Comparative Efficacy of Plant Extracts and Probiotics on Growth and Gut Health in Chickens with Necrotic Enteritis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Plant Extracts, Probiotics, Antibiotics
2.3. Animals, C. perfringens, and E. maxima
2.4. Experimental Design
2.5. Growth Performance
2.6. Jejunum Lesion Score
2.7. Jejunal Villus Height and Crypt Depth
2.8. Determination of Intestinal-Related Factors Expression Levels
2.9. Statistical Analysis
3. Results
3.1. All Group Growth Performance
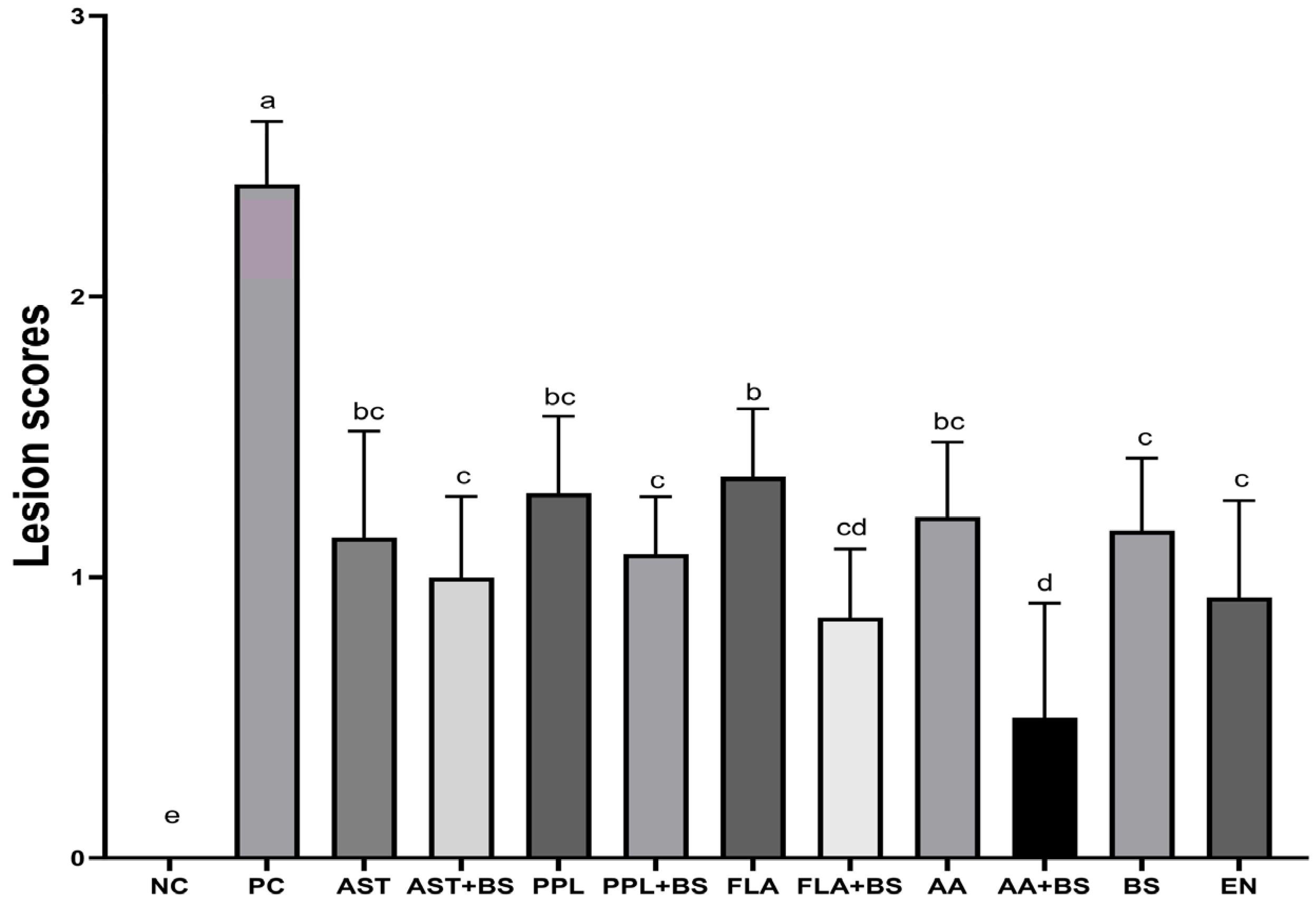
3.2. Jejunal Lesion Scores
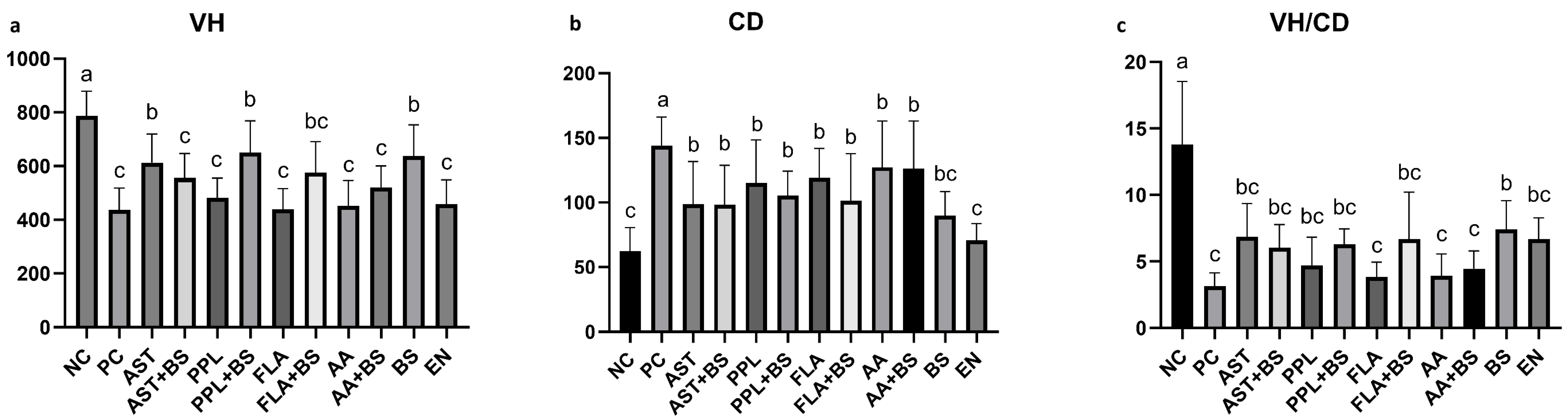
3.3. Jejunal Villus Height and Crypt Depth
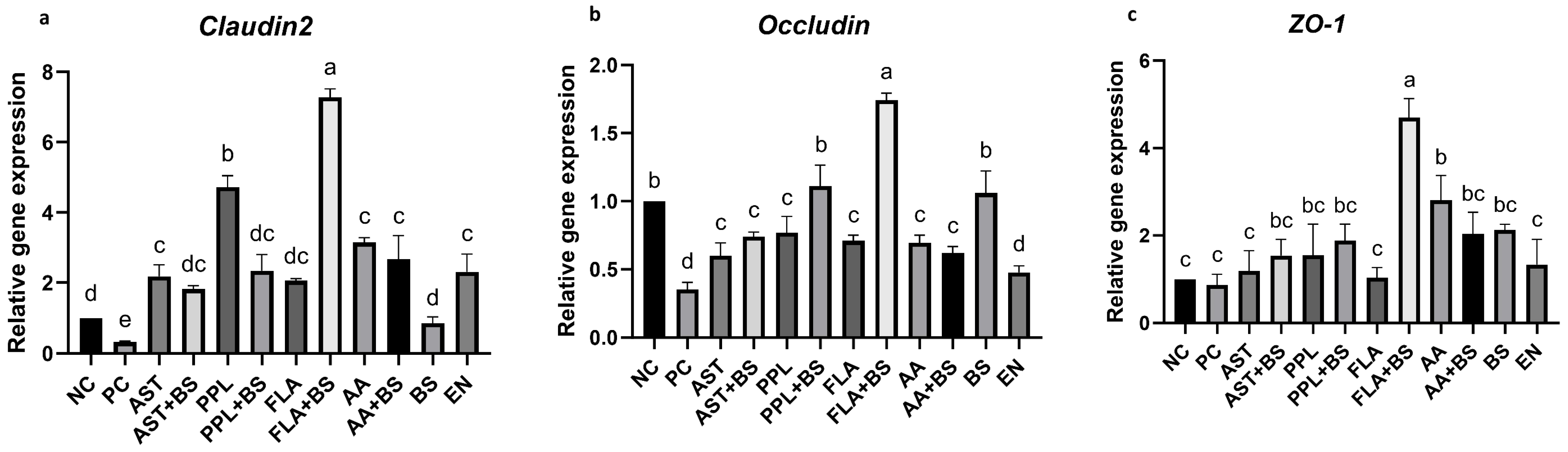
3.4. Tight Junction Proteins
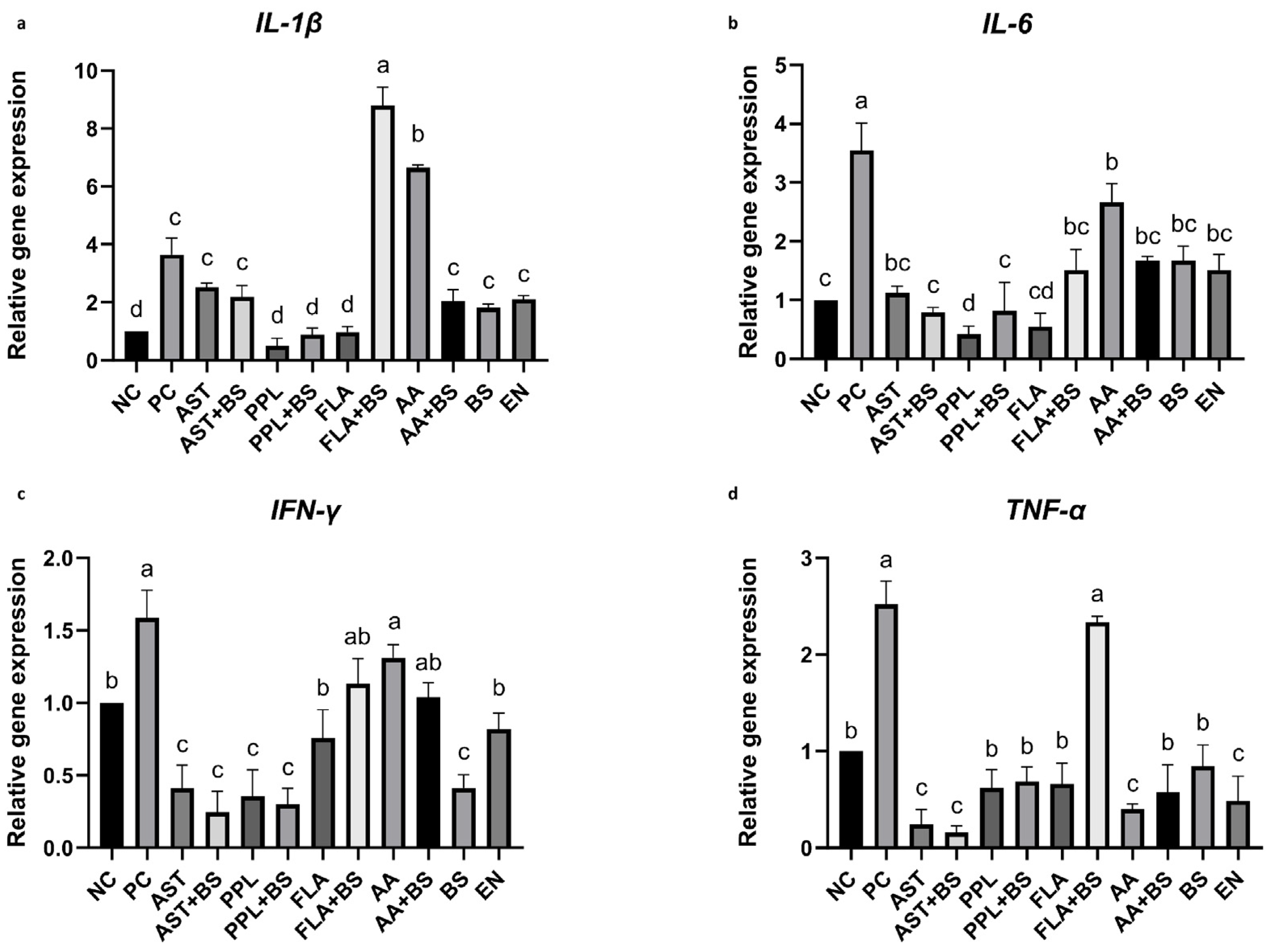
3.5. Proinflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skinner, J.T.; Bauer, S.; Young, V.; Pauling, G.; Wilson, J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010, 54, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lillehoj, H.S.; Jeong, W.; Jeoung, H.Y.; An, D.J. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011, 90, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Dalloul, R.A. Centennial Review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021, 100, 101330. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Chen, Z.; He, Y.; Chen, J.; Hayat, K.; Pan, J.; Lin, H. A reliable and low-cost deep learning model integrating convolutional neural network and transformer structure for fine-grained classification of chicken Eimeria species. Poult. Sci. 2023, 102, 102459. [Google Scholar] [CrossRef]
- Badri, M.; Olfatifar, M.; Hayati, A.; Bijani, B.; Samimi, R.; Abdoli, A.; Nowak, O.; Diaz, D.; Eslahi, A.V. The global prevalence and associated risk factors of Eimeria infection in domestic chickens: A systematic review and meta-analysis. Vet. Med. Sci. 2024, 10, e1469. [Google Scholar] [CrossRef]
