Effects of Sophora alopecuroides in a High-Concentrate Diet on the Liver Immunity and Antioxidant Function of Lambs According to Transcriptome Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diet Composition
2.2. Feeding Management
2.3. Sample Collection and Determination Indicators
2.3.1. The Rumen pH Value
2.3.2. Liver Function Indicators
2.3.3. LPS and LBP in the Serum and Liver and Inflammatory Signaling Factors in the Liver
2.3.4. Liver Antioxidant Indicators
2.3.5. Liver Tissue Sections
2.3.6. Liver Transcriptome Sequencing
2.3.7. Expression of Differential Genes in Liver Tissue (q-PCR Verification)
2.4. Data Analysis
3. Results
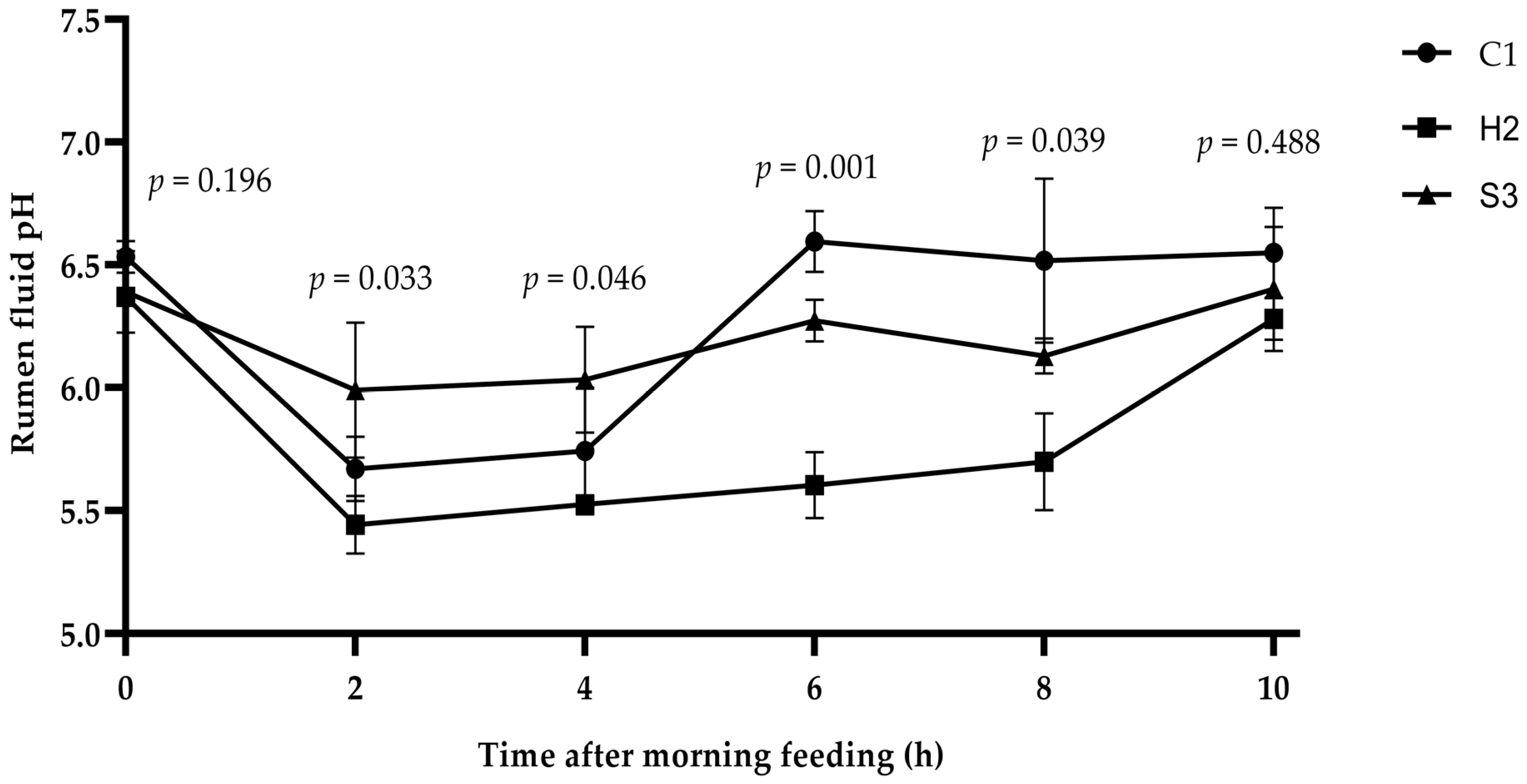
