Analysis of lncRNAs and Their Regulatory Network in Skeletal Muscle Development of the Yangtze River Delta White Goat
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Preparation
2.2. Observation of Muscle Tissue Sections
2.3. Determination of Meat Quality
2.3.1. pH Value
2.3.2. Meat Color
2.3.3. Shear Force
2.3.4. Drip Loss
2.4. Extraction of Total RNA and Construction of Library
2.5. Quality Control and Comparison of Reference Genomes
2.6. Identification and Differential Expression Analysis of lncRNAs
2.7. Prediction of Differentially Expressed lncRNA Target Genes and Enrichment Analysis of GO and KEGG
2.8. Construction of lncRNA–miRNA–mRNA ceRNA Networks
2.9. Quantitative Real-Time PCR (qPCR) Analysis
2.10. Statistical Analysis
3. Results
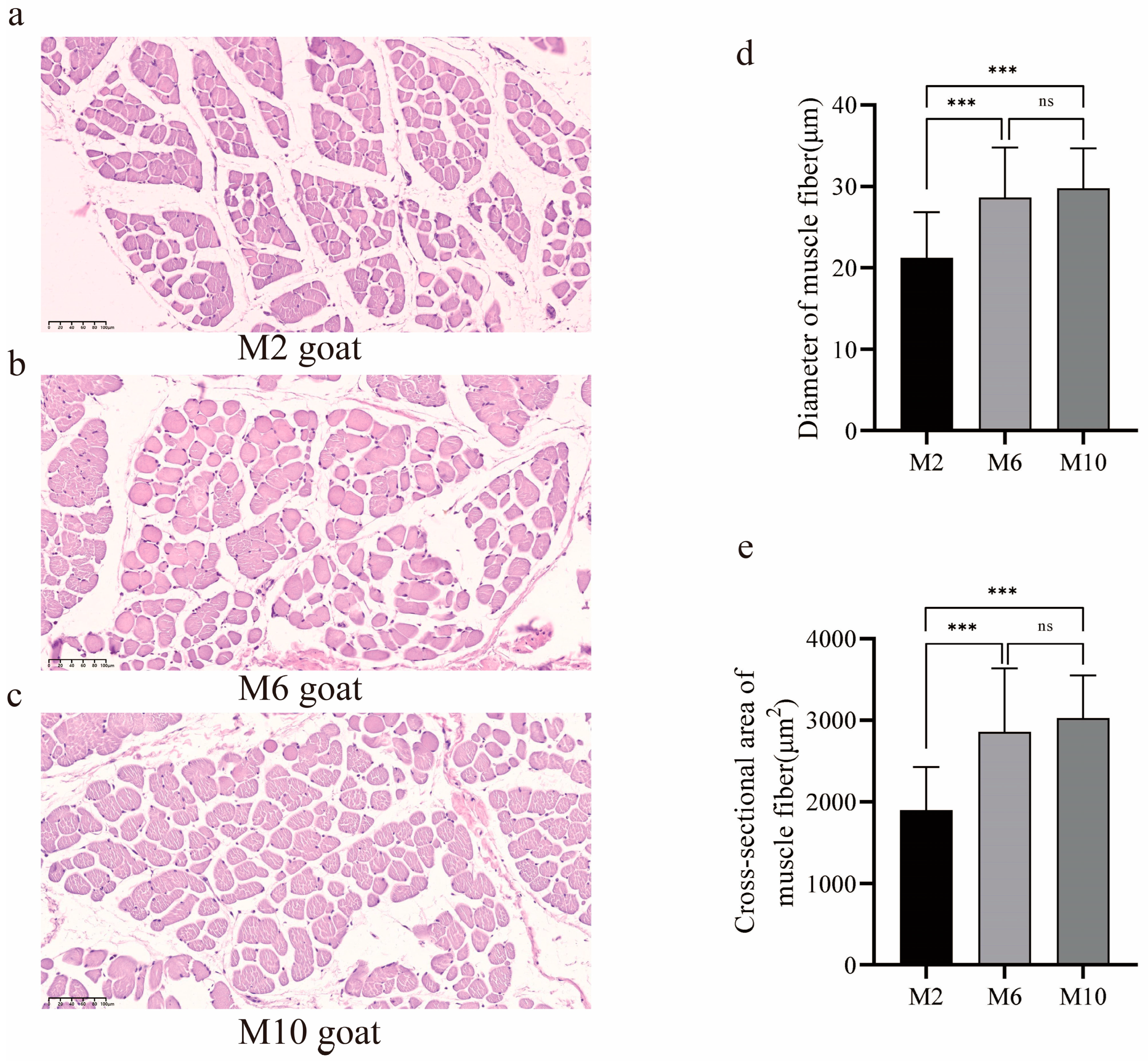
3.1. Observation of Muscle Fiber Characteristics in Goats of Different Ages
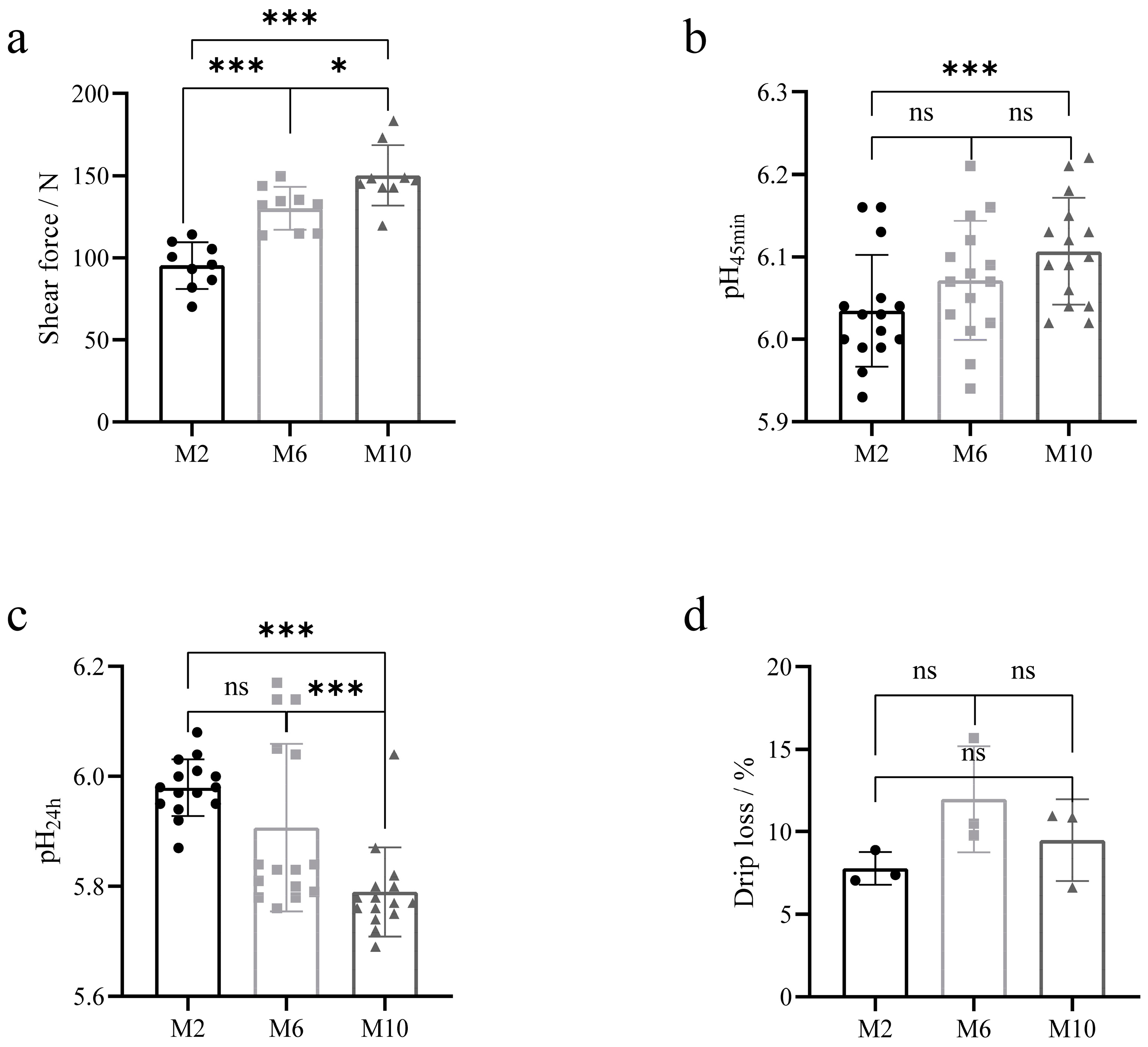
3.2. Quality Comparison of Goat Meat of Different Ages
3.3. Quality Control and Comparative Analysis of Sequencing Data
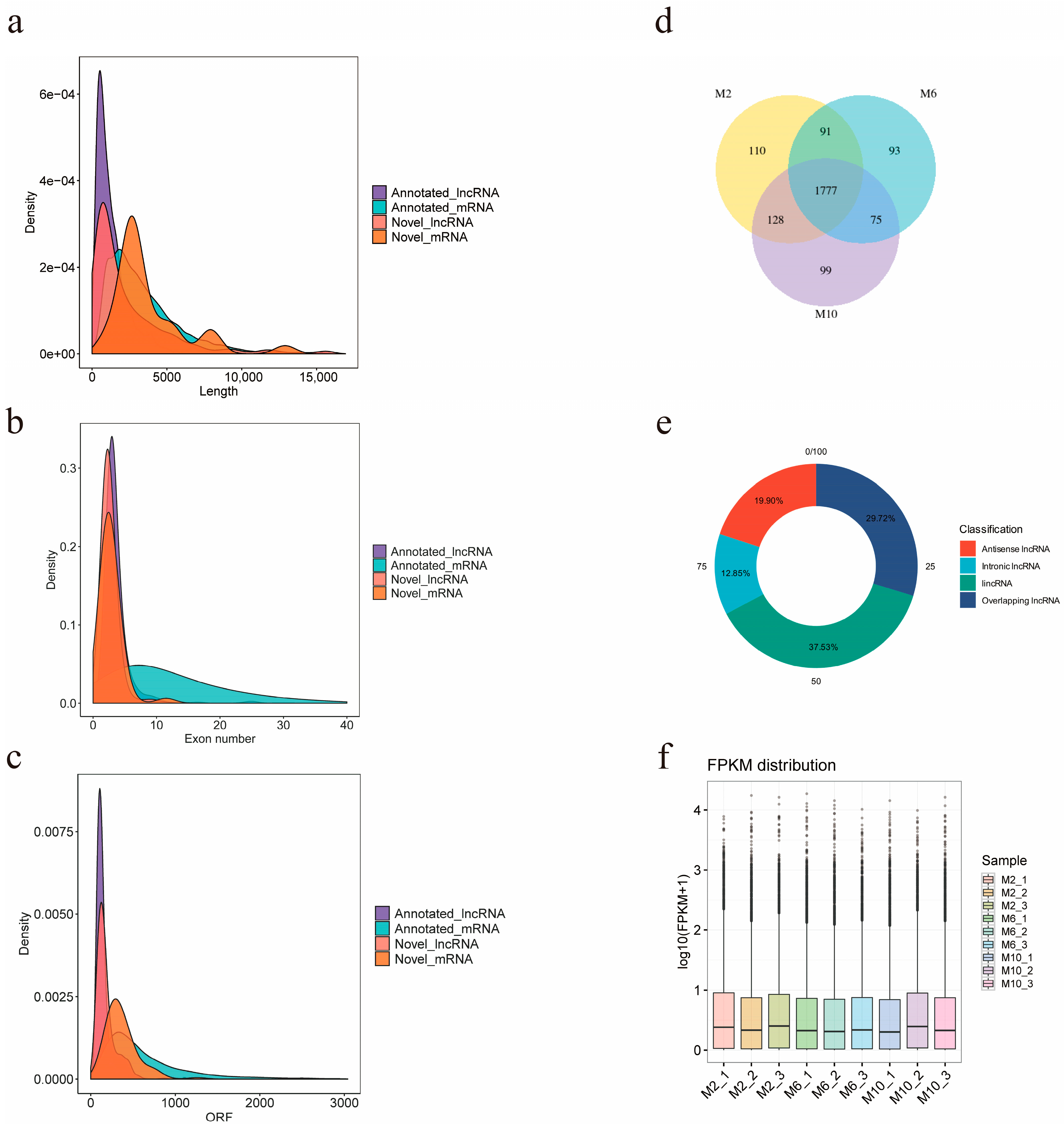
3.4. Screening and Identification of lncRNA
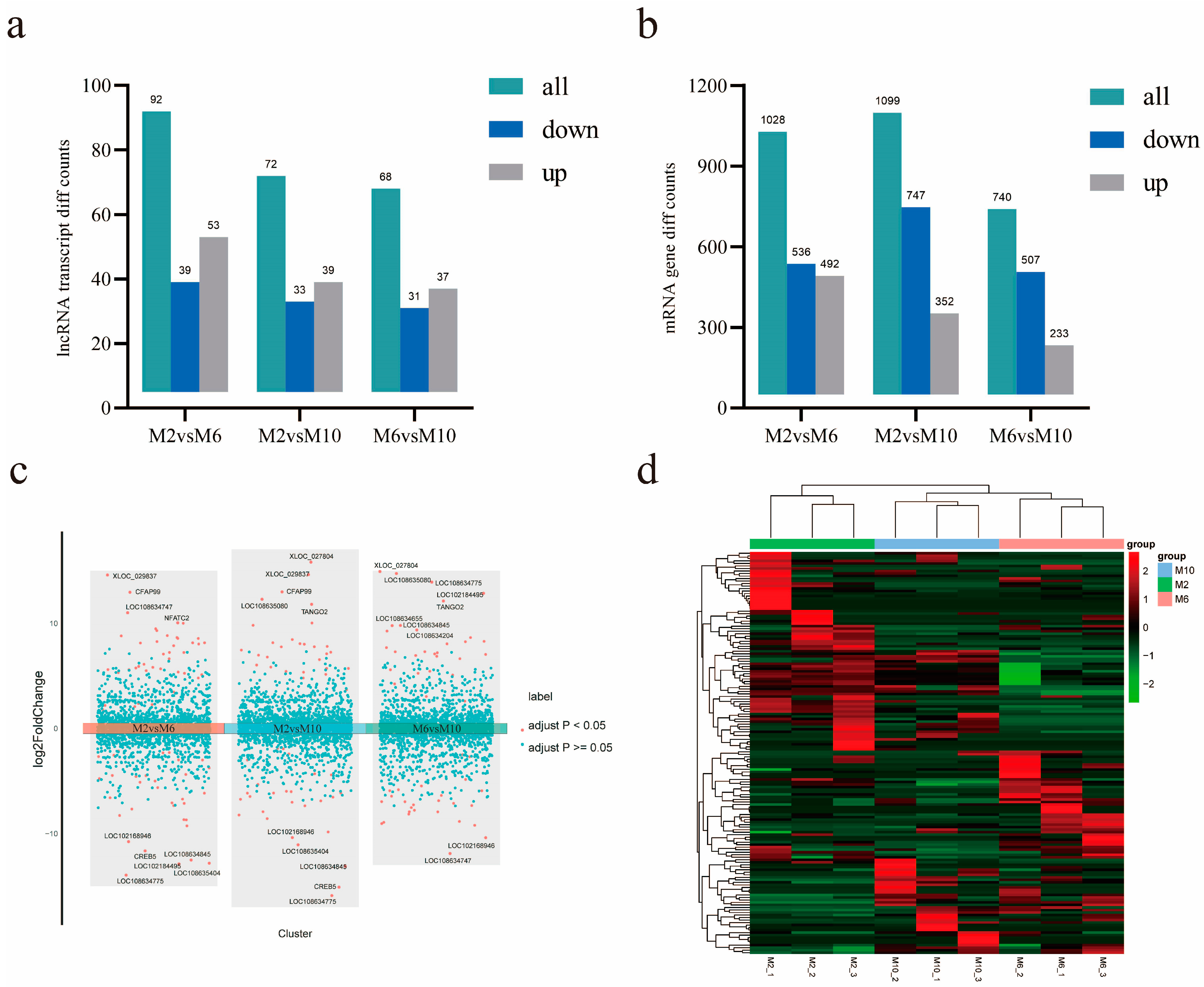
3.5. Analysis of Differentially Expressed mRNA and lncRNA
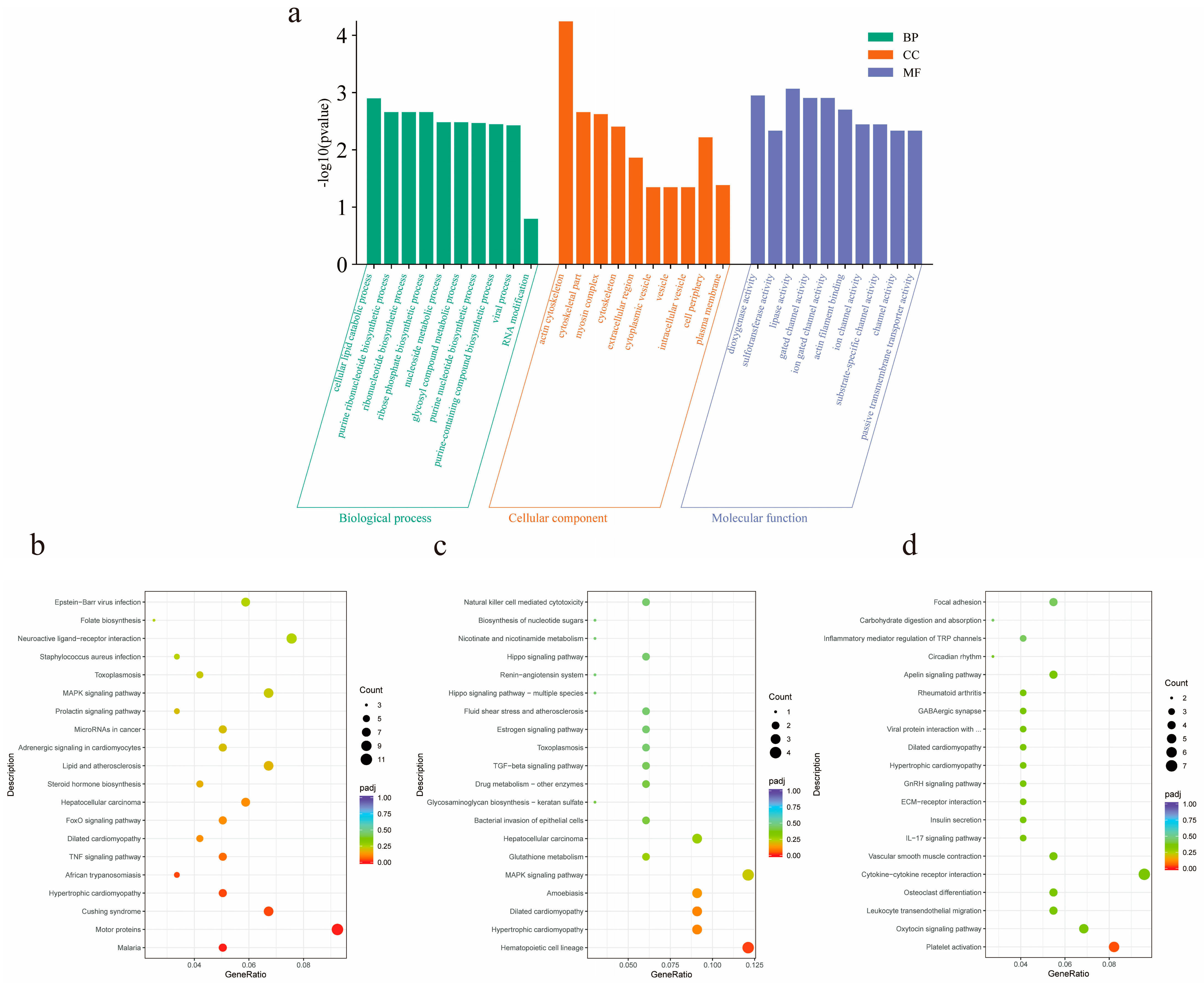
3.6. Prediction and Enrichment Analysis of lncRNA Target Genes
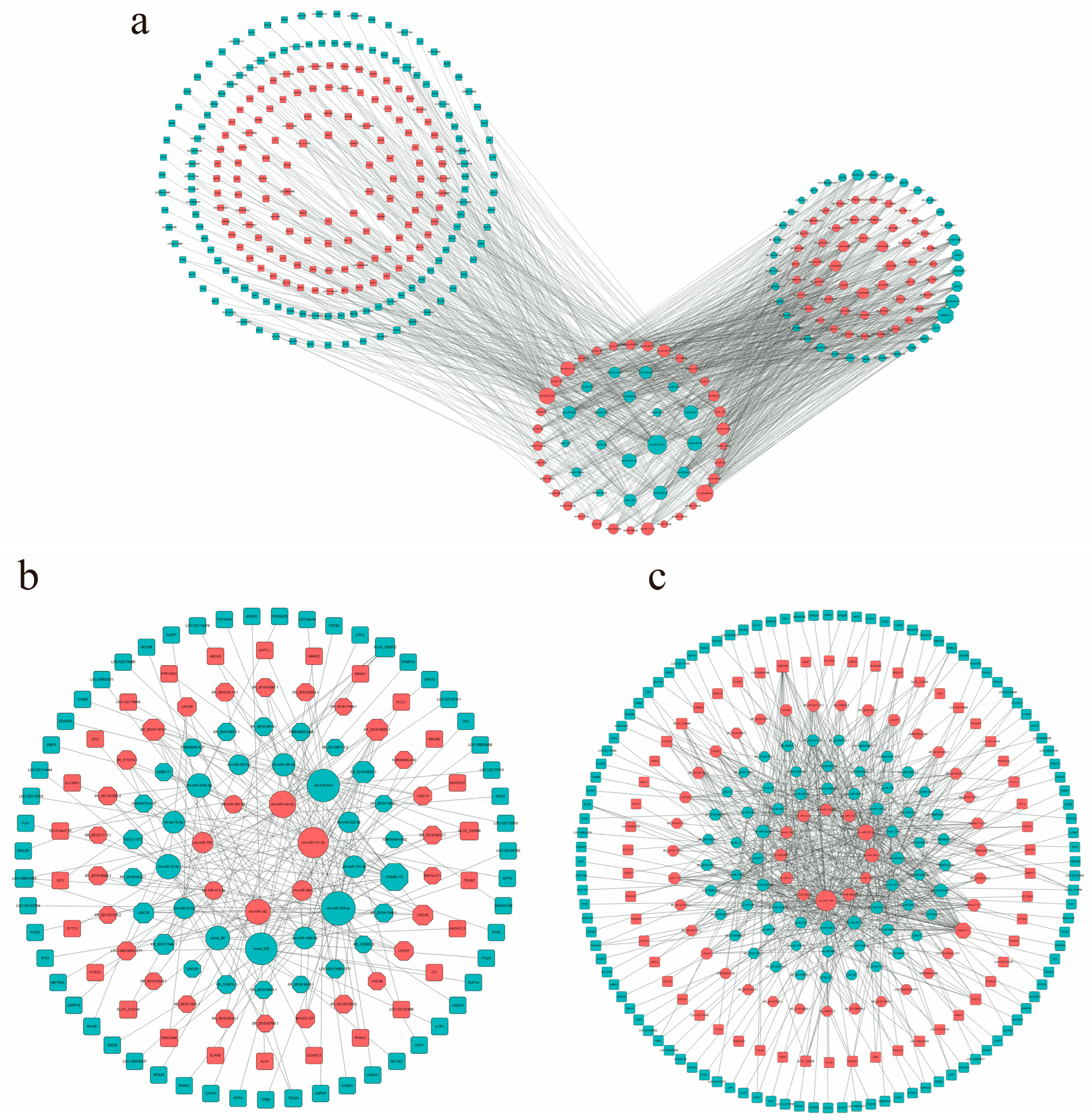
3.7. Construction of lncRNA–miRNA–mRNA ceRNA Network
3.8. Verification of Differentially Expressed lncRNAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crespi, M. Long non-coding RNAs reveal new regulatory mechanisms controlling gene expression. Comptes Rendus Biol. 2022, 345, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar] [PubMed]
- Mattick, J.S. Non-coding RNAs: The architects of eukaryotic complexity. EMBO Rep. 2001, 2, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Sunkin, S.M.; Mehler, M.F.; Mattick, J.S. Specific expression of long noncoding RNAs in the mouse brain. Proc. Natl. Acad. Sci. USA 2008, 105, 716–721. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef]
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; Horlings, H.M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621–629. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 925–934. [Google Scholar] [CrossRef]
