Ruminal Crude Protein Degradation Determined in Sacco and by Co-Incubation of Streptomyces griseus Protease and Carbohydrases
Abstract
Simple Summary
Abstract
1. Introduction
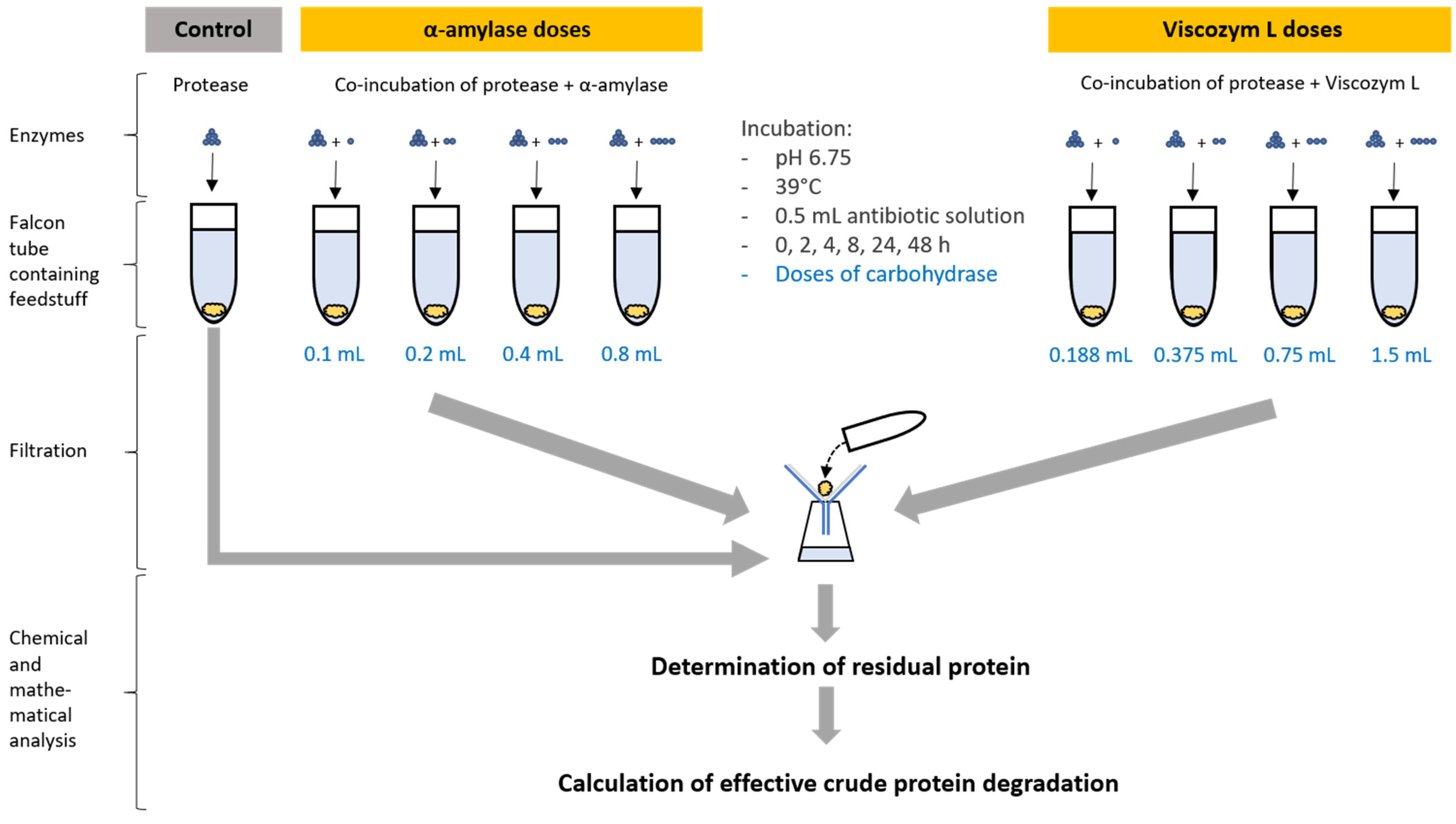
2. Materials and Methods
2.1. Feedstuffs
2.2. In Sacco Procedure
2.3. In Vitro Procedure
2.4. Effective Protein Degradation
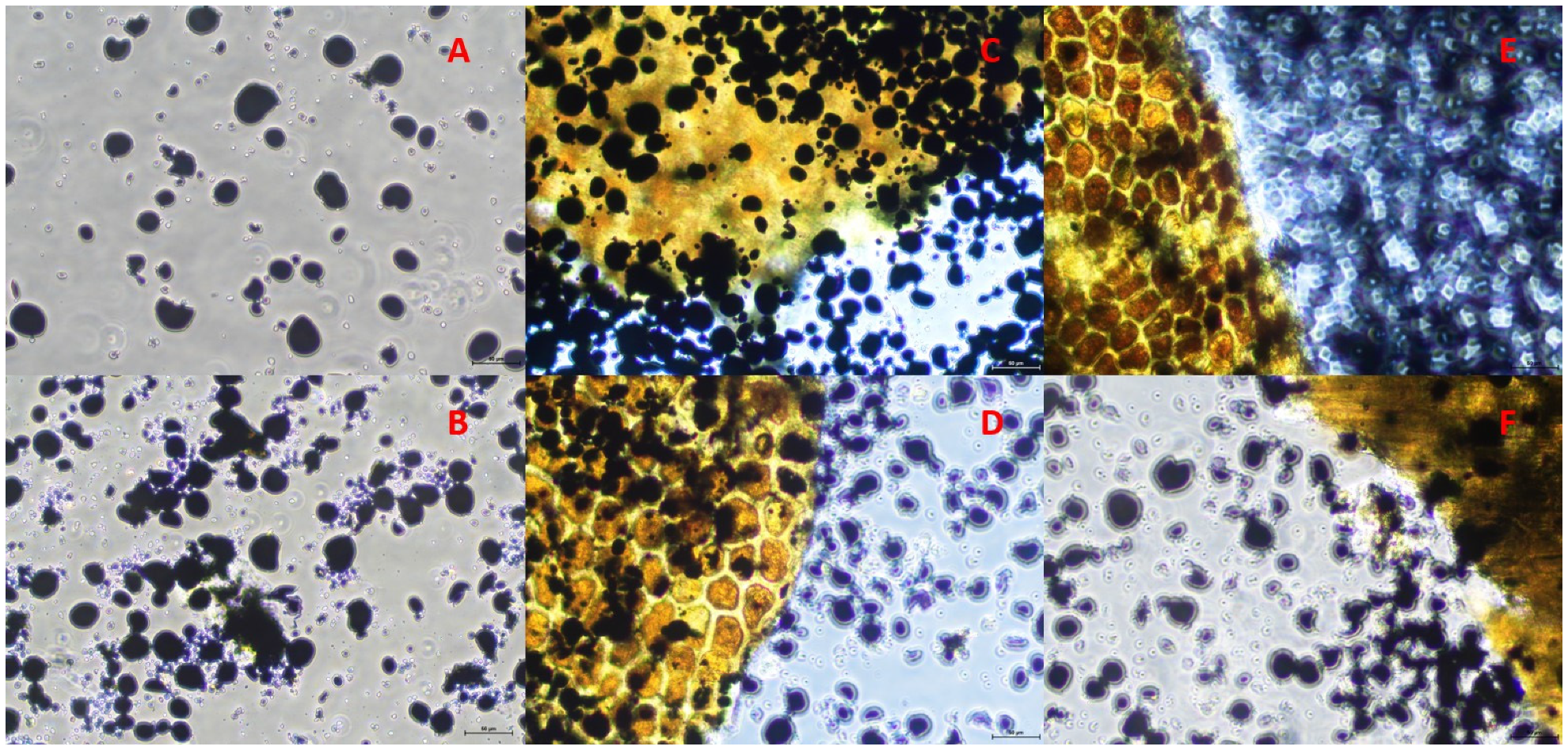
2.5. Microscopy
2.6. Chemical Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hristov, A.N.; Bannink, A.; Crompton, L.A.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.K.; Bayat, A.R.; Yáñez-Ruiz, D.R.; et al. Invited Review: Nitrogen in Ruminant Nutrition: A Review of Measurement Techniques. J. Dairy Sci. 2019, 102, 5811–5852. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.D.; Varga, G.A.; Clark, J.H.; Firkins, J.L.; Huber, J.T.; Palmquist, D.L. Evaluation of Chemical and Physical Properties of Feeds That Affect Protein Metabolism in the Rumen. J. Dairy Sci. 1994, 77, 2762–2786. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, U.; Sniffen, C.J.; Stern, M.D.; Van Soest, P.J. Evaluation of a Mathematical Model of Rumen Digestion and an in Vitro Simulation of Rumen Proteolysis to Estimate the Rumen-Undegraded Nitrogen Content of Feedstuffs. Br. J. Nutr. 1983, 50, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Hvelplund, T.; Weisbjerg, M. In Situ Techniques for the Estimation of Protein Degradability. In Forage Evaluation in Ruminant Nutrition; Owen, E., Axford, R.F.E., Omed, H.M., Eds.; CABI: Wallingford, UK, 2000; pp. 233–258. [Google Scholar]
- Michalet-Doreau, B.; Ould-Bah, M.Y. In Vitro and in Sacco Methods for the Estimation of Dietary Nitrogen Degradability in the Rumen: A Review. Anim. Feed Sci. Technol. 1992, 40, 57–86. [Google Scholar] [CrossRef]
- Nocek, J.E. In Situ and Other Methods to Estimate Ruminal Protein and Energy Digestibility: A Review. J. Dairy Sci. 1988, 71, 2051–2069. [Google Scholar] [CrossRef]
- Licitra, G.; Lauria, F.; Carpino, S.; Schadt, I.; Sniffen, C.J.; Van Soest, P.J. Improvement of the Streptomyces Griseus Method for Degradable Protein in Ruminant Feeds. Anim. Feed Sci. Technol. 1998, 72, 1–10. [Google Scholar] [CrossRef]
