Association Between Dairy Production System and Milk Functionality Based on Analysis of miRNAs in Exosomes from Milk
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Milk-Derived Extracellular Vesicles (MDEVs)
2.2. Characterisation of MDEVs
2.2.1. Size and Concentration
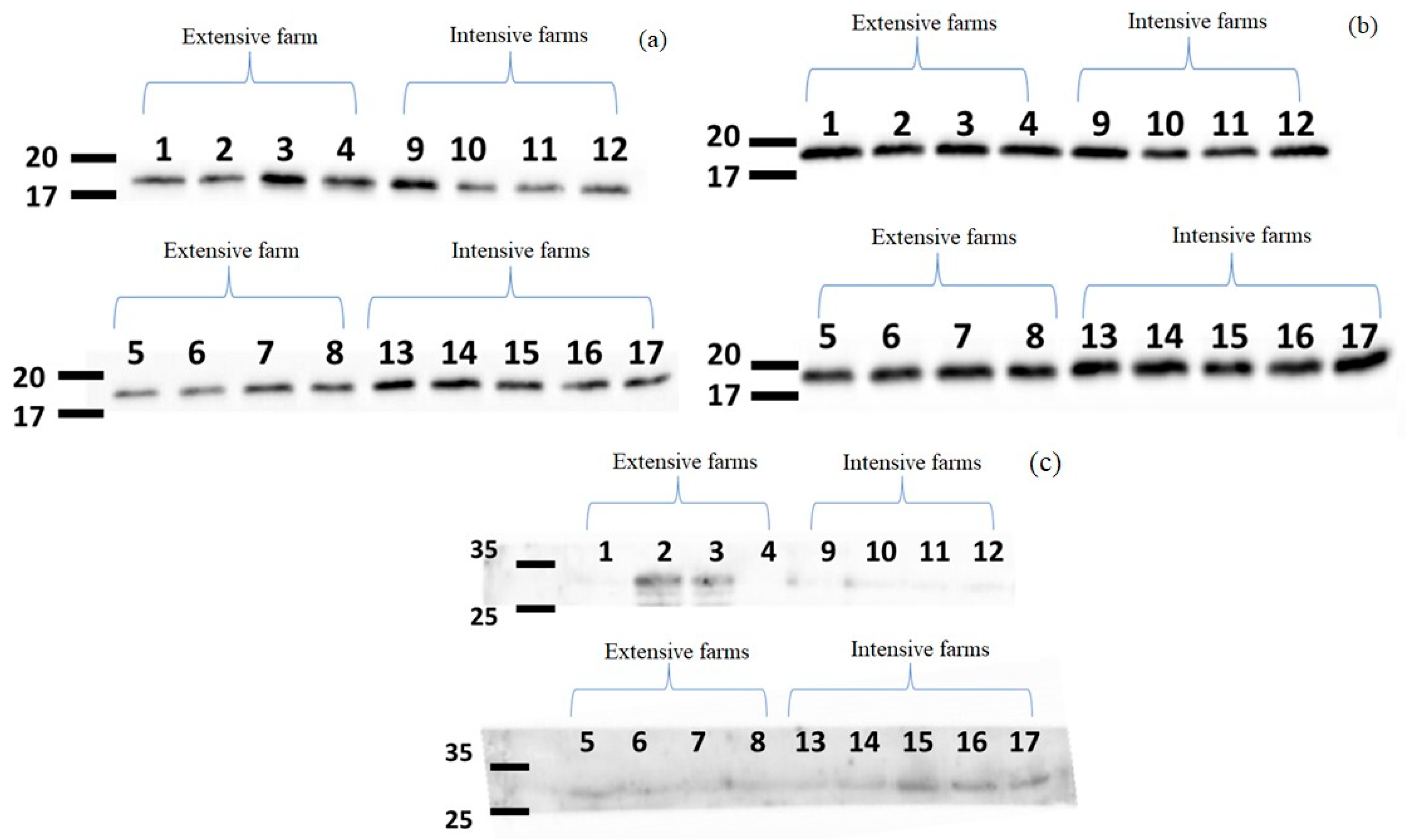
2.2.2. MDEVs Specific Protein Determination
2.3. Analysis of miRNAs in MDEVs
2.3.1. miRNAs Extraction
2.3.2. Quantitative Reverse-Transcription Polymerase Chain Reaction (RT-qPCR)
2.4. Prediction of Genes Targeted by miRNAs from MDEVs
2.5. Statistical Analysis
3. Results
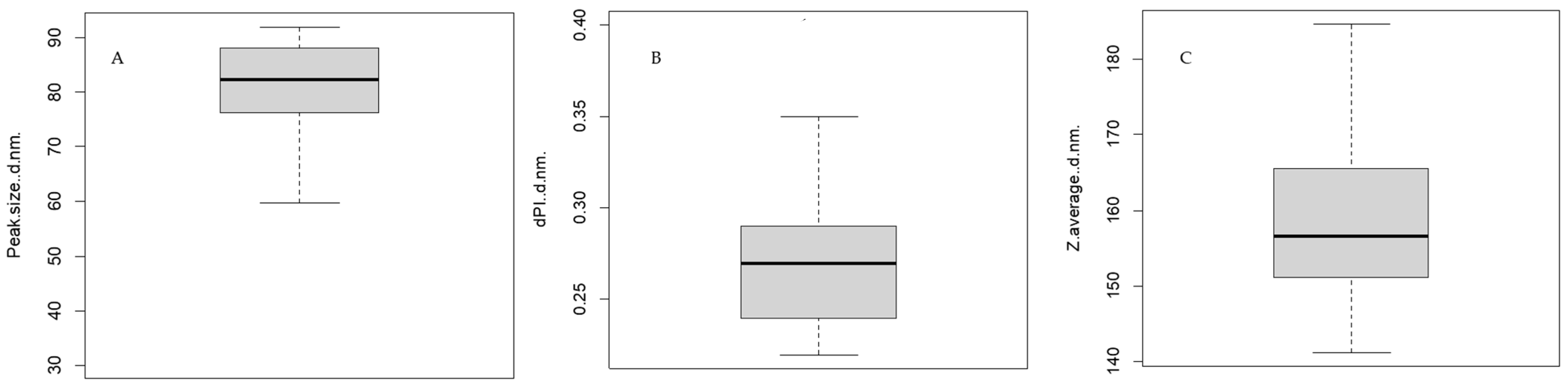
3.1. Confirmation and Characterisation of Exosomes in Milk Samples
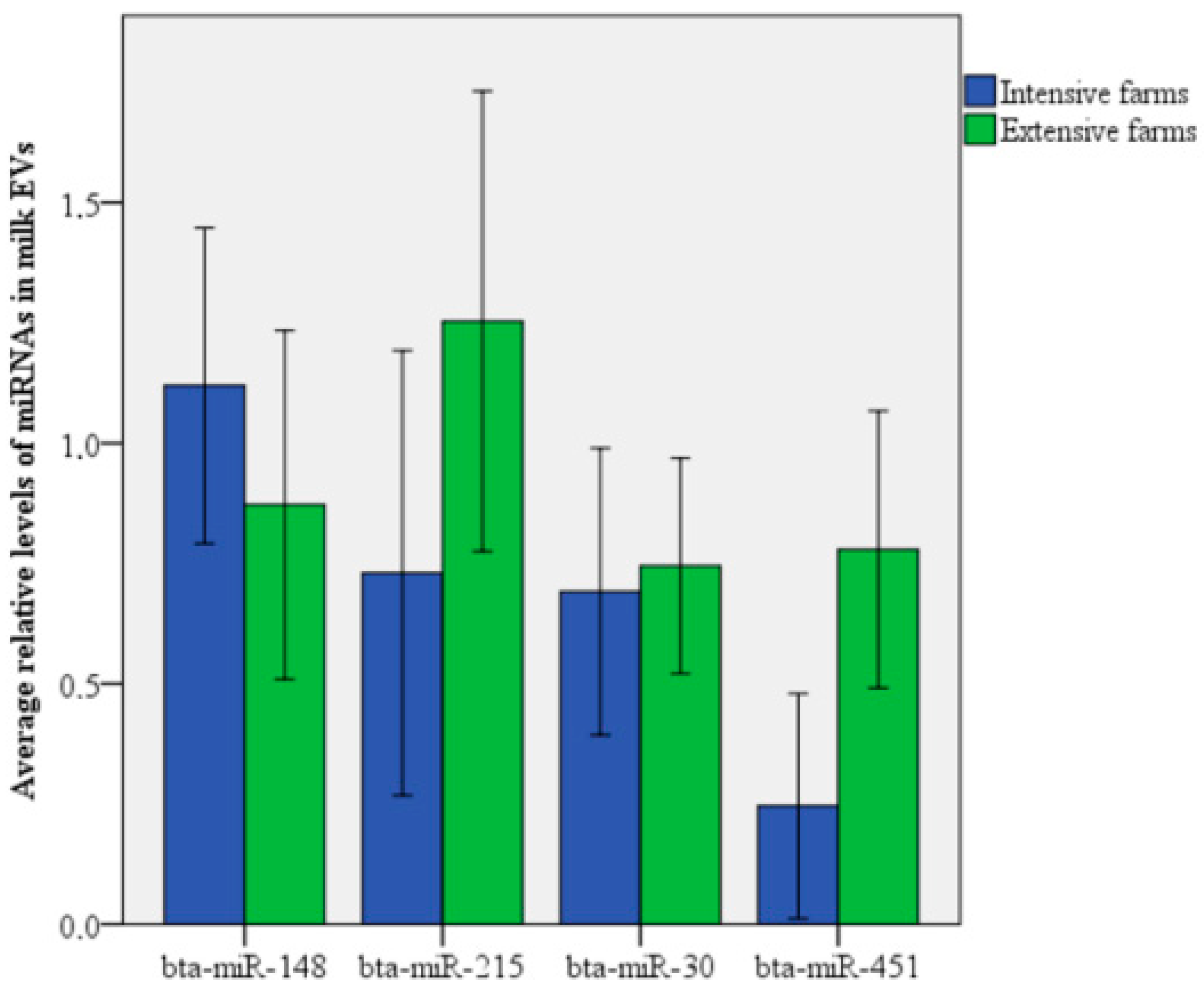
3.2. Relative Levels of miRNAs in MDEVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariza, A.B.; Juaristi, J.L. Sistemas y prácticas de manejo en rebaños de vacuno lechero en España. Tierras Castilla Y León Ganad. 2008, 151, 56–60. [Google Scholar]
- Leblanc, B. Analyse Comparée des Performances des Systèmes de Production des Fermes Laitières au Canada et aux États-Unis. Master’s Thesis, Laval University, Québec, QC, Canada, 2012. [Google Scholar]
- Vicente Mainar, F.; Martínez-Fernández, A.; Soldado, A.; De la Roza Delgado, B.; Argamentería Gutiérrez, A. Producción Sostenible de Leche de Vaca Mediante El Aprovechamiento de Los Recursos Naturales y Su Impacto Sobre El Medio Ambiente. In 3er Simposio Internacional del Producción Animal; Univ. Autónoma del Estado México: Ciudad de Mexico, Mexico, 2013; pp. 76–89. [Google Scholar]
- Marshall, C.; Beck, M.; Garrett, K.; Barrell, G.; Al-Marashdeh, O.; Gregorini, P. Grazing dairy cows with low milk urea nitrogen breeding values excrete less urinary urea nitrogen. Sci. Total. Environ. 2020, 739, 139994. [Google Scholar] [CrossRef] [PubMed]
