Efficacy of Allogeneic and Xenogeneic Exosomes for the Treatment of Canine Atopic Dermatitis: A Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Production of cExos and hExos
2.3. Counting and Immunoblotting cExos and hExos
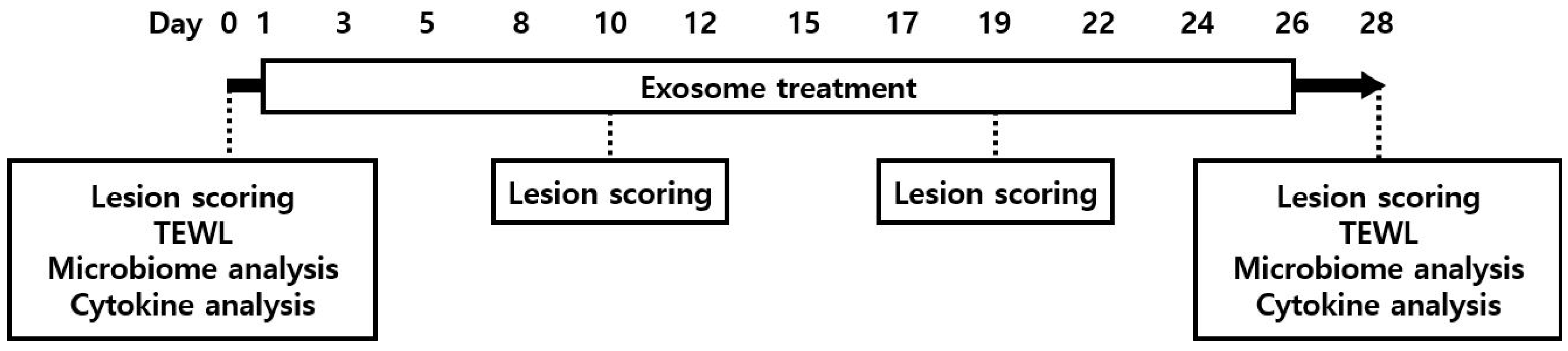
2.4. Study Design
2.5. Evaluation of Skin Lesions
2.6. Evaluation of Skin Barrier Function
2.7. Serum Cytokine Analysis
2.8. Skin Microbiome Analysis
2.9. Statistical Analysis
3. Results
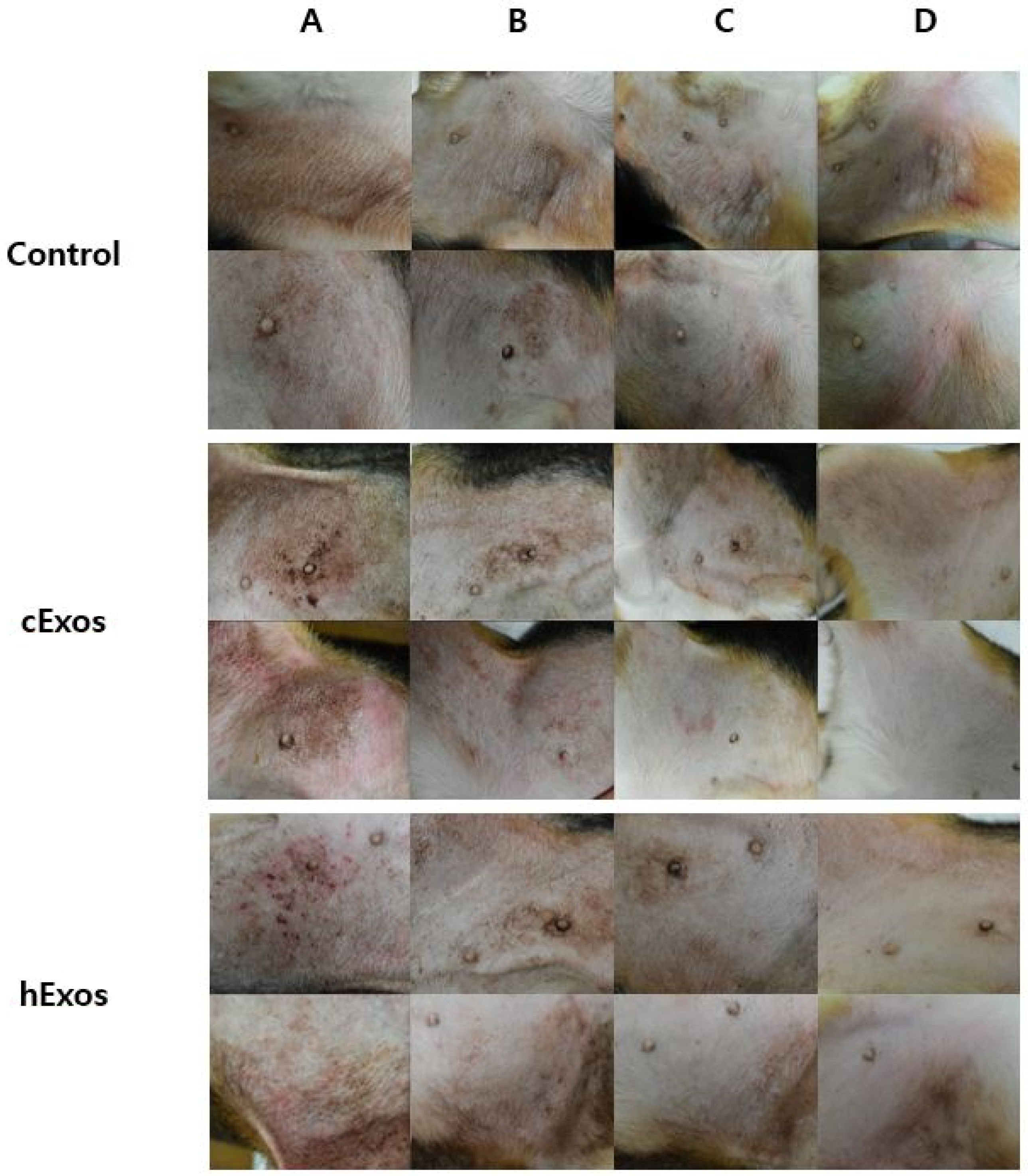
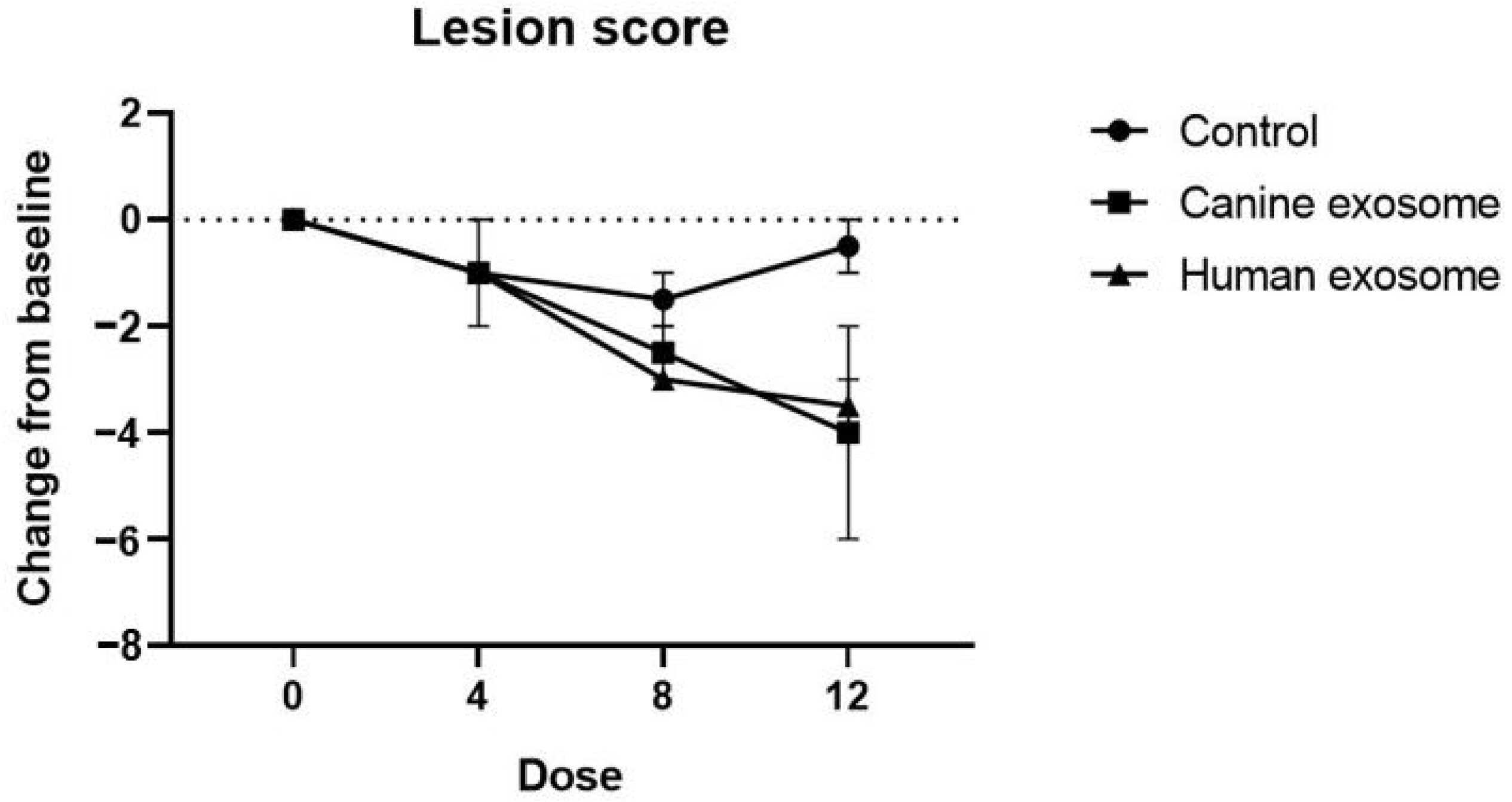
3.1. Evaluation of Skin Lesions
3.2. Evaluation of Skin Barrier Function
3.3. Serum Cytokine Levels
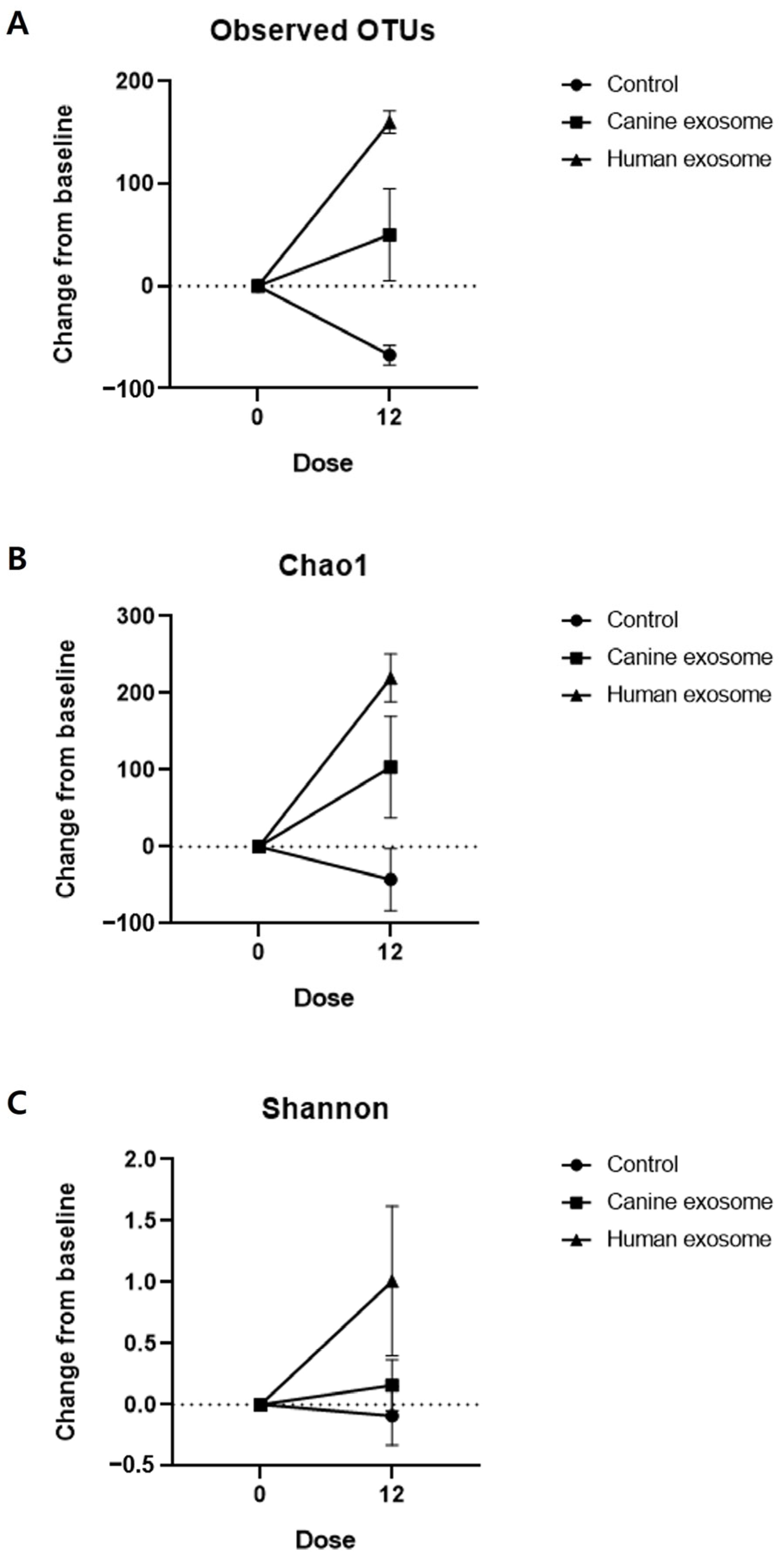
3.4. Skin Microbiome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Olivry, T.; DeBoer, D.J.; Griffin, C.E.; Halliwell, R.E.; Hill, P.B.; Hillier, A.; Marsella, R.; Sousa, C.A. The ACVD Task Force on Canine Atopic Dermatitis: Forewords and Lexicon. Vet. Immunol. Immunopathol. 2001, 81, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Hillier, A.; Griffin, C.E. The ACVD Task Force on Canine Atopic Dermatitis (I): Incidence and Prevalence. Vet. Immunol. Immunopathol. 2001, 81, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Tarpataki, N.; Pápa, K.; Reiczigel, J.; Vajdovich, P.; Vörös, K. Prevalence and Features of Canine Atopic Dermatitis in Hungary. Acta Vet. Hung. 2006, 54, 353–366. [Google Scholar] [CrossRef]
- Olivry, T.; Sousa, C.A. The ACVD Task Force on Canine Atopic Dermatitis (XIX): General Principles of Therapy. Vet. Immunol. Immunopathol. 2001, 81, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bizikova, P.; Santoro, D.; Marsella, R.; Nuttall, T.; Eisenschenk, M.N.C.; Pucheu-Haston, C.M. Review: Clinical and Histological Manifestations of Canine Atopic Dermatitis. Vet. Dermatol. 2015, 26, 79-e24. [Google Scholar] [CrossRef] [PubMed]
- Deboer, D.J.; Hillier, A. The ACVD Task Force on Canine Atopic Dermatitis (XV): Fundamental Concepts in Clinical Diagnosis. Vet. Immunol. Immunopathol. 2001, 81, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C.; Steffan, J.; Seewald, W.; Picco, F. A Prospective Study on the Clinical Features of Chronic Canine Atopic Dermatitis and Its Diagnosis. Vet. Dermatol. 2010, 21, 23–31. [Google Scholar] [CrossRef]