- Kosinska-Selbi, B.; Mielczarek, M.; Szyda, J. Review: Long non-coding RNA in livestock. Animal 2020, 14, 2003–2013. [Google Scholar] [CrossRef]
- Di Michele, F.; Chillon, I.; Feil, R. Imprinted Long Non-Coding RNAs in Mammalian Development and Disease. Int. J. Mol. Sci. 2023, 24, 13647. [Google Scholar] [CrossRef]
- Rashid, F.; Shah, A.; Shan, G. Long Non-coding RNAs in the Cytoplasm. Genom. Proteom. Bioinform. 2016, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carnes, M.E.; Pins, G.D. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengineering 2020, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Parakati, R.; DiMario, J.X. Repression of myoblast proliferation and fibroblast growth factor receptor 1 promoter activity by KLF10 protein. J. Biol. Chem. 2013, 288, 13876–13884. [Google Scholar] [CrossRef]
- Yun, J.; Huang, X.; Liu, C.; Shi, M.; Li, W.; Niu, J.; Cai, C.; Yang, Y.; Gao, P.; Guo, X.; et al. Genome-wide analysis of circular RNA-mediated ceRNA regulation in porcine skeletal muscle development. BMC Genom. 2023, 24, 196. [Google Scholar] [CrossRef]
- Cao, Y.; Ai, Y.; Zhang, X.; Zhang, J.; Long, X.; Zhu, Y.; Wang, L.; Gu, Q.; Han, H. Genome-wide epigenetic dynamics during postnatal skeletal muscle growth in Hu sheep. Commun. Biol. 2023, 6, 1077. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Chen, G.; Li, J.; Li, M.; Zou, C.; Fang, C.; Li, C. Transcriptome Analysis Suggests the Roles of Long Intergenic Non-coding RNAs in the Growth Performance of Weaned Piglets. Front. Genet. 2019, 10, 196. [Google Scholar] [CrossRef]
- Buckingham, M.; Rigby, P.W.J. Gene Regulatory Networks and Transcriptional Mechanisms that Control Myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Shen, J.; Zhen, H.; Li, L.; Zhang, Y.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Hao, Z.; Li, M.; et al. Identification and characterization of circular RNAs in Longissimus dorsi muscle tissue from two goat breeds using RNA-Seq. Mol. Genet. Genom. 2022, 297, 817–831. [Google Scholar] [CrossRef]
