miR-10a-5p Regulates the Proliferation and Differentiation of Porcine Preadipocytes Targeting the KLF11 Gene
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval Declarations and Sample Collection
2.2. Cell Culture and Differentiation Induction
2.3. Oil Red O Staining
2.4. Quantitative Real-Time Polymerase Chain Reaction
2.5. Transfection
2.6. Cell Proliferation Assay
2.7. Flow Cytometric Analysis
2.8. Dual-luciferase reporter assay
2.9. Western Blotting Analysis
2.10. Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
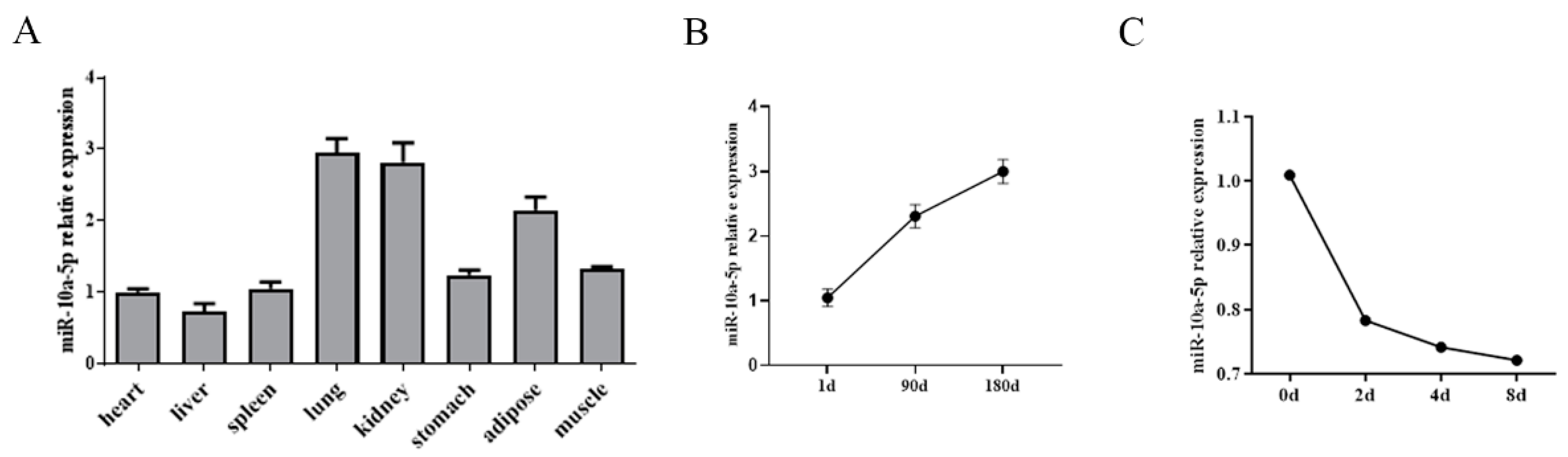
3.1. miR-10a-5p Expression Pattern in Pigs
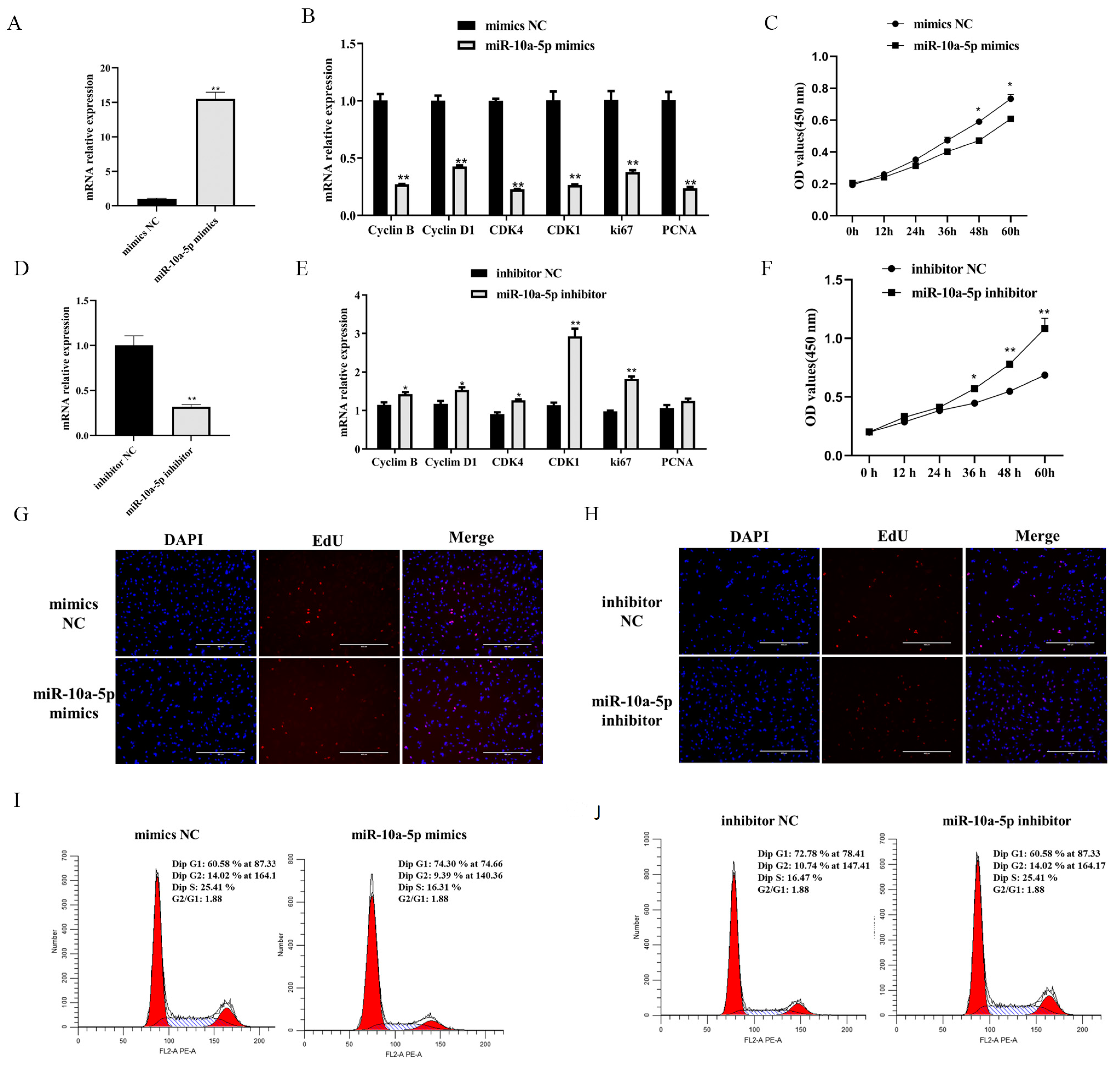
3.2. miR-10a-5p Inhibition of Porcine Preadipocyte Proliferation
3.3. miR-10a-5p Enhancement of Porcine Preadipocyte Differentiation
3.4. KLF11: A Target of miR-10a-5p in Modulating Preadipocyte Proliferation and Differentiation
3.5. KLF11: A Negative Regulator in Preadipocyte Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Cynthia, L.O.; Margaret, D.C.; Lester, R.C.; Margaret, A.M.; Carolyn, J.T.; Katherine, M.F. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar]
- Jannet, K.; Sakari, K.; Claes, W. microRNAs in CNS Disorders. Neuromol. Med. 2009, 11, 162–172. [Google Scholar]
- Jeong, B.C.; Kang, I.H.; Koh, J.T. MicroRNA-302a inhibits adipogenesis by suppressing peroxisome proliferator-activated receptorγ expression. FEBS Lett. 2014, 588, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.M.; Zhang, M.; Tong, M.L.; Yang, L.; Pang, L.X.; Chen, L.; Xu, G.F.; Chi, X.; Hong, Q.; Ni, Y.H.; et al. miR-148a is Associated with Obesity and Modulates Adipocyte Differentiation of Mesenchymal Stem Cells through Wnt Signaling. Sci. Rep. 2015, 22, 9930. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dai, Y.M.; Ji, C.B.; Yang, L.; Shi, C.M.; Xu, G.F.; Pang, L.X.; Huang, F.Y.; Zhang, C.M.; Guo, X.R. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol. Cell Endocrinol. 2014, 393, 65–74. [Google Scholar] [CrossRef]
- Chu, Y.X.; Yao, Y.; Li, X. MiR-370 enhances cell cycle and represses lipid accumulation in porcine adipocytes. Anim. Biotechnol. 2021, 32, 334–342. [Google Scholar] [CrossRef]
- Song, G.X.; Xu, G.F.; Ji, C.B.; Shi, C.M.; Shen, Y.H.; Chen, L.; Zhu, L.J.; Yang, L.; Zhao, Y.P.; Guo, X.R. The role of microRNA-26b in human adipocyte differentiation and proliferation. Gene 2014, 533, 481–487. [Google Scholar] [CrossRef]
- Yang, L.; Shi, C.M.; Chen, L.; Pang, L.X.; Xu, G.F.; Gu, N.; Zhu, L.J.; Guo, X.R.; Ni, Y.H.; Ji, C.B. The biological effects of hsa-miR-1908 in human adipocytes. Mol. Biol. Rep. 2015, 42, 927–935. [Google Scholar] [CrossRef]