- Okon, P.; Bachmann, M.; Wensch-Dorendorf, M.; Titze, N.; Rodehutscord, M.; Rupp, C.; Susenbeth, A.; Greef, J.M.; Zeyner, A. Feed Clusters According to In Situ and In Vitro Ruminal Crude Protein Degradation. Animals 2023, 13, 224. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H.; Steg, A.; Van Vuuren, A.M. Prediction of in Situ Rumen Escape Protein from In Vitro Incubation with Protease from Streptomyces Griseus. J. Sci. Food Agric. 1996, 72, 120–126. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Xu, Q.; Sahari, M.A.; Hamidi, Z. Enzymatic Removal of Starch and Protein during the Extraction of Dietary Fiber from Barley Bran. J. Cereal Sci. 2018, 83, 259–265. [Google Scholar] [CrossRef]
- Okon, P.; Liebscher, S.; Simon, A.H.; Wensch-Dorendorf, M.; Bachmann, M.; Bordusa, F.; Zeyner, A. The Impact of Streptomyces Griseus Protease Reserved for Protein Evaluation of Ruminant Feed on Carbohydrase Activity during Co-Incubation. Animals 2024, 14, 1931. [Google Scholar] [CrossRef]
- Okon, P.; Eckl, J.; Bachmann, M.; Kuhnitzsch, C.; Martens, S.D.; Steinhöfel, O.; Susenbeth, A.; von Soosten, D.; Meyer, U.; Dänicke, S.; et al. Schätzung Des Ruminalen Proteinabbaus In Vitro Mit Kombiniertem Einsatz von Protease Und Kohlenhydratspaltenden Enzyme. VDLUFA-Schriftenreihe 2022, 78, 483–494. [Google Scholar]
- You, Y.; Brody, S.L. Culture and Differentiation of Mouse Tracheal Epithelial Cells. In Epithelial Cell Culture Protocols; Randell, S.H., Fulcher, M.L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 945, pp. 123–143. ISBN 978-1-62703-124-0. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018. [Google Scholar] [CrossRef]
- Okon, P.; Bachmann, M.; Wensch-Dorendorf, M.; Kuhnitzsch, C.; Martens, S.D.; Greef, J.M.; Steinhöfel, O.; Kuhla, B.; Zeyner, A. In Vitro and in Vivo Analyses of the Nutritive Value of Native and Ensiled Partial Crop Field Peas. Anim. Feed Sci. Technol. 2023, 304, 115723. [Google Scholar] [CrossRef]
- Wroblewitz, S.; Hüther, L.; Berk, A.; Lebzien, P.; Kluth, H.; Manderscheid, R.; Erbs, M.; Weigel, H.-J.; Wätzig, H.; Dänicke, S. The Impact of Free Air Carbon Dioxide Enrichment (FACE) on Nutrient Digestibility of Maize Grains in Pigs and Broiler Chickens and on Ruminal in Sacco Degradability. Anim. Feed Sci. Technol. 2014, 196, 128–138. [Google Scholar] [CrossRef]
- GfE. Empfehlungen Zur Energie- Und Nährstoffversorgung Der Milchkühe Und Aufzuchtrinder [Recommendations of Energy and Nutrient Supply for Dairy Cows and Breeding Cattle]; DLG Verlag: Frankfurt am Main, Germany, 2001. [Google Scholar]