- Brant, L.M.S.; De Freitas, J.E.; Jose, E.; Pereira, F.M.; Pina, D.D.S.; Santos, S.A.; Leite, L.C.; Cirne, L.G.A.; Alba, H.D.R.; de Araujo, M.L.G.M.L.; et al. Effects of alternative energy and protein sources on performance, carcass characteristics, and meat quality of feedlot lambs. Livest. Sci. 2021, 251, 104611. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.; Su, R.; Liu, W.; Ren, Y.; Zhang, C.; Du, M.; Zhang, J. Effect of dietary Tartary buckwheat extract supplementation on growth performance, meat quality and antioxidant activity in ewe lambs. Meat Sci. 2017, 134, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, X.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Effects of Herbal Tea Residue on Growth Performance, Meat Quality, Muscle Metabolome, and Rumen Microbiota Characteristics in Finishing Steers. Front. Microbiol. 2022, 12, 821293. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- Mistry, J.; Bateman, A.; Finn, R.D. Predicting active site residue annotations in the Pfam database. BMC Bioinform. 2007, 8, 298. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Wright, M.W. A short guide to long non-coding RNA gene nomenclature. Hum. Genom. 2014, 8, 7. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Wei, C.; Wu, M.; Wang, C.; Liu, R.; Zhao, H.; Yang, L.; Liu, J.; Wang, Y.; Zhang, S.; Yuan, Z.; et al. Long Noncoding RNA Lnc-SEMT Modulates IGF2 Expression by Sponging miR-125b to Promote Sheep Muscle Development and Growth. Cell. Physiol. Biochem. 2018, 49, 447–462. [Google Scholar] [CrossRef]
- Li, R.; Li, B.; Jiang, A.; Cao, Y.; Hou, L.; Zhang, Z.; Zhang, X.; Liu, H.; Kim, K.-H.; Wu, W. Exploring the lncRNAs Related to Skeletal Muscle Fiber Types and Meat Quality Traits in Pigs. Genes 2020, 11, 883. [Google Scholar] [CrossRef]
- Nivelle, M.A.; Beghin, A.S.; Vrinten, P.; Nakamura, T.; Delcour, J.A. Amylose and amylopectin functionality during storage of bread prepared from flour of wheat containing unique starches. Food Chem. 2020, 320, 126609. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kang, G.; Seong, P.-N.; Park, B.; Kang, S.M. Effect of slaughter age on the antioxidant enzyme activity, color, and oxidative stability of Korean Hanwoo (Bos taurus coreanae) cow beef. Meat Sci. 2015, 108, 44–49. [Google Scholar] [CrossRef] [PubMed]