- el Qassim, L.A.; Alonso, J.; Zhao, K.; Le Guillou, S.; Diez, J.; Vicente, F.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Guan, L.; Royo, L.J. Differences in the microRNAs Levels of Raw Milk from Dairy Cattle Raised under Extensive or Intensive Production Systems. Veter- Sci. 2022, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- el Qassim, L.A.; Le Guillou, S.; Royo, L.J. Variation of miRNA Content in Cow Raw Milk Depending on the Dairy Production System. Int. J. Mol. Sci. 2022, 23, 11681. [Google Scholar] [CrossRef]
- Bauman, D.E.; Harvatine, K.J.; Lock, A.L. Nutrigenomics, Rumen-Derived Bioactive Fatty Acids, and the Regulation of Milk Fat Synthesis. Annu. Rev. Nutr. 2011, 31, 299–319. [Google Scholar] [CrossRef]
- Padovani, M.; Lavigne, J.A.; Chandramouli, G.V.; Perkins, S.N.; Barrett, J.C.; Hursting, S.D.; Bennett, L.M.; Berrigan, D. Distinct Effects of Calorie Restriction and Exercise on Mammary Gland Gene Expression in C57BL/6 Mice. Cancer Prev. Res. 2010, 2, 1076–1087. [Google Scholar] [CrossRef]
- Tao, S.; Bubolz, J.W.; do Amaral, B.C.; Thompson, I.M.; Hayen, M.J.; Johnson, S.E.; Dahl, G.E. Effect of heat stress during the dry period on mammary gland development. J. Dairy Sci. 2011, 94, 5976–5986. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef]
- Hwang, I. Cell-Cell Communication Via Extracellular Membrane Vesicles and Its Role in the Immune Response. Mol. Cells 2013, 36, 105–111. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.-Y. Horizontal transfer of microRNAs: Molecular mechanisms and clinical applications. Protein Cell 2012, 3, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Baier, S.R.; Howard, K.M.; Cui, J. Gene regulation by dietary microRNAs. Can. J. Physiol. Pharmacol. 2015, 93, 1097–1102. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.-S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Reif, S.; Shiff, Y.E.; Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J. Transl. Med. 2019, 17, 325. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Laugier, J.; Beauparlant, C.J.; Lambert, M.; Droit, A.; Provost, P. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J. Dairy Sci. 2020, 103, 16–29. [Google Scholar] [CrossRef]