- Paine, C.A.; Crawshaw, R.; Barber, W.P. 9.2 A Complete Exchange Method for the in Sacco Estimation of Rumen Degradability on a Routine Basis. BSAP Occas. Publ. 1982, 6, 177–178. [Google Scholar] [CrossRef]
- Edmunds, B.; Südekum, K.-H.; Spiekers, H.; Schwarz, F.J. Estimating Ruminal Crude Protein Degradation of Forages Using in Situ and in Vitro Techniques. Anim. Feed Sci. Technol. 2012, 175, 95–105. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of Procedures for Nitrogen Fractionation of Ruminant Feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Ansharullah; Hourigan, J.A.; Chesterman, C.F. Application of Carbohydrases in Extracting Protein from Rice Bran. J. Sci. Food Agric. 1997, 74, 141–146. [Google Scholar] [CrossRef]
- Bravo Rodriguez, V.; Jurado Alameda, E.; Martinez Gallegos, J.F.; Reyes Requena, A.; Garcia Lopez, A.I.; Cabral, J.M.S.; Fernandes, P.; Da Fonseca, L.J.P. Modification of the Activity of an A-Amylase from Bacillus Licheniformis by Several Surfactants. Electron. J. Biotechnol. 2006, 9, 567–571. [Google Scholar] [CrossRef]
- Rosset, M.; Prudencio, S.H.; Beléia, A.D.P. Viscozyme L Action on Soy Slurry Affects Carbohydrates and Antioxidant Properties of Silken Tofu. Food Sci. Technol. Int. 2012, 18, 531–538. [Google Scholar] [CrossRef]
- Bachmann, M.; Kuhnitzsch, C.; Okon, P.; Martens, S.D.; Greef, J.M.; Steinhöfel, O.; Zeyner, A. Ruminal In Vitro Protein Degradation and Apparent Digestibility of Energy and Nutrients in Sheep Fed Native or Ensiled + Toasted Pea (Pisum Sativum) Grains. Animals 2019, 9, 401. [Google Scholar] [CrossRef]
- Parand, E.; Spek, J.W. Development of Equations to Estimate Microbial Nitrogen Contamination in Rumen Incubation Residues Using 15N Data and Chemical Composition of Feedstuffs. Anim. Feed Sci. Technol. 2021, 273, 114798. [Google Scholar] [CrossRef]
- McDonald, I. A Revised Model for the Estimation of Protein Degradability in the Rumen. J. Agric. Sci. 1981, 96, 251–252. [Google Scholar] [CrossRef]
- Wulf, M.; Südekum, K.-H. Effects of Chemically Treated Soybeans and Expeller Rapeseed Meal on in Vivo and in Situ Crude Fat and Crude Protein Disappearance from the Rumen. Anim. Feed Sci. Technol. 2005, 118, 215–227. [Google Scholar] [CrossRef]