- England, E.M.; Matarneh, S.K.; Oliver, E.M.; Apaoblaza, A.; Scheffler, T.L.; Shi, H.; Gerrard, D.E. Excess glycogen does not resolve high ultimate pH of oxidative muscle. Meat Sci. 2016, 114, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Joo, S.T.; Ryu, Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010, 86, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Henckel, P.; Karlsson, A.; Jensen, M.T.; Oksbjerg, N.; Petersen, J.S. Metabolic conditions in Porcine longissimus muscle immediately pre-slaughter and its influence on peri- and post mortem energy metabolism. Meat Sci. 2002, 62, 145–155. [Google Scholar] [CrossRef]
- Wicks, J.; Beline, M.; Morales Gomez, J.F.; Luzardo, S.; Silva, S.L.; Gerrard, D. Muscle Energy Metabolism, Growth, and Meat Quality in Beef Cattle. Agriculture 2019, 9, 195. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Fisher, P. Comparison of meat quality characteristics between young and old ostriches. Meat Sci. 2001, 59, 335–337. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.; Faustman, C. Metmyoglobin reducing activity. Meat Sci. 2005, 71, 407–439. [Google Scholar] [CrossRef]
- Bernad, L.; Casado, P.D.; Murillo, N.L.; Picallo, A.B.; Garriz, C.A.; Maceira, N.O. Meat quality traits in the Greater rhea (Rhea americana) as influenced by muscle, sex and age. Poult. Sci. 2018, 97, 1579–1587. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef] [PubMed]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New Insights in Muscle Biology that Alter Meat Quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Dong, Y.; Zhao, W.; Guo, J.; Zhong, T.; Wang, L.; Li, L.; Zhang, H. Genome-wide identification and characterization of long non-coding RNAs in developmental skeletal muscle of fetal goat. BMC Genom. 2016, 17, 666. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.H.; He, Q.J.; Xia, W.L.; Rong, G.; Wang, D.F.; Li, M.; Ji, F.J.; Sun, W.P.; Cao, T.; Zhou, H.L.; et al. Integrated analysis of lncRNA and gene expression in longissimus dorsi muscle at two developmental stages of Hainan black goats. PLoS ONE 2022, 17, e0276004. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, J.; Fan, X.; Chen, J.; Wang, Z.; Liu, X.; Yi, G.; Liu, Y.; Niu, Y.; Zhang, L.; et al. The genome variation and developmental transcriptome maps reveal genetic differentiation of skeletal muscle in pigs. PLoS Genet. 2021, 17, e1009910. [Google Scholar] [CrossRef]