- Griffin, C.E.; Deboer, D.J. The ACVD Task Force on Canine Atopic Dermatitis (XIV): Clinical Manifestations of Canine Atopic Dermatitis. Vet. Immunol. Immunopathol. 2001, 81, 255–269. [Google Scholar] [CrossRef]
- Marsella, R.; Sousa, C.A.; Gonzales, A.J.; Fadok, V.A. Current Understanding of the Pathophysiologic Mechanisms of Canine Atopic Dermatitis. J. Am. Vet. Med. Assoc. 2012, 241, 194–207. [Google Scholar] [CrossRef]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prélaud, P.; International Task Force on Canine Atopic Dermatitis. Treatment of Canine Atopic Dermatitis: 2010 Clinical Practice Guidelines from the International Task Force on Canine Atopic Dermatitis. Vet. Dermatol. 2010, 21, 233–248. [Google Scholar] [CrossRef]
- Jaeger, K.; Linek, M.; Power, H.T.; Bettenay, S.V.; Zabel, S.; Rosychuk, R.A.W.; Mueller, R.S. Breed and Site Predispositions of Dogs with Atopic Dermatitis: A Comparison of Five Locations in Three Continents. Vet. Dermatol. 2010, 21, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Deboer, D.J.; Marsella, R. The ACVD Task Force on Canine Atopic Dermatitis (XII): The Relationship of Cutaneous Infections to the Pathogenesis and Clinical Course of Canine Atopic Dermatitis. Vet. Immunol. Immunopathol. 2001, 81, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T.; Uri, M.; Halliwell, R. Canine Atopic Dermatitis—What Have We Learned? Vet. Rec. 2013, 172, 201–207. [Google Scholar] [CrossRef]
- Linek, M.; Favrot, C. Impact of Canine Atopic Dermatitis on the Health-Related Quality of Life of Affected Dogs and Quality of Life of Their Owners. Vet. Dermatol. 2010, 21, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Noli, C. Assessing Quality of Life for Pets with Dermatologic Disease and Their Owners. Vet. Clin. N. Am.-Small Anim. Pract. 2019, 49, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prélaud, P. Treatment of Canine Atopic Dermatitis: 2015 Updated Guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet. Res. 2015, 11, 210. [Google Scholar] [CrossRef]
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis: Pathogenesis and Treatment. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef]