- VDLUFA. Die Chemische Unterschung von Futtermitteln; Methodenbuch 3; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Conway, E.J.; Byrne, A. An Absorption Apparatus for the Micro-Determination of Certain Volatile Substances: The Micro-Determination of Ammonia. Biochem. J. 1933, 27, 419–429. [Google Scholar] [PubMed]
- Bachmann, M.; Wensch-Dorendorf, M.; Kuhnitzsch, C.; Kleinsteuber, S.; Popp, D.; Thierbach, A.; Martens, S.D.; Steinhöfel, O.; Zeyner, A. Changes in Composition and Diversity of Epiphytic Microorganisms on Field Pea Seeds, Partial Crop Peas, and Whole Crop Peas during Maturation and Ensiling with or without Lactic Acid Bacteria Inoculant. Microbiol. Spectr. 2022, 10, e00953-22. [Google Scholar] [CrossRef]
- Grubješić, G.; Titze, N.; Krieg, J.; Rodehutscord, M. Determination of in Situ Ruminal Crude Protein and Starch Degradation Values of Compound Feeds from Single Feeds. Arch. Anim. Nutr. 2019, 73, 414–429. [Google Scholar] [CrossRef]
- Jiang, F.; Cheng, H.; Liu, D.; Wei, C.; An, W.; Wang, Y.; Sun, H.; Song, E. Treatment of Whole-Plant Corn Silage With Lactic Acid Bacteria and Organic Acid Enhances Quality by Elevating Acid Content, Reducing pH, and Inhibiting Undesirable Microorganisms. Front. Microbiol. 2020, 11, 593088. [Google Scholar] [CrossRef]
- Rupp; Westreicher-Kristen, E.; Susenbeth, A. In Situ and in Vitro Determination of the Protein Value of Feeds for Ruminants. Arch. Anim. Nutr. 2021, 75, 329–344. [Google Scholar] [CrossRef]
- Rupp; Westreicher-Kristen, E.; Susenbeth, A. Effect of Wilting and Lactic Acid Bacteria Inoculant on in Situ and in Vitro Determined Protein Value of Grass Silages. Anim. Feed Sci. Technol. 2021, 282, 115115. [Google Scholar] [CrossRef]
- Tharangani, R.M.H.; Yakun, C.; Zhao, L.S.; Ma, L.; Liu, H.L.; Su, S.L.; Shan, L.; Yang, Z.N.; Kononoff, P.J.; Weiss, W.P.; et al. Corn Silage Quality Index: An Index Combining Milk Yield, Silage Nutritional and Fermentation Parameters. Anim. Feed Sci. Technol. 2021, 273, 114817. [Google Scholar] [CrossRef]
- Delitte, M.; Caulier, S.; Bragard, C.; Desoignies, N. Plant Microbiota Beyond Farming Practices: A Review. Front. Sustain. Food Syst. 2021, 5, 624203. [Google Scholar] [CrossRef]
- Ikeda-Ohtsubo, W.; Brugman, S.; Warden, C.H.; Rebel, J.M.J.; Folkerts, G.; Pieterse, C.M.J. How Can We Define “Optimal Microbiota?”: A Comparative Review of Structure and Functions of Microbiota of Animals, Fish, and Plants in Agriculture. Front. Nutr. 2018, 5, 90. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H.; Mathijssen-Kamman, A.A.; Hindle, V.A. Rumen Escape Protein in Grass and Grass Silage Determined with a Nylon Bag and an Enzymatic Technique. Anim. Feed Sci. Technol. 2004, 111, 1–9. [Google Scholar] [CrossRef]
