Integrated Assessment of Survival, Movement, and Reproduction in Migratory Birds: A Study on Evaluating Reinforcement Success
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Banding and Tracking
2.2. Release into the Wild
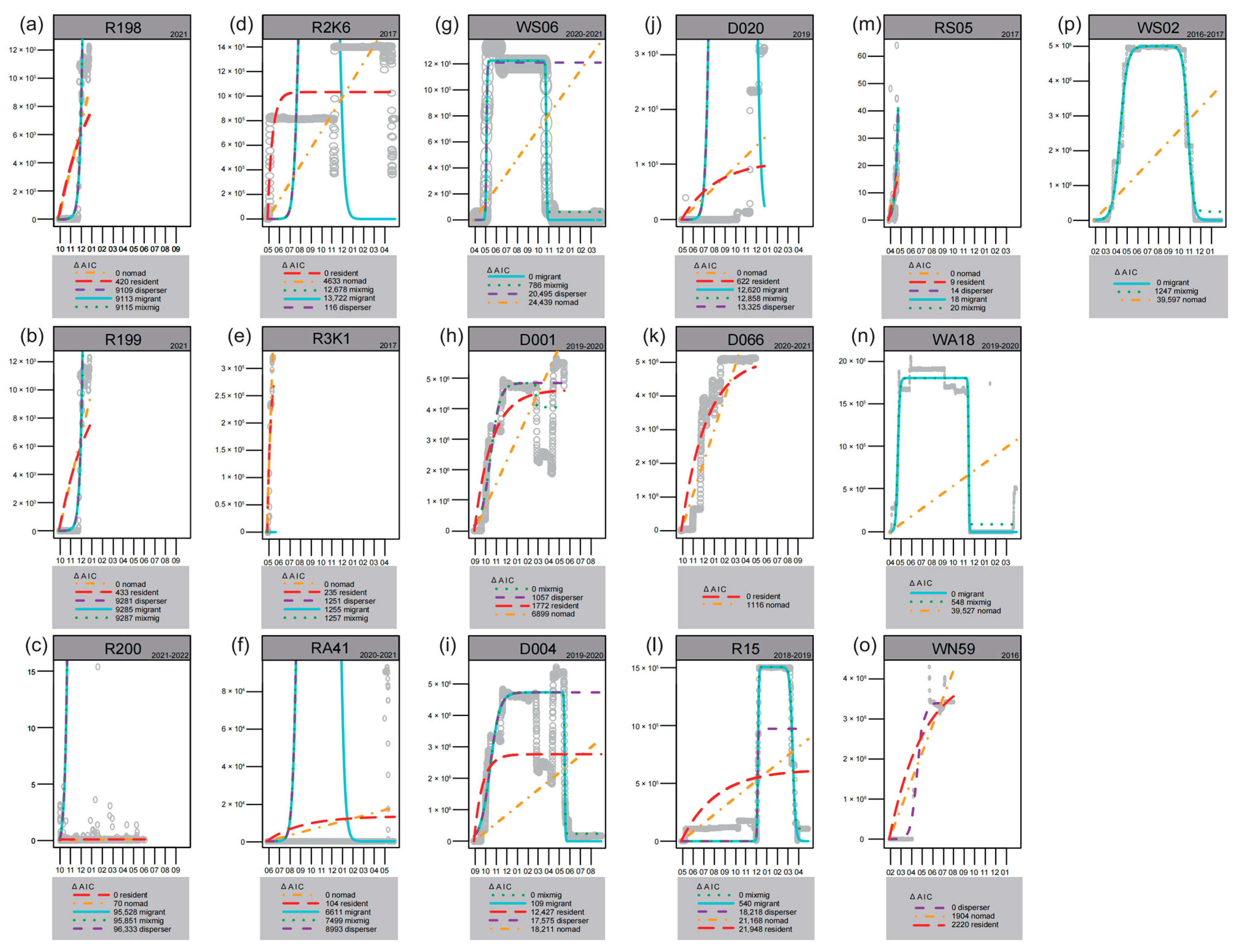
2.3. Data Analysis
3. Results
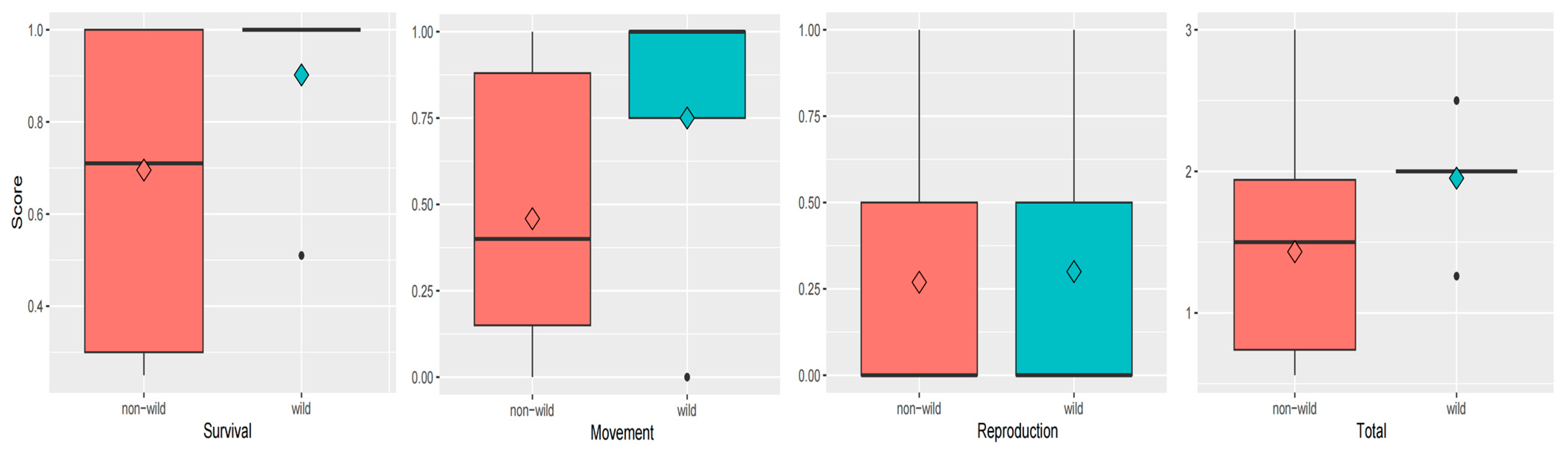
3.1. Survival Score
3.2. Movement Score
3.3. Reproduction Score
3.4. Total Score
4. Discussion
4.1. The Integrated Assessment of Survival, Movement and Reproduction
4.2. The Difference Between Wild and Non-Wild Individuals After Release
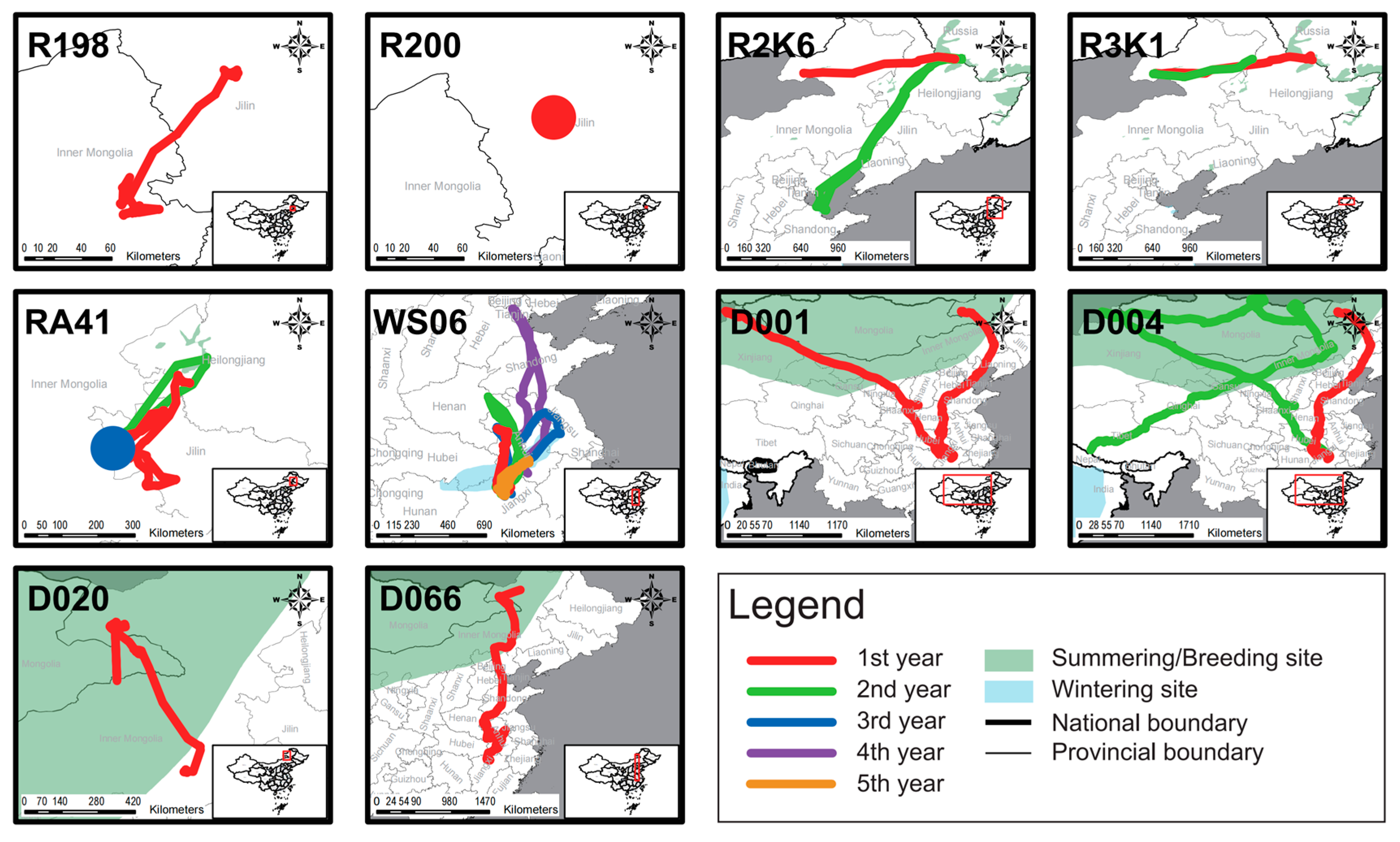
4.3. The Characteristics of Non-Wild Individuals After Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, L.; van de Pol, M.; Osmond, H.L.; Liu, Y.; Cockburn, A.; Kruuk, L. Winter Mortality of a Passerine Bird Increases Following Hotter Summers and During Winters with Higher Maximum Temperatures. Sci. Adv. 2023, 9, eabm0197. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Lopez, A.; Alkemade, R.; Schipper, A.M.; Ingram, D.J.; Verweij, P.A.; Eikelboom, J.A.J.; Huijbregts, M.A.J. The Impact of Hunting on Tropical Mammal and Bird Populations. Science 2017, 356, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, J.R.; Mace, G.M.; Albon, S. Local Extinction in a Small and Declining Population: Wild Dogs in the Serengeti. Proc. Biol. Sci. 1995, 262, 221–228. [Google Scholar] [CrossRef]