- Forsythe, P.; Jackson, H. New Therapies for Canine Atopic Dermatitis. In Pract. 2020, 42, 82–90. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the Barriers of the Skin: Exosome Therapy. Biomater. Res. 2021, 25, 22. [Google Scholar] [CrossRef]
- Mastri, M.; Lin, H.; Lee, T. Enhancing the Efficacy of Mesenchymal Stem Cell Therapy. World J. Stem Cells 2014, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, E.; Cha, M.J.; Hwang, K.C. Cell Adhesion and Long-Term Survival of Transplanted Mesenchymal Stem Cells: A Prerequisite for Cell Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Diomaiuto, E.; Principe, V.; De Luca, A.; Laperuta, F.; Alterisio, C.; Di Loria, A. Exosomes in Dogs and Cats: An Innovative Approach to Neoplastic and Non-Neoplastic Diseases. Pharmaceuticals 2021, 14, 766. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.; Lötvall, J.; Breakefield, X.O.; Andaloussi, S.E.L. Advances in Therapeutic Applications of Extracellular Vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-Exosome: A Novel Cell-Free Therapy for Cutaneous Regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying Extracellular Vesicles Based Therapeutics in Clinical Trials—An ISEV Position Paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Sarko, D.K.; McKinney, C.E. Exosomes: Origins and Therapeutic Potential for Neurodegenerative Disease. Front. Neurosci. 2017, 11, 246618. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, J.H. Establishing an Experimental Model for Canine Atopic Dermatitis through Epicutaneous Application of Dermatophagoides Farinae. Front. Vet. Sci. 2022, 9, 1015915. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.; Choi, Y.; Lim, K.M.; Jeong, Y.; Dayem, A.A.; Lee, Y.; An, J.; Song, K.; Jang, S.B.; et al. Improved Wound Healing and Skin Regeneration Ability of 3,2′-Dihydroxyflavone-Treated Mesenchymal Stem Cell-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 6964. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, Y.; Mirzaaghasi, A.; Heo, J.; Kim, Y.N.; Shin, J.H.; Kim, S.; Kim, N.H.; Cho, E.S.; Yook, J.I.; et al. Exosome-Based Delivery of Super-Repressor IκBα Relieves Sepsis-Associated Organ Damage and Mortality. Sci. Adv. 2020, 6, eaaz6980. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Ryu, S.W.; Choi, H.; You, S.; Park, J.; Choi, C. Manufacturing Therapeutic Exosomes: From Bench to Industry. Mol. Cells 2022, 45, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Dayem, A.A.; Lee, S.; Choi, Y.; Lim, K.M.; Kim, S.; An, J.; Shin, Y.; Park, H.; Jeon, T.I.; et al. Superior therapeutic activity of TGF-β-induced extracellular vesicles against interstitial cystitis. J. Control. Release 2022, 348, 924–937. [Google Scholar] [CrossRef]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B. Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a Simplified Severity Scale for Assessing Skin Lesions of Atopic Dermatitis in Dogs. Vet. Dermatol. 2014, 25, 77-e25. [Google Scholar] [CrossRef]
- Lau-Gillard, P.J.; Hill, P.B.; Chesney, C.J.; Budleigh, C.; Immonen, A. Evaluation of a Hand-Held Evaporimeter (VapoMeter®) for the Measurement of Transepidermal Water Loss in Healthy Dogs. Vet. Dermatol. 2010, 21, 136–145. [Google Scholar] [CrossRef]
- Gedon, N.K.Y.; Mueller, R.S. Atopic Dermatitis in Cats and Dogs: A Difficult Disease for Animals and Owners. Clin. Transl. Allergy 2018, 8, 41. [Google Scholar] [CrossRef]
- Saridomichelakis, M.N.; Olivry, T. An Update on the Treatment of Canine Atopic Dermatitis. Vet. J. 2016, 207, 29–37. [Google Scholar] [CrossRef]
- Marsella, R.; Ahrens, K.; Wilkes, R.; Trujillo, A.; Dorr, M. Comparison of Various Treatment Options for Canine Atopic Dermatitis: A Blinded, Randomized, Controlled Study in a Colony of Research Atopic Beagle Dogs. Vet. Dermatol. 2020, 31, 284-e69. [Google Scholar] [CrossRef]
- Olivry, T.; Sousa, C.A. The ACVD Task Force on Canine Atopic Dermatitis (XX): Glucocorticoid Pharmacotherapy. Vet. Immunol. Immunopathol. 2001, 81, 317–322. [Google Scholar] [CrossRef]
- Nuttall, T.; Reece, D.; Roberts, E. Life-Long Diseases Need Life-Long Treatment: Long-Term Safety of Ciclosporin in Canine Atopic Dermatitis. Vet. Rec. 2014, 174, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Radowicz, S.N.; Power, H.T. Long-Term Use of Cyclosporine in the Treatment of Canine Atopic Dermatitis. Vet. Dermatol. 