- Nicholds, J.F.; McQuain, C.; Hofacre, C.L.; Mathis, G.F.; Fuller, A.L.; Telg, B.E.; Montoya, A.F.; Williams, S.M.; Berghaus, R.D.; Jones, M.K. The Effect of Different Species of Eimeria with Clostridium perfringens on Performance Parameters and Induction of Clinical Necrotic Enteritis in Broiler Chickens. Avian Dis. 2021, 65, 132–137. [Google Scholar] [CrossRef]
- Moore, R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef]
- Williams, R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005, 34, 159–180. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Sharif, S.; Taha-Abdelaziz, K. Probiotics as Alternatives to Antibiotics for the Prevention and Control of Necrotic Enteritis in Chickens. Pathogens 2022, 11, 692. [Google Scholar] [CrossRef]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef]
- Tsiouris, V. Poultry management: A useful tool for the control of necrotic enteritis in poultry. Avian Pathol. 2016, 45, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.I.; Arisha, A.H.; El-Hamid, M. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Munch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Wang, J.; Zuo, K.; Xia, T.; Zhang, J.; Xu, X.; Liu, Q.; Li, X. Alpinia officinarum Hance: A comprehensive review of traditional uses, phytochemistry, pharmacokinetic and pharmacology. Front. Pharmacol. 2024, 15, 1414635. [Google Scholar] [CrossRef]
- Guo, F.C.; Kwakkel, R.P.; Williams, C.B.; Suo, X.; Li, W.K.; Verstegen, M.W. Coccidiosis immunization: Effects of mushroom and herb polysaccharides on immune responses of chickens infected with Eimeria tenella. Avian Dis. 2005, 49, 70–73. [Google Scholar] [CrossRef]
- Song, B.; Li, P.; Yan, S.; Liu, Y.; Gao, M.; Lv, H.; Lv, Z.; Guo, Y. Effects of Dietary Astragalus Polysaccharide Supplementation on the Th17/Treg Balance and the Gut Microbiota of Broiler Chickens Challenged With Necrotic Enteritis. Front. Immunol. 2022, 13, 781934. [Google Scholar] [CrossRef]
- El-Demerdash, A.S.; Mohamady, S.N.; Megahed, H.M.; Ali, N.M. Evaluation of gene expression related to immunity, apoptosis, and gut integrity that underlies Artemisia’s therapeutic effects in necrotic enteritis-challenged broilers. 3 Biotech 2023, 13, 181. [Google Scholar] [CrossRef]