- Zhao, W.J.; Li, Z.J.; Liu, Q.; Xie, S.; Li, M.X.; Wang, Y.; Li, C.C. Analysis of long intergenic non-coding RNAs transcriptomic profiling in skeletal muscle growth during porcine embryonic development. Sci. Rep. 2021, 11, 15240. [Google Scholar] [CrossRef]
- Chen, G.; Cheng, X.; Shi, G.; Zola, C.; Chen, L.; Li, J.; Li, M.; Fang, C.; Li, C. Transcriptome Analysis Reveals the Effect of Long Intergenic Noncoding RNAs on Pig Muscle Growth and Fat Deposition. BioMed Res. Int. 2019, 2019, 2951427. [Google Scholar] [CrossRef]
- Wu, T.Y.; Wang, S.H.; Wang, L.H.; Zhang, W.B.; Chen, W.H.; Lv, X.Y.; Li, Y.; Hussain, Z.; Sun, W. Long Noncoding RNA (lncRNA) CTTN-IT1 Elevates Skeletal Muscle Satellite Cell Proliferation and Differentiation by Acting as ceRNA for YAP1 Through Absorbing miR-29a in Hu Sheep. Front. Genet. 2020, 11, 843. [Google Scholar] [CrossRef]
- Li, Z.; Cai, B.; Abdalla, B.A.; Zhu, X.; Zheng, M.; Han, P.; Nie, Q.; Zhang, X. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachexia Sarcopenia Muscle 2019, 10, 391–410. [Google Scholar] [CrossRef]
- Lin, J.; Tao, L.; Deng, L.; Zhou, R.; Lou, S.; Chen, S.; Chen, X.; Lu, C.; Li, P.; Hu, B. Epigenome-wide DNA methylation analysis of myasthenia gravis. FEBS Open Bio. 2023, 13, 1375–1389. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Orom, U.A.; Cesaroni, M.; Beringer, M.; Taatjes, D.J.; Blobel, G.A.; Shiekhattar, R. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature 2013, 494, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, K.K.; Skovsted, G.F.; Perisic, L.; Dreier, R.; Berg, J.O.; Hedin, U.; Rippe, C.; Sward, K. Expression of endothelin type B receptors (EDNRB) on smooth muscle cells is controlled by MKL2, ternary complex factors, and actin dynamics. Am. J. Physiol.-Cell Physiol. 2018, 315, C873–C884. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, A.; Ovilo, C.; Nunez, Y.; Benitez, R.; Lopez-Garcia, A.; Garcia, F.; Felix, M.d.R.; Laranjo, M.; Charneca, R.; Martins, J.M. Transcriptomic Profiling of Skeletal Muscle Reveals Candidate Genes Influencing Muscle Growth and Associated Lipid Composition in Portuguese Local Pig Breeds. Animals 2021, 11, 1423. [Google Scholar] [CrossRef]