- Li, M.; Zhang, N.; Li, J.; Zhang, W.F.; Hei, W.; Ji, M.T.; Yang, Y.; Cao, G.Q.; Guo, X.H.; Li, B.G. MiR-23b Promotes Porcine Preadipocyte Differentiation viaSESN3 and ACSL4. Cells. 2022, 29, 2339. [Google Scholar] [CrossRef]
- Cai, R.; Chao, M.K.; Zhao, T.T.; Li, R.; Zhang, Z.Y.; Yan, W.Y.; Pang, W.J. miR-503 targets MafK to inhibit subcutaneous preadipocyte adipogenesis causing a decrease of backfat thickness in Guanzhong Black pigs. Meat Sci. 2023, 198, 109116. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Liu, K.K.; You, Z.Y.; Zhang, J. MiR-196b-3p and miR-450b-3p are key regulators of adipogenesis in porcine intramuscular and subcutaneous adipocytes. BMC Genom. 2023, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Z.; Xu, X.H.; Lin, N.; Wang, D.W.; Lin, Y.M.; Su, Z.Z.; Lu, H.D. Overexpression of miR-10a-5p facilitates the progression of osteoarthritis. Aging 2020, 12, 5948–5976. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Son, Y.; Kim, S.N.; Song, H.D.; Kim, M.; Park, J.H.; Jung, Y.S.; Ahn, S.Y.; Saha, A.; Granneman, J.G.; et al. MicroRNA-10a-5p regulates macrophage polarization and promotes therapeutic adipose tissue remodeling. Mol. Metab. 2019, 29, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.F.; Sun, J.W.; Zhu, S.P.; Du, Z.W.; Li, D.H.; Li, W.T.; Li, Z.J.; Tian, Y.D.; Kang, X.T.; Sun, G.R. MiRNAs and mRNAs Analysis during Abdominal Preadipocyte Differentiation in Chickens. Animals 2020, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Xu, M.; Shi, X.; Yang, G.; Sun, S.; Li, X. Elevated miR-10a-5p facilitates cell cycle and restrains adipogenic differentiation via targeting Map2k6 and Fasn, respectively. Acta Biochim. Biophys. Sin. 2020, 52, 1227–1235. [Google Scholar] [CrossRef]
- Liu, J.H.; Liang, Y.; Qiao, L.Y.; Xia, D.; Pan, Y.Y.; Liu, W.Z. MiR-128-1-5p regulates differentiation of ovine stromal vascular fraction by targeting the KLF11 5′-UTR. Domest. Anim. Endocrinol. 2022, 80, 106711. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Jiang, H.; Qu, W.; Rui, Y.J. KLF11 protects chondrocytes via inhibiting p38 MAPK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6505–6516. [Google Scholar]
- Brey, C.W.; Nelder, M.P.; Hailemariam, T.; Gaugler, R.; Hashmi, B. Krüppel-like family of transcription factors: An emerging new frontier in fat biology. Int. J. Biol. Sci. 2009, 5, 622–636. [Google Scholar] [CrossRef]