- Sharma, M.K.; Liu, G.; Choppa, V.; Rafieian-Naeini, H.R.; Mahdavi, F.S.; Marshall, B.; Gogal, R.J.; Kim, W.K. Effects of Artemisia annua supplementation on the performance and gut health of laying hens challenged with mixed Eimeria species. Front. Physiol. 2024, 15, 1381548. [Google Scholar] [CrossRef]
- Hafeez, A.; Piral, Q.; Naz, S.; Almutairi, M.H.; Alrefaei, A.F.; Ayasan, T.; Khan, R.U.; Losacco, C. Ameliorative Effect of Pomegranate Peel Powder on the Growth Indices, Oocysts Shedding, and Intestinal Health of Broilers under an Experimentally Induced Coccidiosis Condition. Animals 2023, 13, 3790. [Google Scholar] [CrossRef]
- Youn, H.J.; Noh, J.W. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet. Parasitol. 2001, 96, 257–263. [Google Scholar] [CrossRef]
- Lv, P.; Song, Y.; Liu, C.; Yu, L.; Shang, Y.; Tang, H.; Sun, S.; Wang, F. Application of Bacillus subtilis as a live vaccine vector: A review. J. Vet. Med. Sci. 2020, 82, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Khochamit, N.; Siripornadulsil, S.; Sukon, P.; Siripornadulsil, W. Antibacterial activity and genotypic-phenotypic characteristics of bacteriocin-producing Bacillus subtilis KKU213: Potential as a probiotic strain. Microbiol. Res. 2015, 170, 36–50. [Google Scholar] [CrossRef]
- La Ragione, R.M.; Woodward, M.J. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Vet. Microbiol. 2003, 94, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Begum, F.; Rabaan, A.A.; Aljeldah, M.; Al, S.B.; Alawfi, A.; Alshengeti, A.; Sulaiman, T.; Khan, A. Classification and Multifaceted Potential of Secondary Metabolites Produced by Bacillus subtilis Group: A Comprehensive Review. Molecules 2023, 28, 927. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Ruan, X.; Xie, Y.; Shen, B.; Fang, R.; Zhao, J.; Zhou, Y. Anticoccidial activity of a botanical natural product based on eucalyptus, apigenin and eugenol against Eimeria tenella in broiler chickens. Parasites Vectors 2024, 17, 327. [Google Scholar] [CrossRef]