- Feng, J.; Han, X.; Yuan, Y.; Cho, C.K.; Janeckova, E.; Guo, T.; Pareek, S.; Rahman, M.S.; Zheng, B.; Bi, J.; et al. TGF-β signaling and Creb5 cooperatively regulate Fgf18 to control pharyngeal muscle development. eLife 2022, 11, e80405. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Qi, Y.; Zhu, C.; Zhao, Z.; Zhang, X.; Liu, X.; Li, S.; Zhao, F.; Wang, J.; et al. Long-stranded non-coding RNAs temporal-specific expression profiles reveal longissimus dorsi muscle development and intramuscular fat deposition in Tianzhu white yak. J. Anim. Sci. 2023, 101, skad394. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, H.; Yang, C.; Li, Q.; Qiu, M.; Song, X.; Yu, C.; Jiang, X.; Liu, L.; Hu, C.; et al. Comparative transcriptome analysis reveals regulators mediating breast muscle growth and development in three chicken breeds. Anim. Biotechnol. 2019, 30, 233–241. [Google Scholar] [CrossRef]
- Tran, L.; Hanavan, P.D.; Campbell, L.E.; De Filippis, E.; Lake, D.F.; Coletta, D.K.; Roust, L.R.; Mandarino, L.J.; Carroll, C.C.; Katsanos, C.S. Prolonged Exposure of Primary Human Muscle Cells to Plasma Fatty Acids Associated with Obese Phenotype Induces Persistent Suppression of Muscle Mitochondrial ATP Synthase β Subunit. PLoS ONE 2016, 11, e0160057. [Google Scholar] [CrossRef]
- Ji, H.; Wang, H.; Ji, Q.M.; Ji, W.B.; Luo, X.L.; Wang, J.B.; Chai, Z.X.; Xin, J.W.; Cai, X.; Wu, Z.J.; et al. Differential expression profile of microRNA in yak skeletal muscle and adipose tissue during development. Genes Genom. 2020, 42, 1347–1359. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, C.; Su, X.; Wang, H.; Yang, W.; Zan, L. MEF2A Regulates the MEG3-DIO3 miRNA Mega Cluster-Targeted PP2A Signaling in Bovine Skeletal Myoblast Differentiation. Int. J. Mol. Sci. 2019, 20, 2748. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Z.; Wang, P.; Li, W.; Zhao, S.; Liu, Y.; Chu, M. Estrogen-mediated oar-miR-485-5p targets PPP1R13B to regulate myoblast proliferation in sheep. Int. J. Biol. Macromol. 