- Cone, J.W.; Kamman, A.A.; van Gelder, A.H.; Hindle, V.A. Rumen Escape Protein in Concentrate Ingredients Determined with the Nylon Bag and Enzymatic Techniques. Anim. Feed Sci. Technol. 2002, 97, 247–254. [Google Scholar] [CrossRef]
- Gosselink, J.M.J.; Dulphy, J.P.; Poncet, C.; Aufrère, J.; Tamminga, S.; Cone, J.W. Rumen Escape Nitrogen from Forages in Sheep: Comparison of In Situ and In Vitro Techniques Using In Vivo Data. Anim. Feed Sci. Technol. 2004, 116, 35–51. [Google Scholar] [CrossRef]
- Tománková, O.; Kopečný, J. Prediction of Feed Protein Degradation in the Rumen with Bromelain. Anim. Feed Sci. Technol. 1995, 53, 71–80. [Google Scholar] [CrossRef]
- Blumberg, P.M.; Strominger, J.L. Interaction of Penicillin with the Bacterial Cell: Penicillin-Binding Proteins and Penicillin-Sensitive Enzymes. Bacteriol. Rev. 1974, 38, 291–335. [Google Scholar] [CrossRef]
- Böttger, C.; Südekum, K.-H. Review: Protein Value of Distillers Dried Grains with Solubles (DDGS) in Animal Nutrition as Affected by the Ethanol Production Process. Anim. Feed Sci. Technol. 2018, 244, 11–17. [Google Scholar] [CrossRef]
- Von Keyserlingk, M.A.G.; Swift, M.L.; Puchala, R.; Shelford, J.A. Degradability Characteristics of Dry Matter and Crude Protein of Forages in Ruminants. Anim. Feed Sci. Technol. 1996, 57, 291–311. [Google Scholar] [CrossRef]
- Trop, M.; Birk, Y. The Specificity of Proteinases from Streptomyces griseus (Pronase). Biochem. J. 1970, 116, 19–25. [Google Scholar] [CrossRef]
- Luchini, N.D.; Broderick, G.A.; Combs, D.K. Characterization of the Proteolytic Activity of Commercial Proteases and Strained Ruminal Fluid. J. Anim. Sci. 1996, 74, 685. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Phillippe, R.C.; Rode, L.M.; Cheng, K.-J. Effect of the Protein Matrix on the Digestion of Cereal Grains by Ruminal Microorganisms. J. Anim. Sci. 1993, 71, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, O.K.; Kaasgaard, S.G.; Palmén, L.G.; Vidal, B.C.; Hamaker, B.R. Enzyme Treatments on Corn Fiber from Wet-Milling Process for Increased Starch and Protein Extraction. Ind. Crops Prod. 2021, 168, 113622. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Dalsgaard, S.; Arent, S.; Lorentsen, R.; Knudsen, K.E.B.; Yu, S.; Lærke, H.N. Xylanase and Protease Increase Solubilization of Non-Starch Polysaccharides and Nutrient Release of Corn- and Wheat Distillers Dried Grains with Solubles. Biochem. Eng. J. 2015, 98, 99–106. [Google Scholar] [CrossRef]