- Manjunatha, V.; Nixon, J.E.; Mathis, G.F.; Lumpkins, B.S.; Guzel-Seydim, Z.B.; Seydim, A.C.; Greene, A.K.; Jiang, X. Combined Effect of Nigella sativa and Kefir on the Live Performance and Health of Broiler Chickens Affected by Necrotic Enteritis. Animals 2024, 14, 2074. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S.; Shahin, S.E.; Omar, A.E.; Mohammed, H.A.; Mahmoud, H.I.; Ibrahim, D. Effects of graded levels of microbial fermented or enzymatically treated dried brewer’s grains on growth, digestive and nutrient transporter genes expression and cost effectiveness in broiler chickens. BMC Vet. Res. 2020, 16, 424. [Google Scholar] [CrossRef]
- Prescott, J.F.; Sivendra, R.; Barnum, D.A. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. Can. Vet. J. 1978, 19, 181–183. [Google Scholar]
- Daneshmand, A.; Kermanshahi, H.; Mohammed, J.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M.; Alizadeh, M.; Razmyar, J.; Kulkarni, R.R. Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult. Sci. 2022, 101, 101652. [Google Scholar] [CrossRef]
- Yin, L.; Hussain, S.; Tang, T.; Gou, Y.; He, C.; Liang, X.; Yin, Z.; Shu, G.; Zou, Y.; Fu, H.; et al. Protective Effects of Cinnamaldehyde on the Oxidative Stress, Inflammatory Response, and Apoptosis in the Hepatocytes of Salmonella Gallinarum-Challenged Young Chicks. Oxid. Med. Cell Longev. 2022, 2022, 2459212. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ci, X.; Chen, S.; Xie, Z.; Li, H.; Zhang, H.; Chen, F.; Xie, Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020, 99, 6606–6618. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Han, Q.; Guo, Y.; Zhang, B.; D’Inca, R. Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. Brit J. Nutr. 2016, 116, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qi, L.; Wei, Q.; Shi, F. Maternal stevioside supplementation improves intestinal immune function of chicken offspring potentially via modulating gut microbiota and down-regulating the promoter methylation level of suppressor of cytokine signaling 1 (SOCS1). Anim. Nutr. 2022, 10, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, I.; Lillehoj, H.S. Oral administration of chicken NK-lysin or recombinant chicken IL-7 improves vaccine efficacy of Eimeria tenella elongation factor-1alpha (EF-1alpha) against coccidiosis in commercial broiler chickens. Poult. Sci. 2023, 102, 102611. