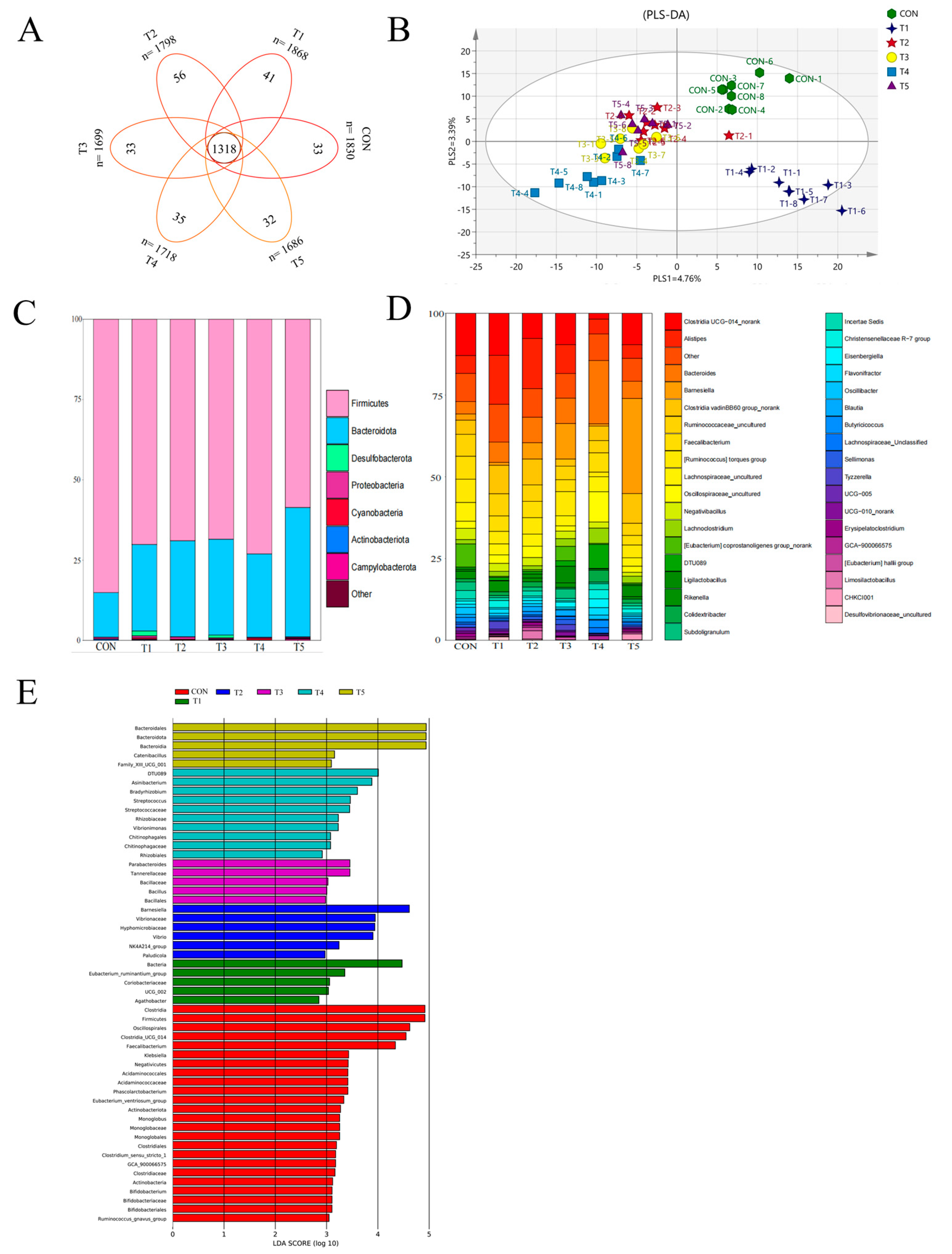
The PLS-DA plot (
Figure 2B) shows that the dietary supplement
L. paracasei LK01 caused a significant change in the microbial profile. It is well known that some bacteria in
Firmicutes help to promote the digestion of cellulose in food and participate in a variety of metabolic pathways in the gut, so they are extremely important for the health of the host [
74]. In contrast,
Bacteroidetes phylum is mainly responsible for catabolizing polysaccharides and dietary fibers in the intestinal tract to produce short-chain fatty acids (SCFAs) such as acetic acid, propionic acid, and butyric acid, which are an important source of energy for the intestinal epithelial cells, as well as positively affecting the maintenance of the intestinal barrier function and the regulation of the immune system [
75]. In addition, a lower ratio of
Firmicutes to
Bacteroidetes (F/B), which is usually associated with a high-fiber diet, is considered a healthier state of the intestinal microbiota and is associated with a lower risk of metabolic diseases [
76]. Our results showed that the experimental group increased the proportion of
Bacteroidetes and significantly reduced the F/B value. In addition, 10
6 group also increased the proportion of Cyanobacteria and Desulfobacterota (
Figure 2C). This is similar to the results of Xu et al. [
7], where the cecal flora of broilers fed
L. paracasei was dominated by
Firmicutes and
Bacteroidetes, accounting for more than 85% of the total number of microorganisms. Thus, our results suggest that dietary supplementation with
L. paracasei LK01 optimized the structure of the cecum flora. At the genus level, we found that
ClostridiaUCG-014 and
Alistipes were the dominant bacteria (
Figure 2D,E); in addition, the 10
6 group significantly increased the abundance of beneficial bacteria such as
Ruminococcaceae,
Lachnospiraceae, and
Faecalibacterium, compared to the CON group. Some Clostridia are very important in the gut, such as butyric acid-producing
Clostridium, which can help maintain the health of intestinal epithelial cells, enhance intestinal barrier function, and regulate the immune system [
77], whereas
Alistipes, a major member of the
Rikenellaceae family, is a bile-resistant organism capable of producing fibrinolysin, digesting gelatin, and fermenting carbohydrates to produce acetic acid, and is often regarded as a beneficial bacterium for the intestinal tract [
78]. These results indicate that dietary supplementation with
L. paracasei LK01 has the potential to promote the secretion of short-chain fatty acids, improve the richness of the cecal flora, increase the abundance of beneficial bacteria, and maintain intestinal health.