- Yin, K.J.; Fan, Y.; Hamblin, M.; Zhang, J.; Zhu, T.; Li, S.; Hawse, J.R.; Subramaniam, M.; Song, C.Z.; Urrutia, R.; et al. Klf11 mediates ppargamma cerebrovascular protection in ischaemic stroke. Brain 2013, 136, 1274–1287. [Google Scholar] [CrossRef]
- Zhang, H.B.; Chen, Q.; Yang, M.; Zhu, B.; Cui, Y.; Xue, Y.; Gong, N.; Cui, A.F.; Wang, M.; Shen, L.; et al. Mouse KLF11 regulates hepatic lipid metabolism. J. Hepatol. 2013, 58, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Yu, Y.; Ao, H.; Zhang, F.X.; Zhao, X.T.; Liu, H.T.; Shi, Y.; Xing, K.; Wang, C.D. Identification of Long Non-Coding RNAs Involved in Porcine Fat Deposition Using Two High-Throughput Sequencing Methods. Genes 2021, 12, 1374. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Liu, H.T.; Zhang, F.X.; Liu, Y.B.; Shi, Y.; Ding, X.D.; Wang, C.D. Identification of key genes affecting porcine fat deposition based on co-expression network analysis of weighted genes. J. Anim. Sci. Biotechnol. 2021, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Iwama, H.; Yamashita, T.; Kobayashi, K.; Fujihara, S.; Fujimori, T.; Kamada, H.; Kobara, H.; Masaki, T. The anti-diabetic drug metformin inhibits pancreatic cancer cell proliferation in vitro and in vivo: Study of the microRNAs associated with the antitumor effect of metformin. Oncol. Rep. 2016, 35, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kobara, H.; Mori, H.; Fujihara, S.; Chiyo, T.; Matsunaga, T.; Nishiyama, N.; Ayaki, M.; Yachida, T.; Morishita, A.; et al. Differences in miRNA expression profiles between GIST and leiomyoma in human samples acquired by submucosal tunneling biopsy. Endosc. Int. Open. 2015, 3, E665–E671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, E.K.; Lee, M.J.; Abdelmohsen, K.; Kim, W.; Kim, M.M.; Srikantan, S.; Martindale, J.L.; Hutchison, E.R.; Kim, H.H.; Marasa, B.S.; et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol. Cell Biol. 2011, 31, 626–638. [Google Scholar] [CrossRef]
- Karbiener, M.; Fischer, C.; Nowitsch, S.; Opriessnig, P.; Papak, C.; Ailhaud, G.; Dani, C.; Amri, E.-Z.; Scheideler, M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem. Biophys. Res. Commun. 2009, 390, 247–251. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, A.Y.; Lee, H.W.; Son, Y.H.; Son, Y.H.; Lee, G.Y.; Lee, J.W.; Lee, Y.S.; Kim, J.B. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem. Biophys. Res. Commun. 2010, 392, 323–328. [Google Scholar] [CrossRef]
- Pan, S.F.; Yang, X.J.; Jia, Y.M.; Li, R.S.; Zhao, R.Q. Microvesicle-shuttled miR-130b reduces fat deposition in recipient primary cultured porcine adipocytes by inhibiting PPAR-gamma expression. J. Cell Physiol. 2014, 229, 631–639. [Google Scholar] [CrossRef]