- Kong, D.J.; Wu, F.; Shan, P.F.; Gao, J.Y.; Yan, D.; Luo, W.X.; Yang, X.J. Status and Distribution Changes of the Endangered Green Peafowl (Pavo muticus) in China Over the Past Three Decades (1990s-2017). Avian Res. 2018, 9, 18. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.Z.; Wang, D.J.; Schaller, G.B.; Wu, Y.L.; Harris, R.B.; Zhang, K.J.; Lv, Z. Distribution and Population Status of Przewalski’s gazelle, Procapra przewalskii (Cetartiodactyla, Bovidae). Mammalia 2013, 77, 31–40. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation Translocations, version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013; p. 2. [Google Scholar]
- Tavecchia, G.; Viedma, C.; Martínez-Abraín, A.; Bartolomé, M.; Gómez, J.; Oro, D. Maximizing Re-introduction Success: Assessing the Immediate Cost of Release in a Threatened Waterfowl. Biol. Conserv. 2009, 142, 3005–3012. [Google Scholar] [CrossRef]
- Abrahms, B.; Teitelbaum, C.S.; Mueller, T.; Converse, S.J. Ontogenetic Shifts from Social to Experiential Learning Drive Avian Migration Timing. Nat. Commun. 2021, 12, 7326. [Google Scholar] [CrossRef]
- Wild, T.A.; Wikelski, M.; Tyndel, S.; Alarcon-Nieto, G.; Klump, B.C.; Aplin, L.M.; Meboldt, M.; Williams, H.J. Internet on Animals: Wi-Fi-enabled Devices Provide a Solution for Big Data Transmission in Biologging. Methods Ecol. Evol. 2023, 14, 87–102. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Dou, H.S.; Liu, S.T.; Guo, Y.M. Rectification of Abnormal Migration Recorded in Hand-reared Red-crowned Cranes (Grus japonensis). Waterbirds 2019, 42, 425–430. [Google Scholar] [CrossRef]
- Shephard, J.M.; Rycken, S.; Almalik, O.; Struyf, K.; Van Erp-van Der Kooij, L. Migration Strategies Revealed by Satellite Tracking among Descendants of a Population of European White Stork (Ciconia ciconia) Reintroduced to Belgium. J. Ornithol. 2015, 156, 943–953. [Google Scholar] [CrossRef]
