Does Exposure to Summer Season at Different Stages of Intrauterine Development and Maternal Parity Affect Health and First-Lactation Milk Production of Female Offspring of Holstein Cows? †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Herds and Dataset Acquisition
2.2. Study Design
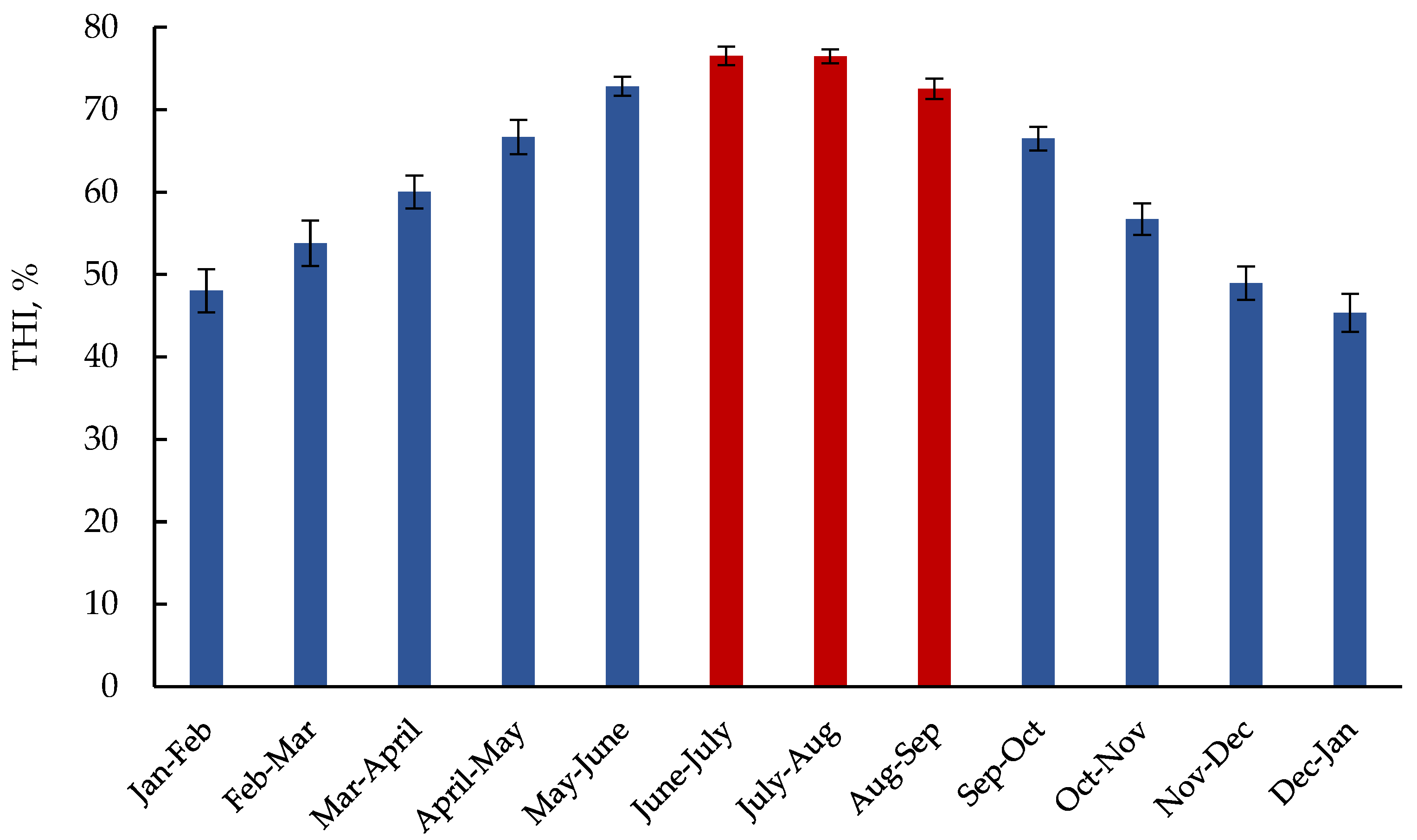
2.3. Meteorological Data
2.4. Management of Dams
2.5. Management of Daughters
Binary-Coded Variables
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Maternal Summer Season Exposure
4.2. Maternal Parity Effect
4.3. Future Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opsomer, G.; Van Eetvelde, M.; Kamal, M.; Van Soom, A. Epidemiological evidence for metabolic programming in dairy cattle. Reprod. Fertil. Dev. 2017, 29, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Recio, O.; Ugarte, E.; Bach, A. Trans-generational effect of maternal lactation during pregnancy: A Holstein cow model. PLoS ONE 2012, 7, e51816. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, P.; De Vries, A. Season of conception is associated with future survival, fertility, and milk yield of Holstein cows. J. Dairy Sci. 2017, 100, 6631–6639. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Cretney, E.C.; Kropp, J.; Khateeb, K.; Berg, M.A.; Peñagaricano, F.; Magness, R.; Radunz, A.E.; Khatib, H. Maternal diet during pregnancy induces gene expression and DNA methylation changes in fetal tissues in sheep. Front. Genet. 2013, 4, 49. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Developmental programming of health and disease. Proc. Nutr. Soc. 2006, 65, 97–105. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. Board-invited review: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.T.; Dahl, G.E. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 2016, 99, 8443–8450. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- IPCC. Climate Change: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Polsky, L.; von Keyserlingk, M.A. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Tao, S.; Dahl, G.E. Invited review: Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef]
- Ouellet, V.; Boucher, A.; Dahl, G.E.; Laporta, J. Consequences of maternal heat stress at different stages of embryonic and fetal development on dairy cows’ progeny. Anim. Front. 2021, 11, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Caton, J.S.; Redmer, D.A.; Grazul-Bilska, A.T.; Vonnahme, K.A.; Borowicz, P.P.; Luther, J.S.; Wallace, J.M.; Wu, G.; Spencer, T.E. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J. Physiol. 2006, 572, 51–58. [Google Scholar] [CrossRef]
- Laporta, J.; Ferreira, F.C.; Ouellet, V.; Dado-Senn, B.; Almeida, A.K.; De Vries, A.; Dahl, G.E. Late-gestation heat stress impairs daughter and granddaughter lifetime performance. J. Dairy Sci. 2020, 103, 7555–7568. [Google Scholar] [CrossRef]
- Duncan, N.B.; Stoecklein, K.S.; Foote, A.P.; Meyer, A.M. Dam parity affects fetal growth, placental size, and neonatal metabolism in spring-born beef calves. J. Anim. Sci. 2023, 101, skac399. [Google Scholar] [CrossRef]