- Liu, S.H.; Yang, Y.; Wu, J.R. TNFalpha-induced up-regulation of miR-155 inhibits adipogenesis by down-regulating early adipogenic transcription factors. Biochem. Biophys. Res. Commun. 2011, 414, 618–624. [Google Scholar] [CrossRef]
- Yang, Z.; Bian, C.; Zhou, H.; Huang, S.; Wang, S.; Liao, L.; Zhao, R.C. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev. 2011, 20, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Li, X.Y.; He, H.R.; Zhang, Y.; He, H.; Sun, C.J.; Zhang, X.Y.; Wang, X.Z.; Kan, Z.Y.; Su, Y.; et al. miR-10a-5p inhibits chicken granulosa cells proliferation and Progesterone (P4) synthesis by targeting MAPRE1 to suppress CDK2. Theriogenology 2022, 192, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Kang, M.S.; Bai, X.P. TCF21 regulates miR-10a-5p/LIN28B signaling to block the proliferation and invasion of melanoma cells. PLoS ONE 2021, 16, e0255971. [Google Scholar] [CrossRef] [PubMed]
- Vaher, H.; Runnel, T.; Urgard, E.; Aab, A.; Badosa, G.C.; Maslovskaja, J.; Abram, K.; Raam, L.; Kaldvee, B.; Annilo, T.; et al. miR-10a-5p is increased in atopic dermatitis and has capacity to inhibit keratinocyte proliferation. Allergy 2019, 74, 2146–2156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, M. MEK6 Overexpression Exacerbates Fat Accumulation and Inflammatory Cytokines in High-Fat Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 13559. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.C.; Bensinger, S.J.; Villanueva, C.J.; Wroblewski, K.; Tontonoz, P. Inhibition of adipocyte differentiation by Nur77, Nurr1, and Nor1. Mol. Endocrinol. 2008, 22, 2596–2608. [Google Scholar] [CrossRef][Green Version]
- Bi, P.P.; Shan, T.Z.; Liu, W.Y.; Yue, F.; Yang, X.; Liang, X.R.; Wang, J.H.; Li, J.; Carlesso, N.; Liu, X.Q.; et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat. Med. 2014, 20, 911–918. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Li, X.; Du, Y.; Li, Y.; Zhu, J.; Lin, Y. miR-10a-5p Inhibits the Differentiation of Goat Intramuscular Preadipocytes by Targeting KLF8 in Goats. Front. Mol. Biosci. 2021, 8, 700078. [Google Scholar] [CrossRef]
- Correa, L.F.; Zheng, Y.; Delaney, A.A.; Khan, Z.; Shenoy, C.C.; Daftary, G.S. TGF-β Induces Endometriotic Progression via a Noncanonical, KLF11-Mediated Mechanism. Endocrinology 2016, 157, 3332–3343. [Google Scholar] [CrossRef][Green Version]
- Loft, A.; Forss, I.; Siersbæk, M.S.; Schmidt, S.F.; Larsen, A.B.; Madsen, J.G.S.; Didier, F.; Pisani, D.F.; Nielsen, R.; Aagaard, M.M.; et al. Browning of human adipocytes requires KLF11 and reprogramming of PPARγ superenhancers. Genes Dev. 2015, 29, 7–22. [Google Scholar] [CrossRef]