2005, 16, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Little, P.R.; King, V.L.; Davis, K.R.; Cosgrove, S.B.; Stegemann, M.R. A Blinded, Randomized Clinical Trial Comparing the Efficacy and Safety of Oclacitinib and Ciclosporin for the Control of Atopic Dermatitis in Client-Owned Dogs. Vet. Dermatol. 2015, 26, 23-e8. [Google Scholar] [CrossRef]
- Cosgrove, S.B.; Cleaver, D.M.; King, V.L.; Gilmer, A.R.; Daniels, A.E.; Wren, J.A.; Stegemann, M.R. Long-Term Compassionate Use of Oclacitinib in Dogs with Atopic and Allergic Skin Disease: Safety, Efficacy and Quality of Life. Vet. Dermatol. 2015, 26, 171-e35. [Google Scholar] [CrossRef] [PubMed]
- Moyaert, H.; Van Brussel, L.; Borowski, S.; Escalada, M.; Mahabir, S.P.; Walters, R.R.; Stegemann, M.R. A Blinded, Randomized Clinical Trial Evaluating the Efficacy and Safety of Lokivetmab Compared to Ciclosporin in Client-Owned Dogs with Atopic Dermatitis. Vet. Dermatol. 2017, 28, 593-e145. [Google Scholar] [CrossRef] [PubMed]
- Tamamoto-Mochizuki, C.; Paps, J.S.; Olivry, T. Proactive Maintenance Therapy of Canine Atopic Dermatitis with the Anti-IL-31 Lokivetmab. Can a Monoclonal Antibody Blocking a Single Cytokine Prevent Allergy Flares? Vet. Dermatol. 2019, 30, 98-e26. [Google Scholar] [CrossRef]
- Fischer, N.; Rostaher, A.; Favrot, C. Intralymphatic Immunotherapy: An Effective and Safe Alternative Route for Canine Atopic Dermatitis. Schweiz. Arch. Tierheilkd. 2016, 158, 646–652. [Google Scholar] [CrossRef]
- Fischer, N.M.; Rostaher, A.; Favrot, C. A Comparative Study of Subcutaneous, Intralymphatic and Sublingual Immunotherapy for the Long-Term Control of Dogs with Nonseasonal Atopic Dermatitis. Vet. Dermatol. 2020, 31, 365-e96. [Google Scholar] [CrossRef]
- Griffin, C.E.; Hillier, A. The ACVD Task Force on Canine Atopic Dermatitis (XXIV): Allergen-Specific Immunotherapy. Vet. Immunol. Immunopathol. 2001, 81, 363–383. [Google Scholar] [CrossRef]
- Gonzales, A.J.; Humphrey, W.R.; Messamore, J.E.; Fleck, T.J.; Fici, G.J.; Shelly, J.A.; Teel, J.F.; Bammert, G.F.; Dunham, S.A.; Fuller, T.E.; et al. Interleukin-31: Its Role in Canine Pruritus and Naturally Occurring Canine Atopic Dermatitis. Vet. Dermatol. 2013, 24, 48-e12. [Google Scholar] [CrossRef]