- Klaassen, R.H.G.; Hake, M.; Strandberg, R.; Koks, B.J.; Trierweiler, C.; Exo, K.; Bairlein, F.; Alerstam, T. When and Where does Mortality Occur in Migratory Birds? Direct Evidence from Long-term Satellite Tracking of Raptors. J. Anim. Ecol. 2014, 83, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, R.; Szyszkoski, E.; Zimorski, S. Winter Distribution Dynamics and Implications to a Reintroduced Population of Migratory Whooping Cranes. J. Fish Wildl. Manag. 2014, 5, 1538027542. [Google Scholar] [CrossRef]
- Both, C.; Bouwhuis, S.; Lessells, C.M.; Visser, M.E. Climate Change and Population Declines in a Long-distance Migratory bird. Nature 2006, 441, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Blackburn, T.M. Birds, Body Size and the Threat of Extinction. Philos. Trans. R. Soc. B Biol. Sci. 1997, 347, 205–212. [Google Scholar] [CrossRef]
- Bragina, E.V.; Balan, I.V.; Kuznetsova, N.V.; Parilov, M.P.; Slaght, J.C. Reintroduction of Parent-reared and Semi-wild Chicks of Red-crowned Grus japonensis and White-naped Cranes Antigone vipio in Russia: Lessons from 29 Years of Experience. Ornithol. Sci. 2022, 21, 53–62. [Google Scholar] [CrossRef]
- Insee, J.; Kamolnorranath, S.; Baicharoen, S.; Chumpadang, S.; Sawasu, W.; Wajjwalku, W. PCR-based Method for Sex Identification of Eastern Sarus Crane (Grus antigone sharpii): Implications for Reintroduction Programs in Thailand. Zool. Sci. 2014, 31, 95–100. [Google Scholar] [CrossRef][Green Version]