2023, 236, 123987. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, X.Y.; Shen, X.X.; Zhang, Y.; Zhang, Y.; Ye, L.; Li, D.Y.; Zhu, Q.; Yin, H.D. CircCCDC91 regulates chicken skeletal muscle development by sponging miR-15 family via activating IGF1-PI3K/AKT signaling pathway. Poult. Sci. 2022, 101, 101803. [Google Scholar] [CrossRef] [PubMed]
- Freire, P.P.; Cury, S.S.; Lopes, L.O.; Fernandez, G.J.; Liu, J.M.; de Moraes, L.N.; de Oliveira, G.; Oliveira, J.S.; de Moraes, D.; Cabral-Marques, O.; et al. Decreased miR-497-5p Suppresses IL-6 Induced Atrophy in Muscle Cells. Cells 2021, 10, 3527. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Wang, X.; Wang, D.; Zhang, T.; Zhang, Y.; Wang, X.; Chen, Z. Identification of novel pathways and immune profiles related to sarcopenia. Front. Med. 2023, 10, 928285. [Google Scholar] [CrossRef]
- Keren, A.; Tamir, Y.; Bengal, E. The p38 MAPK signaling pathway: A major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 2006, 252, 224–230. [Google Scholar] [CrossRef]
- Cohen, S.; Lee, D.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. Trim32 reduces PI3K-Akt-Fox0 signaling in muscle atrophy by promoting plakoglobin-PI3K dissociation. J. Cell Biol. 2014, 204, 747–758. [Google Scholar] [CrossRef]

| Gene Name | Primer Type | Sequence of Primers |
|---|---|---|
| TCONS_00062881 | Forward | ATCCGCAGCAGGTCTCCAAG |
| Reverse | AGCCCTTAGAGCCAATCCTTATCC | |
| TCONS_00058684 | Forward | CCTGCGTCCTCTCCTCTTGG |
| Reverse | CGGATGAAGCGGAACGGATATG | |
| TCONS_00017558 | Forward | TTCCTCAGCCCACTCCAAACAAAC |
| Reverse | GCGAGCACAGAGCCCAAACC | |
| TCONS_00056090 | Forward | TAGAGGAAGGAAGCGGGAAGGAAG |
| Reverse | TCGGTTTAGCCAACAGGGTCTTTG | |
| ACTB | Forward | AGCCTTCCTTCCTGGGCATGGA |
| Reverse | GGACAGCACCGTGTTGGCGTAA |
| Item | Group | |||
|---|---|---|---|---|
| 2 m | 6 m | 10 m | p-Value | |
| Lightness (L*) | 43.66 ± 4.44 a | 44.61 ± 4.52 a | 35.08 ± 1.15 b | <0.01 |
| Redness (a*) | 13.12 ± 3.48 a | 19.11 ± 6.25 b | 4.93 ± 1.01 c | <0.01 |
| Yellowness (b*) | 8.69 ± 2.94 b | 16.10 ± 3.58 a | 10.23 ± 0.73 b | <0.01 |
| pH45min | 6.03 ± 0.068 b | 6.07 ± 0.072 ab | 6.11 ± 0.065 a | 0.006 |
| pH24h | 5.98 ± 0.051 a | 5.91 ± 0.152 a | 5.79 ± 0.081 b | <0.01 |