- Lim, L.P.; Glasner, M.E.; Yekta, S.; Burge, C.B.; Bartel, D.P. Vertebrate MicroRNA Genes. Science 2003, 299, 1540. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Bourdon, C.; Bardou, P.; Aujean, E.; Le Guillou, S.; Tosser-Klopp, G.; Le Provost, F. RumimiR: A detailed microRNA database focused on ruminant species. Database 2019, 2019, baz099. [Google Scholar] [CrossRef]
- Sethupathy, P.; Corda, B.; Hatzigeorgiou, A.G. TarBase: A comprehensive database of experimentally supported animal microRNA targets. RNA 2005, 12, 192–197. [Google Scholar] [CrossRef]
- Didiano, D.; Hobert, O. Perfect seed pairing is not a generally reliable predictor for miRNA-target interactions. Nat. Struct. Mol. Biol. 2006, 13, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, K.; Koh, Y.Q.; Almughlliq, F.B.; Peiris, H.N.; Mitchell, M.D. A method for the isolation and enrichment of purified bovine milk exosomes. Reprod. Biol. 2017, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Golan-Gerstl, R.; Shiff, Y.E.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Elbaum-Shiff, Y.; Koroukhov, N.; Shilo, I.; Musseri, M.; Golan-Gerstl, R. Cow and Human Milk-Derived Exosomes Ameliorate Colitis in DSS Murine Model. Nutrients 2020, 12, 2589. [Google Scholar] [CrossRef]
- Shiff, Y.E.; Reif, S.; Marom, R.; Shiff, K.; Reifen, R.; Golan-Gerstl, R. MiRNA-320a is less expressed and miRNA-148a more expressed in preterm human milk compared to term human milk. J. Funct. Foods 2019, 57, 68–74. [Google Scholar] [CrossRef]
- Muroya, S.; Ogasawara, H.; Hojito, M. Grazing Affects Exosomal Circulating MicroRNAs in Cattle. PLoS ONE 2015, 10, e0136475. [Google Scholar] [CrossRef]
- De La Torre-Santos, S. Identificación de Biomarcadores Específicos Para Autentificar El Origen y El Sistema de Alimen-tación Del Vacuno Lechero. Ph.D. Thesis, Zaragoza University, Zaragoza, Spain, 2021. [Google Scholar]