- Bafandeh, M.; Makiabadi, M.J.M.; Gharagozlou, F.; Vojgani, M.; Mobedi, E.; Akbarinejad, V. Developmental programming of production and reproduction in dairy cows: I. Association of maternal parity with offspring’s birth weight, milk yield, reproductive performance and AMH concentration during the first lactation period. Theriogenology 2023, 210, 34–41. [Google Scholar] [CrossRef]
- Wathes, D.C. Developmental programming of fertility in cattle—Is it a cause for concern? Animals 2022, 12, 2654. [Google Scholar] [CrossRef]
- García-Ispierto, I.; López-Gatius, F.; Bech-Sabat, G.; Santolaria, P.; Yániz, J.L.; Nogareda, C.; De Rensis, F.; López-Béjar, M. Climate factors affecting conception rate of high producing dairy cows in northeastern Spain. Theriogenology 2007, 67, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, H.; Ahmadi, F.; Babajanzade-Sorati, S.; Alamouti, A.A. Effects of fresh-cow grouping strategy and rumen-protected glucose on production performance, reproductive variables and risk of culling in Holstein cows. Vet. Med. Sci. 2023, 9, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutritional Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Bellows, R.; Lammoglia, M. Effects of severity of dystocia on cold tolerance and serum concentrations of glucose and cortisol in neonatal beef calves. Theriogenology 2000, 53, 803–813. [Google Scholar] [CrossRef]
- Gröhn, Y.; Eicker, S.; Hertl, J. The association between previous 305-day milk yield and disease in New York State dairy cows. J. Dairy Sci. 1995, 78, 1693–1702. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef] [PubMed]
- Frizzarin, M.; Gormley, I.C.; Berry, D.P.; McParland, S. Estimation of body condition score change in dairy cows in a seasonal calving pasture-based system using routinely available milk mid-infrared spectra and machine learning techniques. J. Dairy Sci. 2023, 106, 4232–4244. [Google Scholar] [CrossRef] [PubMed]
- Makiabadi, M.J.M.; Bafandeh, M.; Gharagozlou, F.; Vojgani, M.; Mobedi, E.; Akbarinejad, V. Developmental programming of production and reproduction in dairy cows: II. Association of gestational stage of maternal exposure to heat stress with offspring’s birth weight, milk yield, reproductive performance and AMH concentration during the first lactation period. Theriogenology 2023, 212, 41–49. [Google Scholar]
- Bauman, D.E.; Currie, W.B. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Rowson, A.R.; Daniels, K.M.; Ellis, S.E.; Hovey, R.C. Growth and development of the mammary glands of livestock: A veritable barnyard of opportunities. Semin. Cell Dev. Biol. 2012, 23, 557–566. [Google Scholar] [CrossRef]
- Akers, R.M. Lactation and the Mammary Gland, 1st ed.; Blackwell Publishing Co.: Ames, IA, USA, 2002. [Google Scholar]
- de Barros, F.R.; Paula-Lopes, F.F. Cellular and epigenetic changes induced by heat stress in bovine preimplantation embryos. Mol. Reprod. Dev. 2018, 85, 810–820. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V. Impact of heat stress on embryonic development during first 16 days of gestation in dairy cows. Sci. Rep. 2021, 11, 14839. [Google Scholar] [CrossRef]
- Sakatani, M.; Bonilla, L.; Dobbs, K.B.; Block, J.; Ozawa, M.; Shanker, S.; Yao, J.; Hansen, P.J. Changes in the transcriptome of morula-stage bovine embryos caused by heat shock: Relationship to developmental acquisition of thermotolerance. Reprod. Biol. Endocrinol. 2013, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miętkiewska, K.; Kordowitzki, P.; Pareek, C.S. Effects of heat stress on bovine oocytes and early embryonic development—An update. Cells 2022, 11, 4073. [Google Scholar] [CrossRef]