- Mudrik, E.A.; Kashentseva, T.A.; Postelnykh, K.A.; Politov, D.V. Genetic Monitoring of the Captive Breeding and Reintroduction of the Siberian Crane, Russia’s Endemic Species (Leucogeranus leucogeranus, Gruidae). Biol. Bull. 2023, 50, 1995–2001. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Cui, D.Y.; Li, C.R.; Chen, G.Y.; Zhao, Y.Q.; Lu, C.H. Reinforcement Project and Breeding Cases of Introduced Endangered Red-crowned Cranes Grus japonensis in Yancheng National Nature Reserve, China. Ornithol. Sci. 2020, 19, 93–97. [Google Scholar] [CrossRef]
- Servanty, S.; Converse, S.J.; Bailey, L.L. Demography of a Reintroduced Population: Moving toward Management Models for an Endangered Species, the Whooping Crane. Ecol. Appl. 2014, 24, 927–937. [Google Scholar] [CrossRef]
- Kreger, M.D.; Hatfield, J.S.; Estevez, I.; Gee, G.F.; Clugston, D.A. Behavioral Profiles of the Captive Juvenile Whooping Crane as an Indicator of Post-release Survival. Zoo Biol. 2006, 25, 11–24. [Google Scholar] [CrossRef]
- Strobel, B.N.; Giorgi, G.F. Nest-Site Selection Patterns of Coexisting Sandhill and Whooping Cranes in Wisconsin. J. Fish Wildl. Manag. 2017, 8, 588–595. [Google Scholar] [CrossRef]
- Ma, Z.J. The Research Methods and Study Advances of Bird Migration. Biol. Bull. 2009, 3, 5–9. [Google Scholar] [CrossRef]
- Wu, T.T.; Gao, Z.Y.; Qian, F.W.; Guo, X.Y.; Wang, Z.X. Migration of Semi-free-ranging Red-crowned Cranes in Zhalong National Nature Reserve, Heilongjiang Province. Terr. Ecosyst. Conserv. 2023, 4, 54–65. [Google Scholar] [CrossRef]