| Shear force (N) | 98.68 ± 10.70 a | 130.06 ± 13.08 b | 150.10 ± 18.44 c | <0.05 |
| Drip loss% | 7.8 ± 1.0 | 12.0 ± 3.2 | 9.5 ± 2.5 | >0.05 |
| Sample Name | Raw Reads | Clean Reads | Q20 (%) | Q30 (%) | Total Mapping (%) |
|---|---|---|---|---|---|
| M2-1 | 91,139,898 | 89,638,430 | 98.46 | 95.95 | 86,129,394 (96.09%) |
| M2-2 | 87,557,804 | 86,071,772 | 98.11 | 95.22 | 82,055,643 (95.33%) |
| M2-3 | 83,098,176 | 81,743,532 | 98.03 | 95.12 | 77,764,683 (95.13%) |
| M6-1 | 86,928,100 | 85,492,412 | 98.03 | 95.13 | 81,247,096 (95.03%) |
| M6-2 | 89,144,180 | 87,765,748 | 97.99 | 95.01 | 83,546,691 (95.19%) |
| M6-3 | 87,391,674 | 86,154,582 | 98.07 | 95.17 | 81,960,778 (95.13%) |
| M10-1 | 82,018,490 | 80,757,046 | 98.21 | 95.45 | 77,024,837 (95.38%) |
| M10-2 | 89,452,288 | 88,011,694 | 98.03 | 95.06 | 83,682,629 (95.08%) |
| M10-3 | 89,534,296 | 88,120,994 | 97.94 | 94.94 | 83,555,700 (94.82%) |
| lncRNA | miRNA | mRNA |
|---|---|---|
| CREB5-IT3 | chi-miR-758 | WNT2 |
| XR_001297299.2 | chi-miR-758 | ADCY2 |
| CREB5-IT3 | chi-miR-758 | LRRTM3 |
| CREB5-IT3 | chi-miR-127-5p | OPTN |
| 108635404-AS2 | chi-miR-127-5p | CITED1 |
| 108634775-AS1 | chi-miR-127-5p | S100A1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Sun, J.; Malyar, R.M.; Shi, F. Analysis of lncRNAs and Their Regulatory Network in Skeletal Muscle Development of the Yangtze River Delta White Goat. Animals 2024, 14, 3125. https://doi.org/10.3390/ani14213125
Tang W, Sun J, Malyar RM, Shi F. Analysis of lncRNAs and Their Regulatory Network in Skeletal Muscle Development of the Yangtze River Delta White Goat. Animals. 2024; 14(21):3125. https://doi.org/10.3390/ani14213125
Chicago/Turabian StyleTang, Wenjun, Jiahao Sun, Rahmani Mohammad Malyar, and Fangxiong Shi. 2024. "Analysis of lncRNAs and Their Regulatory Network in Skeletal Muscle Development of the Yangtze River Delta White Goat" Animals 14, no. 21: 3125. https://doi.org/10.3390/ani14213125
APA StyleTang, W., Sun, J., Malyar, R. M., & Shi, F. (2024). Analysis of lncRNAs and Their Regulatory Network in Skeletal Muscle Development of the Yangtze River Delta White Goat. Animals, 14(21), 3125. https://doi.org/10.3390/ani14213125






