Potential Tick Defense Associated with Skin and Hair Characteristics in Korean Water Deer (Hydropotes inermis argyropus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection
2.2. Sample Collection and Hair Analysis
2.3. Hair Morphology Examination
2.4. Histopathological Analysis
2.5. Statistical Analysis
3. Results
3.1. Variation in Tick Infestation between Roe Deer and Korean Water Deer
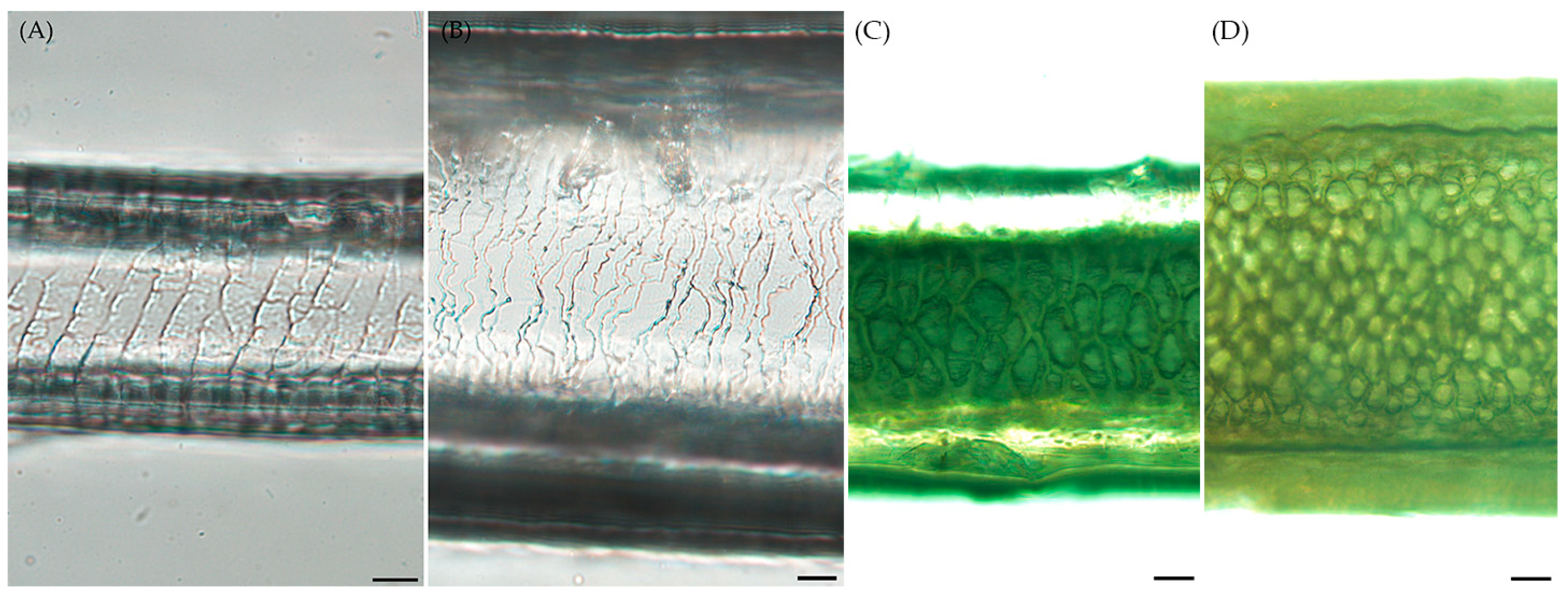
3.2. Morphological Distrinction in Hair of Roe Deer and Korean Water Deer
3.3. Comparison of Hair Diameter and Length in Roe Deer and Korean Water Deer
3.4. Skin Pathology Comparison between Roe Deer and Korean Water Deer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson, W.L.; Sonenshine, D.E.; Noden, B.H.; Brown, R.N. Ticks (Ixodida). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 603–672. [Google Scholar]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible effects of climate change on ixodid ticks and the pathogens they transmit: Predictions and observations. J. Med. Entomol. 2021, 58, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; VanAcker, M.C.; Fernandez, M.P. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J. Med. Entomol. 2021, 58, 1546–1564. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, A.; Laroche, M.; Degani, M.; Perandin, F.; Bisoffi, Z.; Raoult, D.; Parola, P. Tick-borne pathogens in removed ticks Veneto, northeastern Italy: A cross-sectional investigation. Travel Med. Infect. Dis. 2018, 26, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.I.; Hamer, S.A.; Han, S.; Sidge, J.L.; Hickling, G.J. The contribution of wildlife hosts to the rise of ticks and tick-borne diseases in North America. J. Med. Entomol. 2021, 58, 1565–1587. [Google Scholar] [CrossRef] [PubMed]
- Orkun, Ö.; Çakmak, A. Molecular identification of tick-borne bacteria in wild animals and their ticks in Central Anatolia, Turkey. Comp. Immunol. Microbiol. Infect. Dis. 2019, 63, 58–65. [Google Scholar] [CrossRef]
- Kiffner, C.; Loedige, C.; Alings, M.; Vor, T.; Ruehe, F. Attachment site selection of ticks on roe deer, Capreolus capreolus. Exp. Appl. Acarol. 2011, 53, 79–94. [Google Scholar] [CrossRef]
- Oorebeek, M.; Sharrad, R.; Kleindorfer, S. What attracts larval Ixodes hirsti (Acari: Ixodidae) to their host? Parasitol. Res. 2009, 104, 623–628. [Google Scholar] [CrossRef]
- Carr, A.L.; Salgado, V.L. Ticks home in on body heat: A new understanding of Haller’s organ and repellent action. PLoS ONE 2019, 14, e0221659. [Google Scholar] [CrossRef]
- O’kelly, J.C.; Spiers, W.G. Observations on body temperature of the host and resistance to the tick Boophilus microplus (Acari: Ixodidae). J. Med. Entomol. 1983, 20, 498–505. [Google Scholar] [CrossRef]