- Hoffmann, A.R.; Patterson, A.P.; Diesel, A.; Lawhon, S.D.; Ly, H.J.; Stephenson, C.E.; Mansell, J.; Steiner, J.M.; Dowd, S.E.; Olivry, T.; et al. The Skin Microbiome in Healthy and Allergic Dogs. PLoS ONE 2014, 9, e83197. [Google Scholar] [CrossRef]
- Mocchi, M.; Dotti, S.; Del Bue, M.; Villa, R.; Bari, E.; Perteghella, S.; Torre, M.L.; Grolli, S. Veterinary Regenerative Medicine for Musculoskeletal Disorders: Can Mesenchymal Stem/Stromal Cells and Their Secretome Be the New Frontier? Cells 2020, 9, 1453. [Google Scholar] [CrossRef]
- Toh, W.S.; Lai, R.C.; Hui, J.H.P.; Lim, S.K. MSC Exosome as a Cell-Free MSC Therapy for Cartilage Regeneration: Implications for Osteoarthritis Treatment. Semin. Cell Dev. Biol. 2017, 67, 56–64. [Google Scholar] [CrossRef]
- El-Tookhy, O.S.; Shamaa, A.A.; Shehab, G.G.; Abdallah, A.N.; Azzam, O.M. Histological Evaluation of Experimentally Induced Critical Size Defect Skin Wounds Using Exosomal Solution of Mesenchymal Stem Cells Derived Microvesicles. Int. J. Stem Cells 2017, 10, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.N.; Rosenkrantz, W.S.; Hong, J.H.; Griffin, C.E.; Mendelsohn, C.M. Evaluation of the Potential Use of Adipose-Derived Mesenchymal Stromal Cells in the Treatment of Canine Atopic Dermatitis: A Pilot Study. Vet. Ther. 2010, 11, E1–E14. [Google Scholar] [PubMed]
- Villatoro, A.J.; Hermida-Prieto, M.; Fernández, V.; Fariñas, F.; Alcoholado, C.; Isabel Rodríguez-García, M.; Mariñas-Pardo, L.; Becerra, J. Allogeneic Adipose-Derived Mesenchymal Stem Cell Therapy in Dogs with Refractory Atopic Dermatitis: Clinical Efficacy and Safety. Vet. Rec. 2018, 183, 654. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Gu, N.Y.; Byeon, J.S.; Hyun, B.H.; Lee, J.; Yang, D.K. Immunomodulatory Effects of Canine Mesenchymal Stem Cells in an Experimental Atopic Dermatitis Model. Front. Vet. Sci. 2023, 10, 1201382. [Google Scholar] [CrossRef]
- Kim, J.; Song, Y.; Park, C.H.; Choi, C. Platform Technologies and Human Cell Lines for the Production of Therapeutic Exosomes. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 3–17. [Google Scholar] [CrossRef]