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Rood, J.I.; Moore, R.J.; Titball, R.W. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009, 17, 32–36. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Zhao, Y.; Tao, L.; Liu, H.; Dong, W.; Yang, G.; Li, L. Effects of dietary supplementation with itaconic acid on the growth performance, nutrient digestibility, slaughter variables, blood biochemical parameters, and intestinal morphology of broiler chickens. Poult. Sci. 2022, 101, 101732. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Martin, L.N.; Scheuerlein, A.; Wikelski, M. Immune activity elevates energy expenditure of house sparrows: A link between direct and indirect costs? Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 153–158. [Google Scholar] [CrossRef]
- Jin, M.; Zhao, K.; Huang, Q.; Shang, P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int. J. Biol. Macromol. 2014, 64, 257–266. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Wang, Q.; Yu, S.; Li, Y.; Yao, B.; Yang, X. Astragalus polysaccharides-induced gut microbiota play a predominant role in enhancing of intestinal barrier function of broiler chickens. J. Anim. Sci. Biotechnol. 2024, 15, 106. [Google Scholar] [CrossRef]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Endo, E.H.; Cortez, D.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias, F.B. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res. Microbiol. 2010, 161, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Naz, S.; Raziq, F.; Qudratullah, Q.; Khan, N.A.; Laudadio, V.; Tufarelli, V.; Ragni, M. Prospects of organic acids as safe alternative to antibiotics in broiler chickens diet. Environ. Sci. Pollut. Res. 2022, 29, 32594–32604. [Google Scholar] [CrossRef] [PubMed]