- Ilyashenko, E.I.; Mudrik, E.A.; Andryushchenko, Y.A.; Belik, V.P.; Belyalov, O.V.; Wikelski, M.; Gavrilov, A.E.; Goroshko, O.A.; Guguyeva, E.V.; Korepov, M.V.; et al. Migrations of the Demoiselle Crane (Anthropoides virgo, Gruiformes): Remote Tracking along Flyways and at Wintering Grounds. Biol. Bull. 2022, 49, 863–888. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, H.; Yang, L.F.; Hua, R.; Liu, J.; Liu, Y.; Chen, G.Y.; Cui, D.Y. Indirect Evidence of Migration of the Offspring of Reintroduced Red-crowned Cranes in Yancheng Wetland, Jiangsu Province. Wetl Sci. Manag. 2023, 19, 94–95. [Google Scholar] [CrossRef]
- Ellner, S.P.; Fieberg, J.; Ludwig, D.; Wilcox, C. Precision of Population Viability Analysis. Conserv. Biol. 2002, 16, 258–261. [Google Scholar] [CrossRef]
- Wang, B.C.; Pei, W.; Se, Y.J.; Yang, J.C.; Wang, Y.M.; Yang, H.R.; Fu, H.Y.; Yu, F.Q.; Wang, Z.J.; Lang, X.M.; et al. Colliding with Power Lines Being the Main Reason of the Death of Satellite-Tracked Black-necked Crane Juveniles in Wintering Area. J. Zool. 2021, 56, 161–170. [Google Scholar] [CrossRef]
- Huang, Z.H.; Chen, J.J.; Wen, L.J.; Pu, Z.; Ma, C.X.; Gao, Y.Y.; Guo, Y.M. The Influence of Fence on Black-necked Crane (Grus nigricollis) in Southern Foothills of Qilian Mountains. Biodivers. Sci. 2023, 31, 107–114. [Google Scholar] [CrossRef]
- Donaldson, L.; Bridge, D.J.; Hilton, G.M. High Survival Promotes Persistence in a Reintroduced Population of Common Crane (Grus grus). Ibis 2023, 165, 1129–1144. [Google Scholar] [CrossRef]
- Oldekop, W.; Oldekop, G.; Vahldiek, K.; Klawonn, F.; Rinas, U. Counting Young Birds: A Simple Tool for the Determination of Avian Population Parameters. PLoS ONE 2023, 18, e279899. [Google Scholar] [CrossRef]