- Sedykh, S.E.; Burkova, E.E.; Purvinsh, L.V.; Klemeshova, D.A.; Ryabchikova, E.I.; Nevinsky, G.A. Milk Exosomes: Isolation, Biochemistry, Morphology, and Perspectives of Use. In Extracellular Vesicles and Their Importance in Human Health; IntechOpen: London, UK, 2020; pp. 1–28. [Google Scholar]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Théry, C. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extra-cellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Hvam, M.L.; Primdahl-Bengtson, B.; Boysen, A.T.; Whitehead, B.; Dyrskjøt, L.; Ørntoft, T.F.; Howard, K.A.; Ostenfeld, M.S. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles 2014, 3, 25011. [Google Scholar] [CrossRef]
- Adriano, B.; Cotto, N.M.; Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Milk exosomes: Nature’s abundant nanoplatform for theranostic applications. Bioact. Mater. 2021, 6, 2479–2490. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, M.; Tian, W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016, 49, 3–13. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Scholz-Romero, K.; Reed, S.; Peiris, H.; Koh, Y.; Meier, S.; Walker, C.; Burke, C.; Roche, J.; Rice, G.; et al. Plasma exosome profiles from dairy cows with divergent fertility phenotypes. J. Dairy Sci. 2016, 99, 7590–7601. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.-Y.; Nan, X.-M.; Wang, K.; Zhao, Y.-G.; Jiang, L.-S.; Yao, J.-H.; Xiong, B.-H. Replacement of forage fiber with non-forage fiber sources in dairy cow diets changes milk extracellular vesicle-miRNA expression. Food Funct. 2020, 11, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J. Lipid Res. 2018, 59, 1325–1340. [Google Scholar] [CrossRef]