- Abdel, B.S.; Ashour, E.A.; Abd, E.M.; El-Mekkawy, M.M. Effect of different levels of pomegranate peel powder and probiotic supplementation on growth, carcass traits, blood serum metabolites, antioxidant status and meat quality of broilers. Anim. Biotechnol. 2022, 33, 690–700. [Google Scholar] [CrossRef]
- Wang, Y.; Shou, Z.; Fan, H.; Xu, M.; Chen, Q.; Tang, Q.; Liu, X.; Wu, H.; Zhang, M.; Yu, T.; et al. Protective effects of oxymatrine against DSS-induced acute intestinal inflammation in mice via blocking the RhoA/ROCK signaling pathway. Biosci. Rep. 2019, 39, BSR20182297. [Google Scholar] [CrossRef]
- Jia, Y.Q.; Yuan, Z.W.; Zhang, X.S.; Dong, J.Q.; Liu, X.N.; Peng, X.T.; Yao, W.L.; Ji, P.; Wei, Y.M.; Hua, Y.L. Total alkaloids of Sophora alopecuroides L. ameliorated murine colitis by regulating bile acid metabolism and gut microbiota. J. Ethnopharmacol. 2020, 255, 112775. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, J.; Li, Y.; Ding, Y.; Lian, J.; Dong, Q.; Qu, Q.; Lv, W.; Guo, S. Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers. Poult. Sci. 2023, 102, 102714. [Google Scholar] [CrossRef]
- Gu, Y.; Lu, J.; Sun, W.; Jin, R.; Ohira, T.; Zhang, Z.; Tian, X. Oxymatrine and its metabolite matrine contribute to the hepatotoxicity induced by radix Sophorae tonkinensis in mice. Exp. Ther. Med. 2019, 17, 2519–2528. [Google Scholar] [CrossRef]
- Hezil, N.; Baazize-Ammi, D.; Abdelli, A.; Adel, A.; Kebbal, S.; Gharbi, I.; Djezzar, R.; Guetarni, D. Effects of Artemisia absinthium on broiler chicken coccidiosis: A systematic review and meta-analysis. Avian Pathol. 2024, 53, 350–358. [Google Scholar] [CrossRef]
- Ghimire, S.; Subedi, K.; Zhang, X.; Wu, C. Efficacy of Bacillus subtilis probiotic in preventing necrotic enteritis in broilers: A systematic review and meta-analysis. Avian Pathol. 2024, 53, 451–466. [Google Scholar] [CrossRef]
- Smulikowska, S.; Czerwinski, J.; Mieczkowska, A. Effect of an organic acid blend and phytase added to a rapeseed cake-containing diet on performance, intestinal morphology, caecal microflora activity and thyroid status of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2010, 94, 15–23. [Google Scholar] [CrossRef]