- Chae, J.S.; Park, H.; Ahn, S.H.; Han, E.C.; Lee, Y.; Kim, Y.J.; Ahn, E.J.; Oh, H.W.; Lee, H.J.; Choi, C.; et al. The Effect of Super-Repressor IkB-Loaded Exosomes (Exo-SrIκBs) in Chronic Post-Ischemia Pain (CPIP) Models. Pharmaceutics 2023, 15, 553. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-ΚB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.A.; Yoon, H.; Kim, M.Y.; Yoo, J.K.; Ahn, S.H.; Park, C.H.; Park, J.; Nam, B.Y.; Park, J.T.; et al. Exosome-Based Delivery of Super-Repressor IκBα Ameliorates Kidney Ischemia-Reperfusion Injury. Kidney Int. 2021, 100, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Radnaa, E.; Yoo, J.K.; Kim, E.; Choi, K.; Kim, Y.; Kim, Y.; Richardson, L.; Choi, C.; Menon, R. Exosomal Delivery of NF-ΚB Inhibitor Delays LPS-Induced Preterm Birth and Modulates Fetal Immune Cell Profile in Mouse Models. Sci. Adv. 2021, 7, 3865–3887. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.F.; Lázaro-Ibáñez, E.; Forsgard, M.A.M.; Shatnyeva, O.; Osteikoetxea, X.; Karlsson, F.; Heath, N.; Ingelsten, M.; Rose, J.; Harris, J.; et al. Extracellular Vesicles Induce Minimal Hepatotoxicity and Immunogenicity. Nanoscale 2019, 11, 6990–7001. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wu, Y.; Zhang, Y.; Yu, M.; Tian, W. Comparison of the Therapeutic Effect of Allogeneic and Xenogeneic Small Extracellular Vesicles in Soft Tissue Repair. Int. J. Nanomed. 2020, 15, 6975–6991. [Google Scholar] [CrossRef]

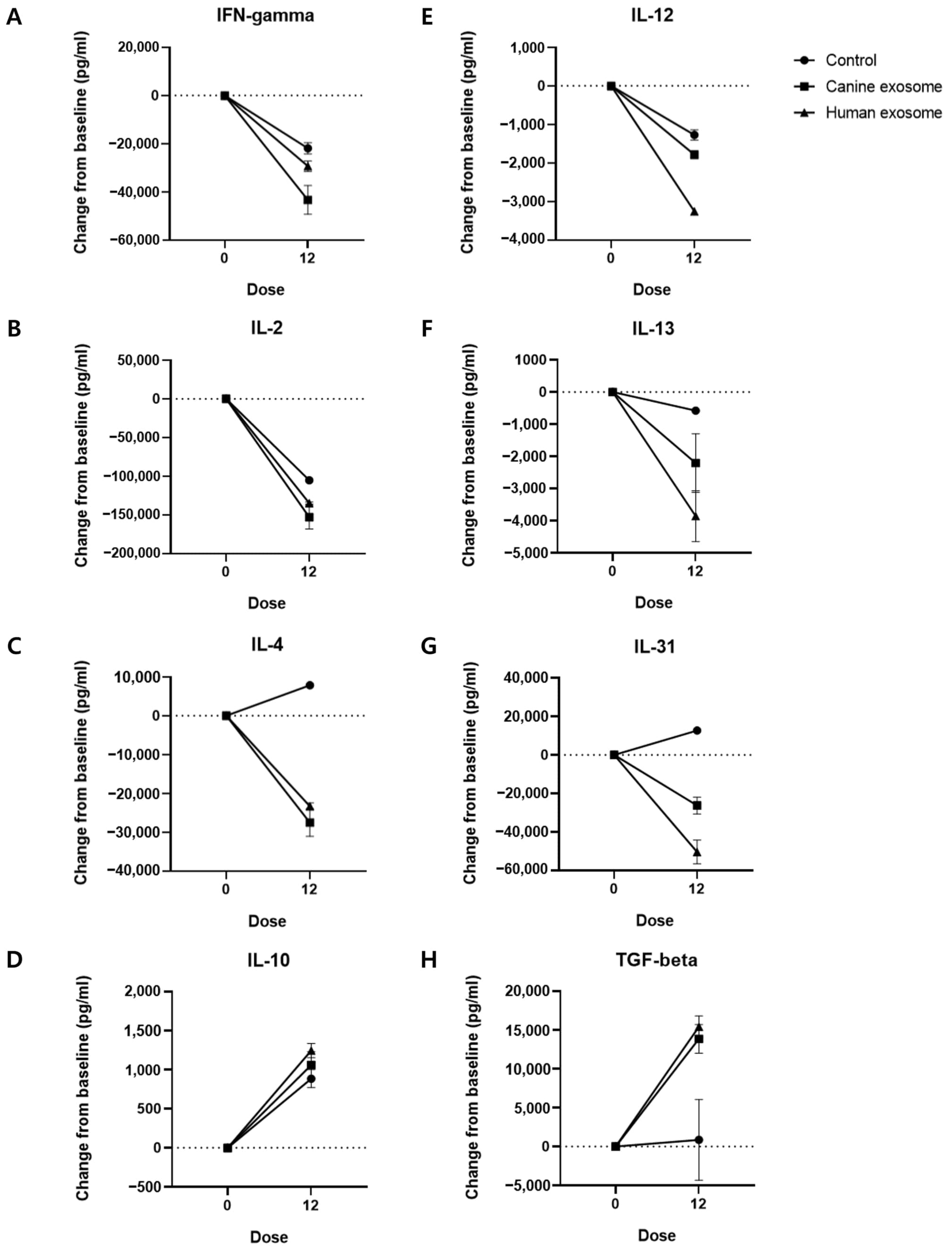

| Cytokine (pg/mL) | Control | cExos | hExos |
|---|---|---|---|
| IFN-γ | −21,903 ± 2328 | −43,335 ± 5973 | −29,327 ± 2188 |
| IL-2 | −105,333 ± 1667 | −153,000 ± 15,333 | −134,833 ± 1500 |
| IL-4 | 7833 ± 833.4 | −27,542 ± 3542 | −23,308 ± 891.7 |
| IL-10 | 884.7 ± 115.4 | 1060 ± 170.4 | 1245 ± 91.2 |
| IL-12 | −1275 ± 131.7 | −1787 ± 107.1 | −3271 ± 66.05 |
| IL-13 | −575.1 ± 56.25 | −2209 ± 908.6 | −3859 ± 791.4 |
| IL-31 | 12,676 ± 369.2 | −26,238 ± 4353 | −50,364 ± 6135 |
| TGF-β | 864.6 ± 5188 | 13,860 ± 1852 | 15,424 ± 1401 |
| Group | Subjects | Treatment | Total Valid Reads | Number of Normalized Reads | Observed OTUs | Chao1 | Shannon | Good’s Coverage (%) |
|---|---|---|---|---|---|---|---|---|
| Control | 1 | Before | 69,584 | 61,927 | 613 | 637.29 | 3.64 | 99.86 |