- Jakobsen, G.V.; Jensen, B.B.; Knudsen, K.E.B.; Canibe, N. Improving the Nutritional Value of Rapeseed Cake and Wheat Dried Distillers Grains with Solubles by Addition of Enzymes during Liquid Fermentation. Anim. Feed Sci. Technol. 2015, 208, 198–213. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme Assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef]
- Liu, J.; Guan, X.; Zhu, D.; Sun, J. Optimization of the Enzymatic Pretreatment in Oat Bran Protein Extraction by Particle Swarm Optimization Algorithms for Response Surface Modeling. LWT Food Sci. Technol. 2008, 41, 1913–1918. [Google Scholar] [CrossRef]
- Saleh, F.; Ohtsuka, A.; Tanaka, T.; Hayashi, K. Carbohydrases Are Digested by Proteases Present in Enzyme Preparations during In Vitro Digestion. J. Poult. Sci. 2004, 41, 229–235. [Google Scholar] [CrossRef]
- Saleh, F.; Ohtsuka, A.; Tanaka, T.; Hayashi, K. Effect of Enzymes of Microbial Origin on In Vitro Digestibilities of Dry Matter and Crude Protein in Maize. J. Poult. Sci. 2003, 40, 274–281. [Google Scholar] [CrossRef]

| α-Amylase | Viscozym® L | |
|---|---|---|
| Enzyme activity | 240 KNU/g 1 | ≥100 FBGU/g 2 |
| Enzyme density | 1.25 g/mL 3 | 1.2 g/mL 4 |
| Enzyme activity per dose | 0.1 mL~30 KNU 0.2 mL~60 KNU 0.4 mL~120 KNU 0.8 mL~240 KNU | 0.188 mL~22.56 FBGU 0.375 mL~45 FBGU 0.750 mL~90 FBGU 1.5 mL~180 FBGU |
| Reference | Cone et al. [9] | Ansharullah et al. [21] |
| Feedstuff | DM | CA | CP | AEE | CF | aNDFom | ADFom | Starch |
|---|---|---|---|---|---|---|---|---|
| Rapeseed meal | 880 | 75 | 374 | 38 | 156 | 249 | 227 | n.a. |
| DDGS | 901 | 47 | 321 | 79 | 75 | 293 | 120 | n.a. |
| Wheat grain | 984 | 19 | 140 | 24 | 25 | 145 | 34 | 743 |
| Corn grain | 876 | 14 | 93 | 56 | 27 | 119 | 13 | 783 |
| Grass silage | 377 | 103 | 151 | 41 | 289 | 500 | 329 | n.a. |
| PCFPS | 610 | 69 | 153 | 22 | 220 | 308 | 274 | 201 |
| Corn silage | 328 | 38 | 74 | 32 | 188 | 398 | 217 | 443 |
| Feedstuff | DM | pH | Lactic Acid | Acetic Acid | n-Butyric Acid | NH3-N |