- Haarløv, N. Life cycle and distribution pattern of Lipoptena cervi (L.)(Dipt., Hippobosc.) on Danish deer. Oikos 1964, 15, 93–129. [Google Scholar] [CrossRef]
- De Castro, J.J.; Capstick, P.B.; Nokoe, S.; Kiara, H.; Rinkanya, F.; Slade, R.; Okello, O.; Bennun, L. Towards the selection of cattle for tick resistance in Africa. Exp. Appl. Acarol. 1991, 12, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, C.J.; Nicolau, C.V.J.; Cardoso, V.L.; Pinheiro, M.G. Haircoat characteristics and tick infestation on gyr (zebu) and crossbred (holdstein x gyr) cattle. Arch. Zootec. 2002, 51, 389–392. [Google Scholar]
- Martinez, M.L.; Machado, M.A.; Nascimento, C.S.; Silva, M.V.G.B.; Teodoro, R.L.; Furlong, J.; Prata, M.C.A.; Campos, A.L.; Guimarães, M.F.M.; Azevedo, A.L.S.; et al. Association of BoLA-DRB3. 2 alleles with tick (Boophilus microplus) resistance in cattle. Genet. Mol. Res. 2006, 5, 513–524. [Google Scholar] [PubMed]
- Alkathiri, B.; Lee, S.H. Review of ticks (families: Ixodidae and Argasidae) in the Republic of Korea. J. Biomed. Transl. Res. 2022, 23, 91–108. [Google Scholar]
- Kim, H.C.; Han, S.H.; Chong, S.T.; Klein, T.A.; Choi, C.Y.; Nam, H.Y.; Chae, Y.H.; Lee, H.; Ko, S.; Kang, J.G.; et al. Ticks collected from selected mammalian hosts surveyed in the Republic of Korea during 2008–2009. Korean J. Parasitol. 2011, 49, 331. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, E.J. Internationally vulnerable Korean water deer (Hydropotes inermis argyropus) can act as an ecological filter by endozoochory. Glob. Ecol. Conserv. 2020, 24, e01368. [Google Scholar] [CrossRef]
- Kim, K.Y. Spatio-Temporal Distribution of Ectoparasites in Relation to Habitat use Characteristics of Mammalian Hosts in Cultivated Land and Nearby Habitats in Gangwon-do. Ph.D. Thesis, Kangwon National University, Chuncheon, Republic of Korea, December 2022. [Google Scholar]
- Crocker, E.J. A new technique for the rapid simultaneous examination of medullae and cuticular patterns of hairs. Microscope 1998, 46, 169–173. [Google Scholar]
- Cornally, A.; Lawton, C. A guide to the identification of Irish mammal hair. In Irish Wildlife Manuals; No. 92.; Marnell, F., Jeffrey, R., Eds.; National Parks and Wildlife Service, Department of the Arts, Heritage, Regional, Rural and Gaeltacht Affairs: Dublin, Ireland, 2016; pp. 1–40. ISSN 1393-6670. [Google Scholar]
- De Marinis, A.M.; Asprea, A. Hair identification key of wild and domestic ungulates from southern Europe. Wildl. Biol. 2006, 12, 305–320. [Google Scholar] [CrossRef]
- Korea Meteorological Administration. Available online: http://www.climate.go.kr/home/bbs_new/view.php?code=96&bname=monthweather_new&vcode=302&skind=&sword=&category1=&category2= (accessed on 8 November 2023).