- Holland, M.; Odde, K. Factors affecting calf birth weight: A review. Theriogenology 1992, 38, 769–798. [Google Scholar] [CrossRef]
- Wallace, J.M.; Luther, J.S.; Milne, J.S.; Aitken, R.P.; Redmer, D.A.; Reynolds, L.P.; Hay, W.W. Nutritional modulation of adolescent pregnancy outcome—A review. Placenta 2006, 27, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Aboujaoude, C.; Peñagaricano, F.; Santos, J.E.P.; DeVries, T.J.; McBride, B.W.; Ribeiro, E.S. Associations between maternal characteristics and health, survival, and performance of dairy heifers from birth through first lactation. J. Dairy Sci. 2020, 103, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Velazquez, M.A.; Eckert, J.J. Embryos, DOHaD and David Barker. J. Dev. Orig. Health Dis. 2015, 6, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Pinal, C. The developmental origins of adult disease. Matern. Child Nutr. 2005, 1, 130–141. [Google Scholar] [CrossRef]
- Attupuram, N.M.; Kumaresan, A.; Narayanan, K.; Kumar, H. Cellular and molecular mechanisms involved in placental separation in the bovine: A review. Mol. Reprod. Dev. 2016, 83, 287–297. [Google Scholar] [CrossRef]
- Fuerst-Waltl, B.; Reichl, A.; Fuerst, C.; Baumung, R.; Sölkner, J. Effect of maternal age on milk production traits, fertility, and longevity in cattle. J. Dairy Sci. 2004, 87, 2293–2298. [Google Scholar] [CrossRef]
- Miglior, F.; Fleming, A.; Malchiodi, F.; Brito, L.F.; Martin, P.; Baes, C.F. A 100-Year Review: Identification and genetic selection of economically important traits in dairy cattle. J. Dairy Sci. 2017, 100, 10251–10271. [Google Scholar] [CrossRef]
- Beard, J.K.; Musgrave, J.A.; Hanford, K.J.; Funston, R.N.; Mulliniks, J.T. The effect of dam age on heifer progeny performance and longevity. Transl. Anim. Sci. 2019, 3 (Suppl. S1), 1710–1713. [Google Scholar] [CrossRef]
- Beiranvand, H.; Mahnani, A.; Kahyani, A.; Dunshea, F.R.; Ahmadi, F. Effects of summer season exposure at different trimesters of gestation on survival and first-lactation milk production capacity of the offspring. In Proceedings of the Joint Asian-Australasian Association of Animal Production (AAAP) and Australian Association of Animal Science (AAAS) Animal Production Congress, Melbourne, Australia, 8–12 July 2024. [Google Scholar]

| Category | N | Gestation Exposure in Summer | p-Value | ||
|---|---|---|---|---|---|
| First Trimester | Second Trimester | Third Trimester | |||
| Dam parity | |||||
| Nulliparous | 4525 | 37.2 ± 0.14 a | 37.5 ± 0.12 a | 36.6 ± 0.10 b | <0.01 |
| Parous | 6321 | 38.6 ± 0.13 a | 38.7 ± 0.10 a | 38.2 ± 0.09 b | <0.01 |
| Birth weight categorization | |||||
| <35 kg | 3084 | 32.4 ± 0.12 | 32.1 ± 0.10 | 32.1 ± 0.08 | 0.06 |
| >35 kg | 7762 | 40.0 ± 0.07 a | 40.1 ± 0.06 a | 39.8 ± 0.05 b | 0.01 |
| Category | N | Incidence (%) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Dam parity | ||||
| Nulliparous | 3740 | 48.5 | Referent | 0.08 |
| Parous | 4943 | 49.6 | 1.09 (0.99–1.19) | |
| Gestation exposure in summer | ||||
| First trimester | 1829 | 50.4 | Referent | 0.31 |