- Thompson, H.; Glass, M.; Wellington, M.; Boardman, K.; Olsen, G. Effects of Release Techniques on Parent-reared Whooping Cranes in the Eastern Migratory Population. Proc. N. Am. Crane Workshop 2022, 15, 53–71. [Google Scholar]
- Szyszkoski, E.K.; Thompson, H.L. A Roundtrip Long-distance Movement within One Season by a Nonmigratory Whooping Crane (Grus americana). Wilson J. Ornithol. 2022, 134, 97. [Google Scholar] [CrossRef]
- Mueller, T.; O’Hara, R.B.; Converse, S.J.; Urbanek, R.P.; Fagan, W.F. Social Learning of Migratory Performance. Science 2013, 341, 999–1002. [Google Scholar] [CrossRef] [PubMed]
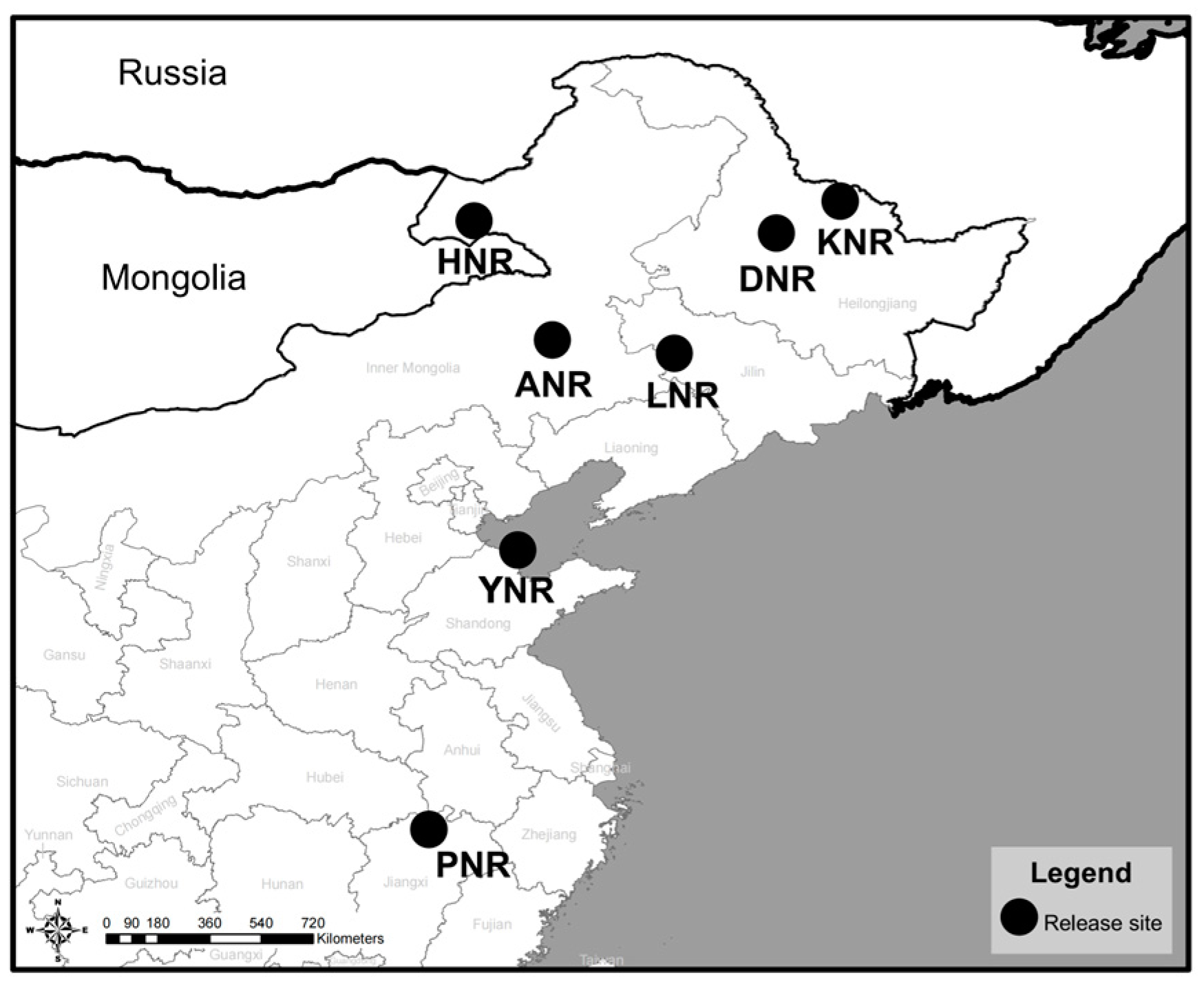

| Species | ID | Release Age | Release Time | Release Area | Tracked Time | Data Volume |
|---|---|---|---|---|---|---|
| RCC | R198 | 1− | 15 Sep. 2021 | LNR N | 15 Sep. 2021–13 Dec. 2021 | 2131 |
| RCC | R199 | 1− | 15 Sep. 2021 | LNR N | 15 Sep. 2021–13 Dec. 2021 | 2143 |
| RCC | R200 F | 1+ | 15 Sep. 2021 | LNR N | 15 Sep. 2021–19 May 2022 | 3692 |
| RCC | R2K6 | 1+ | 16 May 2016 | HNR S | 17 May 2016–12 Jun. 2016 | 154 |
| 2+ | 24 Apr. 2017 | HNR S | 24 Apr. 2017–17 May 2017 | 559 | ||
| RCC | R3K1 | 1+ | 16 May 2016 | HNR S | 16 May 2016–20 Jun. 2016 | 268 |
| 2+ | 24 Apr. 2017 | HNR S | 24 Apr. 2017–12 May 2017 | 437 | ||
| RCC | R1K7 F | NA | 16 May 2016 | HNR S | - | - |
| RCC | RA41 F | 2+ | 10 May 2018 | ANR N | 10 May 2018–10 Mar. 2023 | 26,919 |
| WNC | WS06 F | 2+ | 20 Jan. 2017 | PNR W | 20 Jan. 2017–3 Nov. 2021 | 19,384 |
| WNC | W283 F | 1+ | 28 Jun. 2010 | DNR S | - | - |