|---|---|---|---|---|---|---|
| Grass silage | 377 | 4.35 | 266.8 (14.1) | 21.4 (0.0) | 1.5 (0.0) | 1.8 (0.0) |
| PCFPS | 610 | 4.38 | 91.6 (3.5) | 13.7 (0.1) | n.d. | 1.0 (0.0) |
| Corn silage | 328 | 3.80 | 90.9 (1.3) | 17.7 (0.3) | n.d. | 1.5 (0.0) |
| ED2 | ED5 | ED8 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Feedstuff | − | + | p-Value | − | + | p-Value | − | + | p-Value |
| Rapeseed meal | 70 a | 69 a | 0.1030 | 62 a | 60 b | <0.001 | 56 a | 53 b | <0.001 |
| DDGS | 61 a | 61 a | 0.6266 | 55 a | 55 a | 0.0913 | 51 a | 50 b | 0.0299 |
| Wheat grains | 74 a | 71 b | <0.001 | 71 a | 67 b | <0.001 | 67 a | 64 b | <0.001 |
| Corn grains | 28 a | 25 b | 0.0016 | 27 a | 24 b | <0.001 | 27 a | 23 b | <0.001 |
| Grass silage | 73 a | 70 b | <0.001 | 71 a | 68 b | <0.001 | 70 a | 66 b | <0.001 |
| PCFPS | 80 a | 80 a | 0.5976 | 79 a | 78 b | 0.0422 | 78 a | 77 b | 0.0412 |
| Corn silage | 70 a | 65 b | <0.001 | 69 a | 64 b | <0.001 | 68 a | 63 b | <0.001 |
| Range of SE | 0.18–0.42 | 0.16–0.31 | 0.07–0.32 | ||||||
| Feedstuff | In Sacco | SGP Solo | SGP + α-A1 | SGP + α-A2 | SGP + α-A3 | SGP + α-A4 | SGP + V1 | SGP + V2 | SGP + V3 | SGP + V4 | Range of SE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rapeseed meal | ED2 | 85 A | 69 Bb | 70 Bb | 69 Bb | 69 Bb | 69 Bb | 71 Ba | 71 Ba | 72 Ba | 72 Ba | 0.12–0.84 |
| ED5 | 78 A | 60 Bb | 60 Bb | 59 Bb | 59 Bb | 59 Bb | 62 Ba | 61 Ba | 63 Ba | 62 Ba | 0.28–0.33 | |
| ED8 | 72 A | 53 Bb | 54 Bb | 53 Bb | 53 Bb | 52 Bb | 55 Ba | 54 Bb | 56 Ba | 55 Ba | 0.13–1.16 | |
| DDGS | ED2 | 93 A | 61 Bb | 61 Bb | 61 Bb | 61 Bb | 61 Bb | 62 Ba | 61 Bb | 61 Bb | 59 Bb | 0.08–0.55 |
| ED5 | 91 A | 55 Bb | 55 Bb | 55 Bb | 55 Bb | 55 Bb | 56 Ba | 56 Ba | 55 Bb | 54 Bb | 0.16–0.19 | |
| ED8 | 89 A | 50 Bb | 50 Bb | 51 Ba | 50 Bb | 51 Ba | 51 Ba | 52 Ba | 51 Bb | 50 Bb | 0.15–0.17 | |
| Wheat grain | ED2 | 94 A | 71 Bb | 73 Ba | 74 Ba | 74 Ba | 77 Ba | 76 Ba | 78 Ba | 79 Ba | 82 Ba | 0.16–0.70 |
| ED5 | 89 A | 67 Bb | 69 Ba | 70 Ba | 70 Ba | 72 Ba | 72 Ba | 74 Ba | 74 Ba | 78 Ba | 0.31–0.36 | |
| ED8 | 84 A | 64 Bb | 66 Ba | 67 Ba | 67 Ba | 68 Ba | 68 Ba | 70 Ba | 71 Ba | 75 Ba | 0.30–0.34 | |
| Corn grain | ED2 | 85 A | 25 Bb | 27 Bb | 27 Bb | 27 Ba | 28 Ba | 27 Bb | 25 Bb | 25 Bb | 25 Bb | 0.52–0.60 |
| ED5 | 74 A | 24 Bb | 26 Bb | 26 Ba | 26 Ba | 26 Ba | 25 Ba | 25 Ba | 25 Bb | 25 Ba | 0.10–3.32 | |