- Spickett, A.M.; De Klerk, D.; Enslin, C.B.; Scholtz, M.M. Resistance of Nguni, Bonsmara and Hereford cattle to ticks in a bushveld region of South Africa. Onderstepoort J. Vet. Res. 1989, 56, 245–250. [Google Scholar]
- Marufu, M.C.; Qokweni, L.; Chimonyo, M.; Dzama, K. Relationships between tick counts and coat characteristics in Nguni and Bonsmara cattle reared on semiarid rangelands in South Africa. Ticks Tick Borne Dis. 2011, 2, 172–177. [Google Scholar] [CrossRef]
- Gasparin, G.; Miyata, M.; Coutinho, L.L.; Martinez, M.L.; Teodoro, R.L.; Furlong, J.; Silva, M.V.G.B.; Regitano, L.C.A. Mapping of quantitative trait loci controlling tick [Riphicephalus (Boophilus) microplus] resistance on bovine chromosomes 5, 7 and 14. Anim. Genet. 2007, 38, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Tabor, A.E.; Ali, A.; Rehman, G.; Rocha Garcia, G.; Zangirolamo, A.F.; Malardo, T.; Jonsson, N.N. Cattle tick Rhipicephalus microplus-host interface: A review of resistant and susceptible host responses. Front. Cell. Infect. Microbiol. 2017, 7, 506. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Choi, T.Y.; Woo, D.; Min, M.S.; Sugita, S.; Lee, H. Species identification key of Korean mammal hair. J. Vet. Med. Sci. 2014, 76, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, D.; Gebhardt, R.; Petney, T.; Norra, S. On the complexity of measuring forests microclimate and interpreting its relevance in habitat ecology: The example of Ixodes ricinus ticks. Parasit. Vectors 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2013, 41, 317–320. [Google Scholar] [CrossRef]
- Lehane, M.J. Host-insect interactions. In The Biology of Blood-Sucking in Insects, 2nd ed.; Lehane, M.J., Ed.; Cambridge University Press: Cambridge, MA, USA, 2005; pp. 116–149. [Google Scholar]
- Verhulst, N.O.; Qiu, Y.T.; Beijleveld, H.; Maliepaard, C.; Knights, D.; Schulz, S.; Berg-Lyons, D.; Lauber, L.C.; Verduijn, W.; Haasnoot, G.W.; et al. Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS ONE 2011, 6, e28991. [Google Scholar] [CrossRef]

| Species | No. of Adult Ixodid Ticks | No. of Blood-Feeding Ixodid Ticks |
|---|---|---|
| WD (n = 5) | 25.2 ± 10.69 | 9.8 ± 4.18 |
| RD (n = 5) | 231.8 ± 81.81 ** | 85.2 ± 40.08 * |
| Parameter | Body Part | RD Hair (n = 20) | WD Hair (n = 20) |
|---|---|---|---|
| Diameter (μm) (Mean ± SD) | Head | 136.49 ± 17.92 *** | 216.65 ± 36.08 |
| Neck | 172.45 ± 39.22 *** | 436.63 ± 145.57 | |
| Back | 145.79 ± 17.74 *** | 629.24 ± 26.93 | |
| Axilla | 118.88 ± 15.05 *** | 326.86 ± 43.65 | |
| Total | 144.36 ± 3.47 *** | 409.20 ± 167.56 | |
| Length (mm) (Mean ± SD) | Head | 15.02 ± 3.04 *** | 9.15 ± 1.49 |
| Neck | 19.13 ± 3.55 | 20.56 ± 3.04 | |
| Back | 20.33 ± 3.50 * | 24.03 ± 3.80 | |
| Axilla | 25.92 ± 4.56 | 25.59 ± 3.02 | |
| Total | 19.87 ± 5.24 | 19.98 ± 6.49 |
| Parameter | Tissue | RD | WD |
|---|---|---|---|
| Area (1000 μm2) (Mean ± SD) | Sebaceous gland (n = 24) | 111.11 ± 20.12 *** | 314.52 ± 22.84 |
| Blood vessel (n = 24) | 9.25 ± 1.29 *** | 42.26 ± 6.53 | |
| Sweat gland (n = 24) | 203.24 ± 33.73 ** | 310.49 ± 29.61 | |
| Distance (μm) (Mean ± SD) | Epidermis (n = 64) | 62.23 ± 22.11 | 65.00 ± 12.48 |
| Primary hair follicle (n = 64) | 390.08 ± 151.33 | 389.94 ± 119.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Kim, K.-Y.; Kim, G.; Moon, S.; Park, Y.-C.; Cho, H.-S.; Oh, Y. Potential Tick Defense Associated with Skin and Hair Characteristics in Korean Water Deer (Hydropotes inermis argyropus). Animals 2024, 14, 185. https://doi.org/10.3390/ani14020185
Lee S-J, Kim K-Y, Kim G, Moon S, Park Y-C, Cho H-S, Oh Y. Potential Tick Defense Associated with Skin and Hair Characteristics in Korean Water Deer (Hydropotes inermis argyropus). Animals. 2024; 14(2):185. https://doi.org/10.3390/ani14020185
Chicago/Turabian StyleLee, Sang-Joon, Ki-Yoon Kim, Gyurae Kim, Subin Moon, Yung-Chul Park, Ho-Seong Cho, and Yeonsu Oh. 2024. "Potential Tick Defense Associated with Skin and Hair Characteristics in Korean Water Deer (Hydropotes inermis argyropus)" Animals 14, no. 2: 185. https://doi.org/10.3390/ani14020185
APA StyleLee, S.-J., Kim, K.-Y., Kim, G., Moon, S., Park, Y.-C., Cho, H.-S., & Oh, Y. (2024). Potential Tick Defense Associated with Skin and Hair Characteristics in Korean Water Deer (Hydropotes inermis argyropus). Animals, 14(2), 185. https://doi.org/10.3390/ani14020185