- Bozack, A.K.; Colicino, E.; Rodosthenous, R.; Bloomquist, T.R.; Baccarelli, A.A.; Wright, R.O.; Lee, A.G. Associations between maternal lifetime stressors and negative events in pregnancy and breast milk-derived extracellular vesicle mi-croRNAs in the programming of intergenerational stress mechanisms (PRISM) pregnancy cohort. Epigenetics 2021, 16, 389–404. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, Q.; Chen, X.; Li, C.; Cao, B.; Ou, R.; Hadano, S.; Shang, H.-F. Aberration of miRNAs Expression in Leukocytes from Sporadic Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2016, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, S.; He, J.; Liu, X.; Qu, Y.; Yan, W.; Fan, J.; Li, R.; Xi, H.; Fu, W.; et al. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget 2015, 6, 14993–15007. [Google Scholar] [CrossRef]
- Minna, E.; Romeo, P.; Dugo, M.; De Cecco, L.; Todoerti, K.; Pilotti, S.; Perrone, F.; Seregni, E.; Agnelli, L.; Neri, A.; et al. miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma. Oncotarget 2016, 7, 12731–12747. [Google Scholar] [CrossRef]
- Ma, Y.G.; Han, Y.Z.; Zhang, Z.S.; Yu, Y.; Xu, X.F.; Yuan, L. MiR-451 regulates proliferation and migration of colorectal cells by targeting MIF. Chin. J. Oncol. 2020, 42, 312–318. [Google Scholar]
- Zeng, T.; Peng, L.; Chao, C.; Fu, B.; Wang, G.; Wang, Y.; Zhu, X. miR-451 inhibits invasion and proliferation of bladder cancer by regulating EMT. Int. J. Clin. Exp. Pathol. 2014, 7, 7653–7662. [Google Scholar]

| Characteristic | Farm ID | Number of Cows | Average Daily Milk Production per Cow, L | Grazing | Daily Corn Silage per Cow, kg Fresh Matter | Daily Grass Silage per Cow, kg Fresh Matter | Daily Straw per Cow, kg Fresh Matter | Daily Vetch per Cow, kg Fresh Matter | Daily Alfalfa per Cow, kg Fresh Matter | Daily Concentrate per Cow, kg Fresh Matter |
|---|---|---|---|---|---|---|---|---|---|---|
| Extensive | 1 | 41 | 21 | Yes | 0 | 5 | 0 | 3 | 0 | 6 |
| 2 | 38 | 23 | Yes | 0 | 0 | 0 | 1 | 1 | 7 | |
| 3 | 35 | 15 | Yes | 0 | 0 | 0 | 0 | 0 | 4.5 | |
| 4 | 61 | 25 | Yes | 0 | 5 | 0 | 0 | 0 | 10 | |