| DC | D001 | NA | 28 Aug. 2019 | HNR S | 28 Aug. 2019–14 May 2020 | 5191 |
| DC | D004 | NA | 28 Aug. 2019 | HNR S | 28 Aug. 2019–14 Oct. 2020 | 9715 |
| DC | D020 | NA | 17 Apr. 2019 | HNR S | 17 Apr. 2019–12 Dec. 2019 | 2213 |
| DC | D066 | NA | 8 Sep. 2020 | HNR S | 8 Sep. 2020–10 Apr. 2021 | 5108 |
| RCC | * R15 F | NA | 15 Apr. 2018 | LNR N | 15 Apr. 2018–16 Apr. 2023 | 19,310 |
| RCC | * RS05 F | 4+ | 14 Mar. 2017 | YNR W | 14 Mar. 2017–11 Apr. 2017 | 243 |
| WNC | * WA18 | 1+ | 18 Mar. 2018 | LNR N | 18 Mar. 2018–17 Mar. 2023 | 34,610 |
| WNC | * WN59 | 1+ | 20 Jan. 2016 | PNR W | 20 Jan. 2016–24 Jul. 2016 | 808 |
| WNC | * WS02 | 1+ | 20 Jan. 2016 | PNR W | 20 Jan. 2016–4 Apr. 2018 | 19,630 |
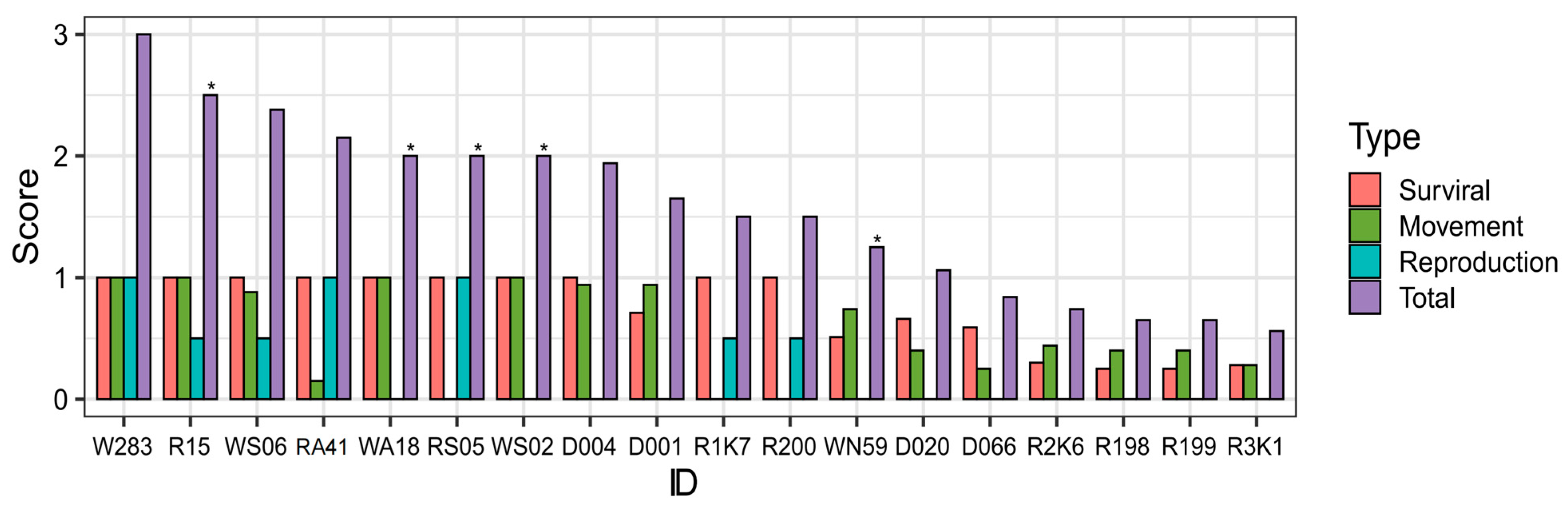
| ID | Survival | Movement | Reproduction | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Not Die | Die | Total | Model | Trajectory | Average | |||
| W283 | 1.00 | 0.00 | 1.00 | NA | 1.00 | 1.00 | 1.00 | 3.00 |
| R15 * | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | ≥0.50 | ≥2.50 |
| WS06 | 1.00 | 0.00 | 1.00 | 1.00 | 0.75 | 0.88 | ≥0.50 | ≥2.38 |
| RA41 | 1.00 | 0.00 | 1.00 | 0.30 | 0.00 | 0.15 | 1.00 | 2.15 |
| WA18 * | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | ≥0.00 | ≥2.00 |
| RS05 * | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 | ≥0.00 | 1.00 | ≥2.00 |
| WS02 * | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | ≥0.00 | ≥2.00 |
| D004 | 1.00 | 0.00 | 1.00 | 1.00 | 0.875 | 0.94 | ≥0.00 | ≥1.94 |
| D001 | 0.71 | 0.00 | 0.71 | 1.00 | 0.875 | 0.94 | ≥0.00 | ≥1.65 |