- Xu, L.; Liao, J.; Li, X.; Zhu, L.; Wang, X.; Xu, B.; Li, L.; Ze, X.; Sun, H.; Li, J. Exploring the mechanism of probiotics in enhancing the utilization of chemical components (or polyphenols) of grape seed extract. Food Chem. 2024, 438, 137982. [Google Scholar] [CrossRef]
| Groups | Diet | Groups | Diet |
|---|---|---|---|
| NC | Basal diet | FLA | Basal diet + 0.2 g/kg Sophora flavescens |
| PC | Basal diet | FLA+BS | Basal diet + 0.2 g/kg Sophora flavescens + 1 g/kg B. subtilis |
| AST | Basal diet + 0.62 g/kg Astragalus | AA | Basal diet + 1 g/kg Artemisia annua |
| AST+BS | Basal diet + 0.62 g/kg Astragalus + 1 g/kg B. subtilis | AA+BS | Basal diet + 1 g/kg Artemisia annua + 1 g/kg B. subtilis |
| PPL | Basal diet + 0.68 g/kg pomegranate peel | BS | Basal diet + 1 g/kg B. subtilis |
| PPL+BS | Basal diet + 0.68 g/kg pomegranate peel + 1 g/kg B. subtilis | EN | Basal diet + 10 mg/kg enramycin |
| Gene | Primer (5′–3′) | Gene Bank Accession Number | |
|---|---|---|---|
| proinflammatory cytokines | IL-6 [29] | CTGTTCGCCTTTCAGACCTACC | HM179640.1 |
| GACCACTTCATCGGGATTTATCA | |||
| IL-1β [30] | GGTCAACATCGCCACCTACA | XM_015297469.3 | |
| CATACGAGATGGAAACCAGCAA | |||
| TNF-α [31] | TGTATGTGCAGCAACCCGTA | NM_204267.2 | |
| CCACACGACAGCCAAGTCAA | |||
| IFN-γ [31] | GATGCCACCTTCTCTCACGA | NM_205427.1 | |
| GGATGTCGTGGGTGGTTTTG | |||
| tight junction proteins | ZO-1 [32] | CTTCAGGTGTTTCTCTTCCTCCTC | XM_413773.4 |
| CTGTGGTTTCATGGCTGGATC | |||
| Occludin [32] | ACGGCAGCACCTACCTCAA | D21837.1 | |
| GGGCGAAGAAGCAGATGAG | |||
| Claudin2 [33] | CTGCTCACCCTCATTGGA | NM_001277622.1 | |
| AACTCACTCTTGGGCTTCTG | |||
| housekeeping gene | β-Actin [29] | CCTGGCACCTAGCACAATGAA | NM_205518.2 |
| GGTTTAGAAGCATTTGCGGTG | |||
| Groups | 14–28 Days | |||||
|---|---|---|---|---|---|---|
| BWG/g | BWGR | p Value | FI/g | FCR | p Value | |
| NC | 1290 ± 60 a | 127.7% | 1 | 2360 ± 45 | 1.830 c | 1 |