| ED8 | 67 A | 23 Bb | 25 Ba | 25 Ba | 25 Ba | 26 Ba | 25 Ba | 25 Bb | 25 Bb | 26 Ba | 0.06–3.99 | |
| Grass silage | ED2 | 91 A | 70 Bb | 70 Bb | 70 Bb | 69 Bb | 69 Ba | 72 Ba | 72 Ba | 72 Ba | 73 Ba | 0.23–0.26 |
| ED5 | 86 A | 68 Bb | 68 Bb | 68 Bb | 67 Bb | 67 Bb | 69 Ba | 69 Ba | 69 Ba | 68 Bb | 0.20–0.23 | |
| ED8 | 83 A | 66 Bb | 66 Bb | 66 Bb | 66 Bb | 66 Bb | 67 Ba | 67 Ba | 67 Ba | 66 Bb | 0.21–0.24 | |
| Partial crop field pea silage | ED2 | 90 A | 80 Bb | 80 Bb | 80 Bb | 80 Bb | 80 Bb | 83 Ba | 83 Ba | 83 Ba | 83 Ba | 0.25–0.29 |
| ED5 | 87 A | 78 Bb | 79 Bb | 79 Bb | 78 Bb | 79 Bb | 81 Ba | 81 Ba | 81 Ba | 82 Ba | 0.16–0.19 | |
| ED8 | 84 A | 77 Bb | 77 Bb | 77 Bb | 77 Bb | 78 Bb | 79 Ba | 80 Ba | 80 Ba | 80 Ba | 0.18–0.21 | |
| Corn silage | ED2 | 92 A | 65 Bb | 68 Ba | 67 Bb | 67 Bb | 68 Ba | 69 Ba | 69 Ba | 69 Ba | 71 Ba | 0.38–0.44 |
| ED5 | 89 A | 64 Bb | 66 Ba | 66 Ba | 66 Ba | 67 Ba | 67 Ba | 67 Ba | 67 Ba | 66 Ba | 0.26–0.30 | |
| ED8 | 86 A | 63 Bb | 65 Ba | 65 Ba | 65 Ba | 66 Ba | 66 Ba | 65 Ba | 66 Ba | 65 Ba | 0.25–0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okon, P.; Wensch-Dorendorf, M.; Bachmann, M.; von Soosten, D.; Meyer, U.; Greef, J.-M.; Dänicke, S.; Zeyner, A. Ruminal Crude Protein Degradation Determined in Sacco and by Co-Incubation of Streptomyces griseus Protease and Carbohydrases. Animals 2024, 14, 2982. https://doi.org/10.3390/ani14202982
Okon P, Wensch-Dorendorf M, Bachmann M, von Soosten D, Meyer U, Greef J-M, Dänicke S, Zeyner A. Ruminal Crude Protein Degradation Determined in Sacco and by Co-Incubation of Streptomyces griseus Protease and Carbohydrases. Animals. 2024; 14(20):2982. https://doi.org/10.3390/ani14202982
Chicago/Turabian StyleOkon, Paul, Monika Wensch-Dorendorf, Martin Bachmann, Dirk von Soosten, Ulrich Meyer, Jörg-Michael Greef, Sven Dänicke, and Annette Zeyner. 2024. "Ruminal Crude Protein Degradation Determined in Sacco and by Co-Incubation of Streptomyces griseus Protease and Carbohydrases" Animals 14, no. 20: 2982. https://doi.org/10.3390/ani14202982
APA StyleOkon, P., Wensch-Dorendorf, M., Bachmann, M., von Soosten, D., Meyer, U., Greef, J.-M., Dänicke, S., & Zeyner, A. (2024). Ruminal Crude Protein Degradation Determined in Sacco and by Co-Incubation of Streptomyces griseus Protease and Carbohydrases. Animals, 14(20), 2982. https://doi.org/10.3390/ani14202982