- Cao, S.; Fernandez-Zapico, M.E.; Jin, D.Z.; Puri, V.; Cook, T.A.; Lerman, L.O.; Zhu, X.Y.; Urrutia, R.; Shah, V. KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. J. Biol. Chem. 2005, 280, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ha, S.E.; Wei, L.; Jin, B.; Zogg, H.; Poudrier, S.M.; Jorgensen, B.G.; Park, C.; Ronkon, C.F.; Bartlett, A.; et al. MiR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology 2021, 160, 1662–1678. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Primer Sequence |
|---|---|
| 18S-F | CCCACGGAATCGAGAAAGAG |
| 18S-R | TTGACGGAAGGGCACCA |
| PCNA-F | GTGATTCCACCACCATGTTC |
| PCNA-R | TGAGACGAGTCCATGCTCG |
| ki67-F | AGCCCGTATCGTGTGCAAAA |
| ki67-R | CCTGCATCTGTGTAAGGGCA |
| Cyclin D1-F | GCGAGGAACAGAAGTGCG |
| Cyclin D1-R | TGGAGTTGTCGGTGTAGATGC |
| Cyclin B-F | TGGCTAGTGCAGGTTCAG |
| Cyclin B-R | CAGTCACAAAGGCAAAGT |
| CDK1-F | CCCTCCTGGTCAGTTCAT |
| CDK1-R | TAGGCTTCCTGGTTTCC |
| CDK4-F | GCATCCCAATGTTGTCCG |
| CDK4-R | GGGGTGCCTTGTCCAGATA |
| PPARγ-F | AGAGTATGCCAAGAACATCC |
| PPARγ-R | AGGTCGCTGTCATCTAATTC |
| C/EBPα-F | AGCCAAGAAGTCGGTAGA |
| C/EBPα-R | CGGTCATTGTCACTGGTC |
| aP2-F | AAGTCAAGAGCACCATAACC |
| aP2-R | GATACATTCCACCACCAACT |
| KLF11-F | AAGCGGCATGACAGTGAGAG |
| KLF11-R miR-10a-5p-F U6 | GAGGAGTCATGCACAGAGTTG TACCCTGTAGATCCGAATTTGT AACGCTTCACGAATTTGCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhao, T.; Gao, X.; Ma, S.; Gong, T.; Yang, Y.; Li, M.; Cao, G.; Guo, X.; Li, B. miR-10a-5p Regulates the Proliferation and Differentiation of Porcine Preadipocytes Targeting the KLF11 Gene. Animals 2024, 14, 337. https://doi.org/10.3390/ani14020337
Zhang W, Zhao T, Gao X, Ma S, Gong T, Yang Y, Li M, Cao G, Guo X, Li B. miR-10a-5p Regulates the Proliferation and Differentiation of Porcine Preadipocytes Targeting the KLF11 Gene. Animals. 2024; 14(2):337. https://doi.org/10.3390/ani14020337
Chicago/Turabian StyleZhang, Wanfeng, Tianzhi Zhao, Xinyu Gao, Shuangji Ma, Tianye Gong, Yang Yang, Meng Li, Guoqing Cao, Xiaohong Guo, and Bugao Li. 2024. "miR-10a-5p Regulates the Proliferation and Differentiation of Porcine Preadipocytes Targeting the KLF11 Gene" Animals 14, no. 2: 337. https://doi.org/10.3390/ani14020337
APA StyleZhang, W., Zhao, T., Gao, X., Ma, S., Gong, T., Yang, Y., Li, M., Cao, G., Guo, X., & Li, B. (2024). miR-10a-5p Regulates the Proliferation and Differentiation of Porcine Preadipocytes Targeting the KLF11 Gene. Animals, 14(2), 337. https://doi.org/10.3390/ani14020337