| PC | 810 ± 150 e | 80.2% | <0.0001 | 2260 ± 60 | 2.795 a | <0.0001 |
| AST | 950 ± 120 cd | 94.1% | <0.0001 | 2260 ± 50 | 2.383 b | <0.0001 |
| AST+BS | 950 ± 130 cd | 94.1% | <0.0001 | 2280 ± 45 | 2.401 b | <0.0001 |
| PPL | 1110 ± 120 b | 109.9% | <0.0001 | 2230 ± 70 | 2.007 c | 0.0513 |
| PPL+BS | 880 ± 100 de | 87.1% | <0.0001 | 2220 ± 35 | 2.524 b | <0.0001 |
| FLA | 930 ± 100 d | 92.1% | <0.0001 | 2290 ± 60 | 2.468 b | <0.0001 |
| FLA+BS | 1000 ± 160 c | 99.0% | <0.0001 | 2280 ± 50 | 2.281 b | <0.0001 |
| AA | 910 ± 190 d | 90.1% | <0.0001 | 2240 ± 70 | 2.457 b | <0.0001 |
| AA+BS | 930 ± 130 d | 92.1% | <0.0001 | 2230 ± 50 | 2.403 b | <0.0001 |
| BS | 930 ± 160 d | 92.1% | <0.0001 | 2220 ± 80 | 2.367 b | <0.0001 |
| EN | 1010 ± 110 c | 1 | <0.0001 | 2390 ± 55 | 2.371 b | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Yang, J.; Wang, Q.; Hu, D.; Zhao, Q.; Zhu, S.; Qiao, Y.; Zhao, F.; Wang, Z.; Wang, J.; et al. Comparative Efficacy of Plant Extracts and Probiotics on Growth and Gut Health in Chickens with Necrotic Enteritis. Animals 2024, 14, 3312. https://doi.org/10.3390/ani14223312
Zhang R, Yang J, Wang Q, Hu D, Zhao Q, Zhu S, Qiao Y, Zhao F, Wang Z, Wang J, et al. Comparative Efficacy of Plant Extracts and Probiotics on Growth and Gut Health in Chickens with Necrotic Enteritis. Animals. 2024; 14(22):3312. https://doi.org/10.3390/ani14223312
Chicago/Turabian StyleZhang, Ruiting, Jia Yang, Qingjie Wang, Dandan Hu, Qiping Zhao, Shunhai Zhu, Yu Qiao, Fanghe Zhao, Zhongchuang Wang, Jinwen Wang, and et al. 2024. "Comparative Efficacy of Plant Extracts and Probiotics on Growth and Gut Health in Chickens with Necrotic Enteritis" Animals 14, no. 22: 3312. https://doi.org/10.3390/ani14223312
APA StyleZhang, R., Yang, J., Wang, Q., Hu, D., Zhao, Q., Zhu, S., Qiao, Y., Zhao, F., Wang, Z., Wang, J., Yu, Y., Han, H., Hao, L., & Dong, H. (2024). Comparative Efficacy of Plant Extracts and Probiotics on Growth and Gut Health in Chickens with Necrotic Enteritis. Animals, 14(22), 3312. https://doi.org/10.3390/ani14223312