| 5 | 14 | 25 | Yes | 0 | 5 | 0 | 0 | 0 | 9 | |
| 6 | 30 | 20 | Yes | 0 | 0 | 0 | 0 | 4 | 7 | |
| 7 | 35 | 26 | Yes | 0 | 5 | 0 | 2 | 2.5 | 9 | |
| 8 | 24 | 23 | Yes | 0 | 0 | 0 | 0 | 2 | 8 | |
| Intensive | 9 | 240 | 36 | No | 16 | 10 | 0.5 | 2 | 2 | 12 |
| 10 | 60 | 32 | No | 20 | 20 | 0 | 1.5 | 0 | 10 | |
| 11 | 75 | 34 | No | 25 | 8 | 0 | 0 | 2 | 11.5 | |
| 12 | 50 | 30 | No | 20 | 6 | 1 | 0 | 2 | 7 | |
| 13 | 44 | 34 | No | 20 | 10 | 0 | 1.5 | 0 | 10 | |
| 14 | 66 | 35 | No | 25 | 10 | 0 | 0 | 0 | 12 | |
| 15 | 120 | 36 | No | 30 | 5 | 1 | 2 | 1.5 | 12 | |
| 16 | 85 | 34 | No | 22 | 15 | 1 | 1.5 | 2 | 11 | |
| 17 | 66 | 40 | No | 25 | 10 | 0 | 0 | 2 | 12 |
| Pathway | p | Number of Genes Targeted by miR-451 |
|---|---|---|
| Parkinson’s disease | <0.01 | 2 |
| mTOR signalling pathway | <0.01 | 5 |
| Thyroid cancer | <0.01 | 2 |
| Colorectal cancer | 0.03 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou el qassim, L.; Golan-Gerstl, R.; Reif, S.; Royo, L.J. Association Between Dairy Production System and Milk Functionality Based on Analysis of miRNAs in Exosomes from Milk. Animals 2024, 14, 2960. https://doi.org/10.3390/ani14202960
Abou el qassim L, Golan-Gerstl R, Reif S, Royo LJ. Association Between Dairy Production System and Milk Functionality Based on Analysis of miRNAs in Exosomes from Milk. Animals. 2024; 14(20):2960. https://doi.org/10.3390/ani14202960
Chicago/Turabian StyleAbou el qassim, Loubna, Regina Golan-Gerstl, Shimon Reif, and Luis J. Royo. 2024. "Association Between Dairy Production System and Milk Functionality Based on Analysis of miRNAs in Exosomes from Milk" Animals 14, no. 20: 2960. https://doi.org/10.3390/ani14202960
APA StyleAbou el qassim, L., Golan-Gerstl, R., Reif, S., & Royo, L. J. (2024). Association Between Dairy Production System and Milk Functionality Based on Analysis of miRNAs in Exosomes from Milk. Animals, 14(20), 2960. https://doi.org/10.3390/ani14202960