| Second trimester | 2834 | 49.1 | 0.92 (0.81–1.03) | |
| Third trimester | 4020 | 48.6 | 0.93 (0.83–1.04) | |
| Category | N | Incidence (%) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Dystocia | ||||
| Dam parity | ||||
| Nulliparous | 3740 | 8.50 | Referent | <0.01 |
| Parous | 4943 | 14.0 | 1.73 (1.49–1.99) | |
| Gestation exposure in summer | ||||
| First trimester | 1829 | 11.7 | Referent | 0.30 |
| Second trimester | 2834 | 12.5 | 1.01 (0.84–1.23) | |
| Third trimester | 4020 | 11.1 | 0.90 (0.75–1.08) | |
| Retained placenta | ||||
| Dam parity | ||||
| Nulliparous | 3734 | 4.93 | Referent | <0.01 |
| Parous | 4927 | 3.67 | 0.71 (0.57–0.89) | |
| Gestation exposure in summer | ||||
| First trimester | 1819 | 4.95 | Referent | 0.39 |
| Second trimester | 2827 | 4.03 | 0.85 (0.64–1.13) | |
| Third trimester | 4015 | 4.01 | 0.84 (0.64–1.09) | |
| Metritis | ||||
| Dam parity | ||||
| Nulliparous | 3727 | 17.1 | Referent | 0.02 |
| Parous | 4916 | 18.1 | 1.16 (1.03–1.31) | |
| Gestation exposure in summer | ||||
| First trimester | 1813 | 17.7 | Referent | 0.67 |
| Second trimester | 2823 | 17.4 | 0.94 (0.80–1.10) | |
| Third trimester | 4007 | 17.8 | 0.99 (0.86–1.15) | |
| Dam Parity | N | Gestation Exposure in Summer | p-Value | ||
|---|---|---|---|---|---|
| First Trimester | Second Trimester | Third Trimester | |||
| Nulliparous | 2261 | 10,998 ± 160 c | 11,194 ± 158 b | 11,364 ± 154 a | <0.01 |
| Parous | 2805 | 10,973 ± 159 b | 11,119 ± 155 b | 11,321 ± 152 a | <0.01 |
| Category | N | Hazard Ratio | 95% Confidence Interval | p-Value 1 |
|---|---|---|---|---|
| Dam parity | ||||
| Nulliparous | 4525 | Referent | <0.01 | |
| Parous | 6321 | 1.17 | 1.12–1.23 | |
| Gestation exposure in summer | ||||
| First trimester | 2345 | Referent | 0.01 | |
| Second trimester | 3513 | 0.96 | 0.90–1.02 | |
| Third trimester | 4988 | 0.90 | 0.84–0.95 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beiranvand, H.; Mahnani, A.; Kahyani, A.; Dunshea, F.R.; Ahmadi, F. Does Exposure to Summer Season at Different Stages of Intrauterine Development and Maternal Parity Affect Health and First-Lactation Milk Production of Female Offspring of Holstein Cows? Animals 2024, 14, 3040. https://doi.org/10.3390/ani14203040
Beiranvand H, Mahnani A, Kahyani A, Dunshea FR, Ahmadi F. Does Exposure to Summer Season at Different Stages of Intrauterine Development and Maternal Parity Affect Health and First-Lactation Milk Production of Female Offspring of Holstein Cows? Animals. 2024; 14(20):3040. https://doi.org/10.3390/ani14203040
Chicago/Turabian StyleBeiranvand, Hamed, Abolfazl Mahnani, Ali Kahyani, Frank R. Dunshea, and Farhad Ahmadi. 2024. "Does Exposure to Summer Season at Different Stages of Intrauterine Development and Maternal Parity Affect Health and First-Lactation Milk Production of Female Offspring of Holstein Cows?" Animals 14, no. 20: 3040. https://doi.org/10.3390/ani14203040
APA StyleBeiranvand, H., Mahnani, A., Kahyani, A., Dunshea, F. R., & Ahmadi, F. (2024). Does Exposure to Summer Season at Different Stages of Intrauterine Development and Maternal Parity Affect Health and First-Lactation Milk Production of Female Offspring of Holstein Cows? Animals, 14(20), 3040. https://doi.org/10.3390/ani14203040