| After | 39,789 | 30,956 | 555 | 634.82 | 3.79 | 99.60 | ||
| 2 | Before | 86,295 | 61,927 | 579 | 628.58 | 4.10 | 99.86 | |
| After | 47,260 | 30,956 | 502 | 545.36 | 3.77 | 99.76 | ||
| cExos | 1 | Before | 61,927 | 61,927 | 524 | 532.84 | 3.63 | 99.92 |
| After | 43,676 | 30,956 | 529 | 570.54 | 4.00 | 99.74 | ||
| 2 | Before | 62,883 | 61,927 | 524 | 535.76 | 3.95 | 99.92 | |
| After | 38,454 | 30,956 | 619 | 705.51 | 3.90 | 99.57 | ||
| hExos | 1 | Before | 63,194 | 61,927 | 320 | 343.61 | 1.37 | 99.92 |
| After | 49,001 | 30,956 | 491 | 594.91 | 2.99 | 99.59 | ||
| 2 | Before | 65,333 | 61,927 | 625 | 653.88 | 4.06 | 99.88 | |
| After | 30,956 | 30,956 | 774 | 842.57 | 4.46 | 99.54 |
| Control | cExos | hExos | |
|---|---|---|---|
| Observed OTUs | −67.5 ± 9.5 | 50.0 ± 45.0 | 160.0 ± 11.0 |
| Chao1 | −42.85 ± 40.38 | 103.7 ± 66.03 | 220.0 ± 31.31 |
| Shannon | −0.09 ± 0.24 | 0.16 ± 0.21 | 1.01 ± 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-W.; Lim, K.-M.; Cho, S.-G.; Ryu, B.; Kim, C.-Y.; Park, S.Y.; Jang, K.; Jung, J.H.; Park, C.; Choi, C.; et al. Efficacy of Allogeneic and Xenogeneic Exosomes for the Treatment of Canine Atopic Dermatitis: A Pilot Study. Animals 2024, 14, 282. https://doi.org/10.3390/ani14020282
Kim S-W, Lim K-M, Cho S-G, Ryu B, Kim C-Y, Park SY, Jang K, Jung JH, Park C, Choi C, et al. Efficacy of Allogeneic and Xenogeneic Exosomes for the Treatment of Canine Atopic Dermatitis: A Pilot Study. Animals. 2024; 14(2):282. https://doi.org/10.3390/ani14020282
Chicago/Turabian StyleKim, Sang-Won, Kyung-Min Lim, Ssang-Goo Cho, Bokyeong Ryu, C-Yoon Kim, Seon Young Park, Kyungmin Jang, Jae Heon Jung, Cheolhyoung Park, Chulhee Choi, and et al. 2024. "Efficacy of Allogeneic and Xenogeneic Exosomes for the Treatment of Canine Atopic Dermatitis: A Pilot Study" Animals 14, no. 2: 282. https://doi.org/10.3390/ani14020282
APA StyleKim, S.-W., Lim, K.-M., Cho, S.-G., Ryu, B., Kim, C.-Y., Park, S. Y., Jang, K., Jung, J. H., Park, C., Choi, C., & Kim, J.-H. (2024). Efficacy of Allogeneic and Xenogeneic Exosomes for the Treatment of Canine Atopic Dermatitis: A Pilot Study. Animals, 14(2), 282. https://doi.org/10.3390/ani14020282