| R1K7 | 1.00 | 0.00 | 1.00 | NA | NA | ≥0.00 | ≥0.50 | ≥1.50 |
| R200 | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | ≥0.50 | ≥1.50 |
| WN59 * | 0.51 | 0.00 | 0.51 | 0.60 | 0.875 | 0.75 | ≥0.00 | ≥1.26 |
| D020 | 0.66 | 0.00 | 0.66 | 0.30 | 0.50 | 0.40 | ≥0.00 | ≥1.06 |
| D066 | 0.59 | 0.00 | 0.59 | 0.00 | 0.25 | 0.13 | ≥0.00 | ≥0.84 |
| R2K6 | 0.52 | 0.00 | 0.30 | 0.00 | 0.875 | 0.44 | ≥0.00 | ≥0.74 |
| 0.07 | 0.00 | |||||||
| R198 | 0.25 | 0.00 | 0.25 | 0.30 | 0.50 | 0.40 | ≥0.00 | ≥0.65 |
| R199 | 0.25 | 0.00 | 0.25 | 0.30 | 0.50 | 0.40 | ≥0.00 | ≥0.65 |
| R3K1 | 0.52 | 0.00 | 0.28 | 0.30 | 0.25 | 0.28 | ≥0.00 | ≥0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Wen, L.; Dou, H.; Guo, Y. Integrated Assessment of Survival, Movement, and Reproduction in Migratory Birds: A Study on Evaluating Reinforcement Success. Animals 2024, 14, 3128. https://doi.org/10.3390/ani14213128
Hu G, Wen L, Dou H, Guo Y. Integrated Assessment of Survival, Movement, and Reproduction in Migratory Birds: A Study on Evaluating Reinforcement Success. Animals. 2024; 14(21):3128. https://doi.org/10.3390/ani14213128
Chicago/Turabian StyleHu, Guilin, Lijia Wen, Huashan Dou, and Yumin Guo. 2024. "Integrated Assessment of Survival, Movement, and Reproduction in Migratory Birds: A Study on Evaluating Reinforcement Success" Animals 14, no. 21: 3128. https://doi.org/10.3390/ani14213128
APA StyleHu, G., Wen, L., Dou, H., & Guo, Y. (2024). Integrated Assessment of Survival, Movement, and Reproduction in Migratory Birds: A Study on Evaluating Reinforcement Success. Animals, 14(21), 3128. https://doi.org/10.3390/ani14213128