3.1. Effect of Adding SA to High-Concentrate Diets on the Rumen pH of Sheep
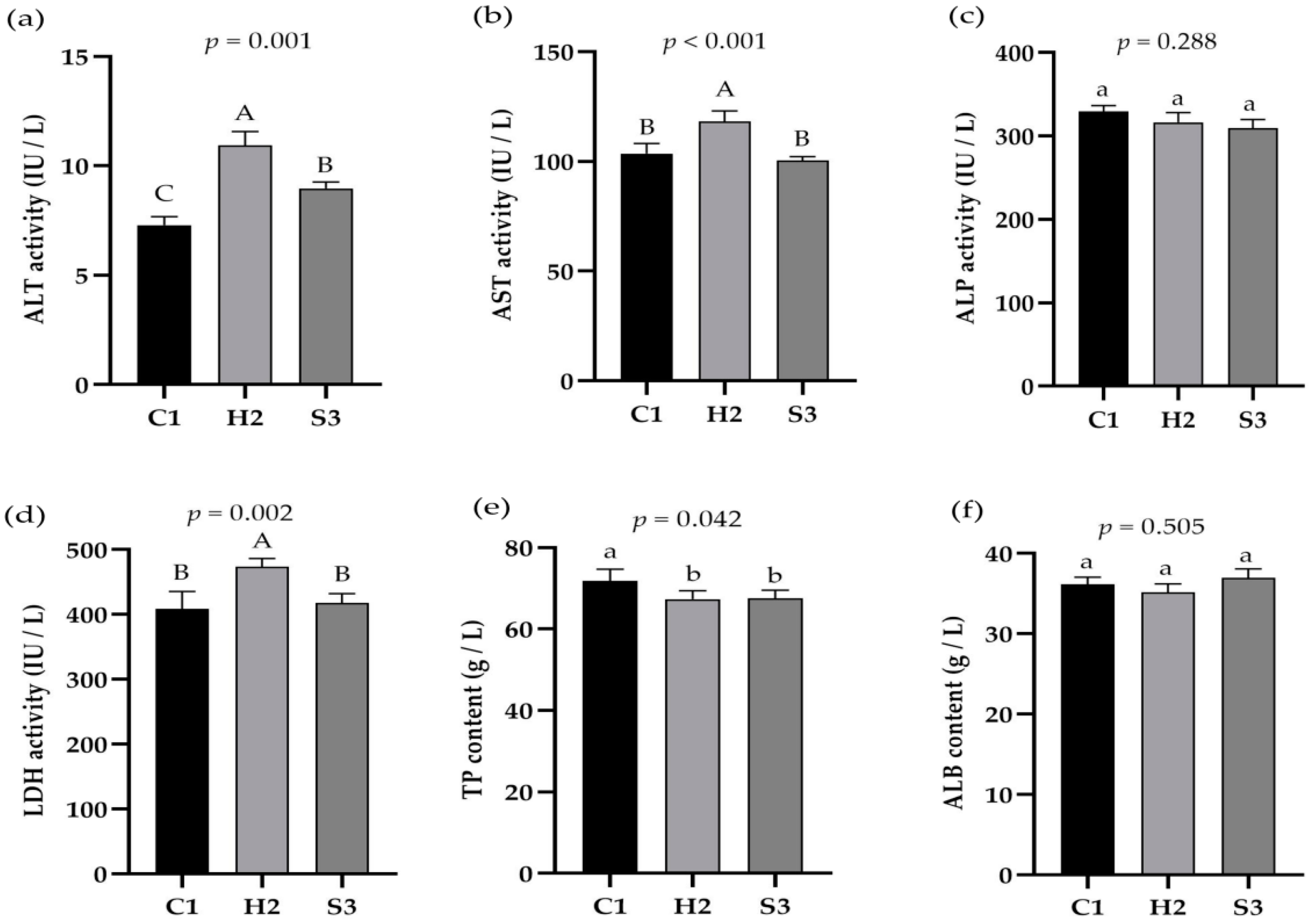
3.2. Effect of Adding SA to High-Concentrate Diets on Liver Function Indices in Sheep
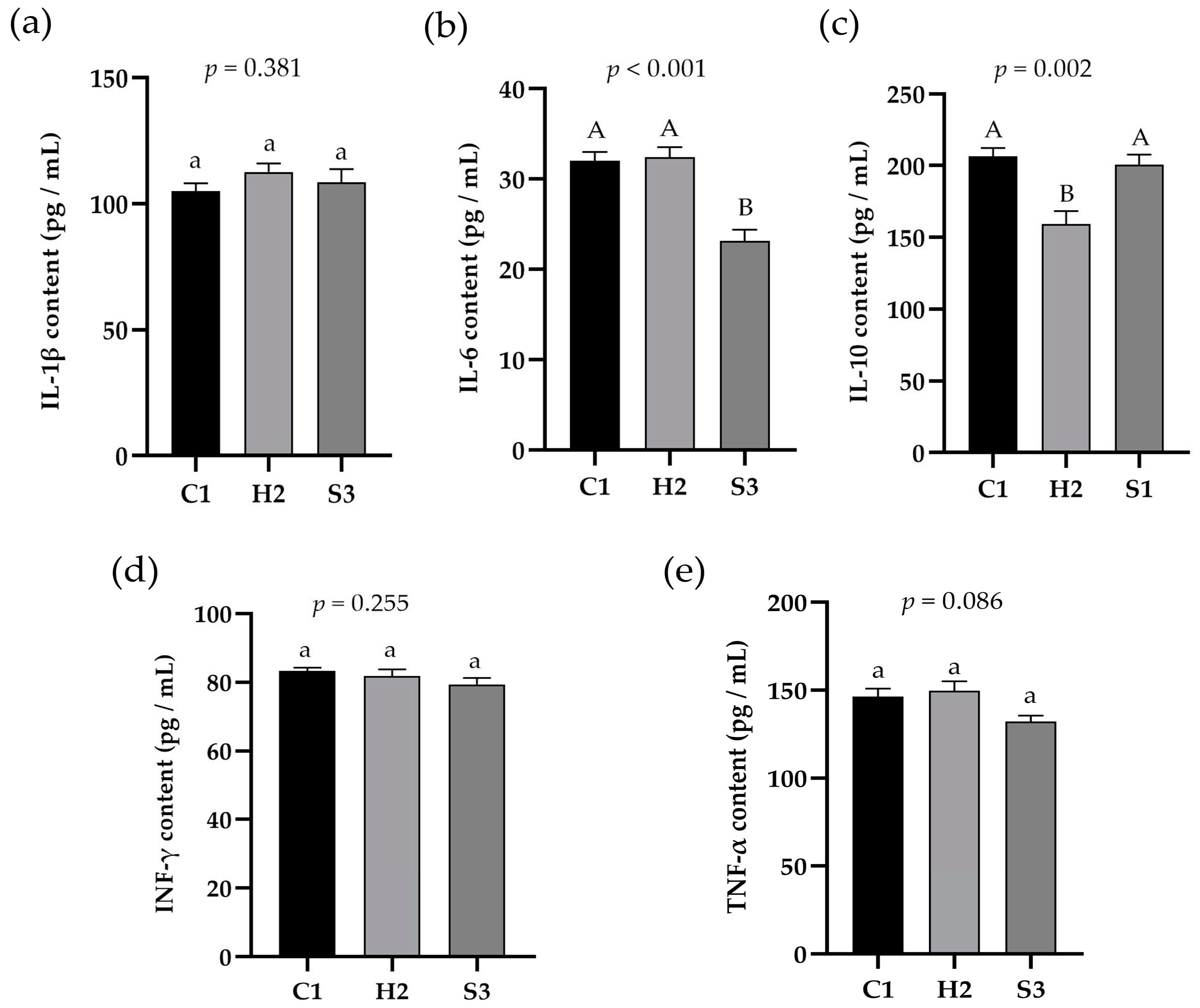
3.3. Effect of Adding SA to High-Concentrate Diets on LPS and LBP in the Serum and Liver and Inflammatory Signal Factors in the Liver of Sheep
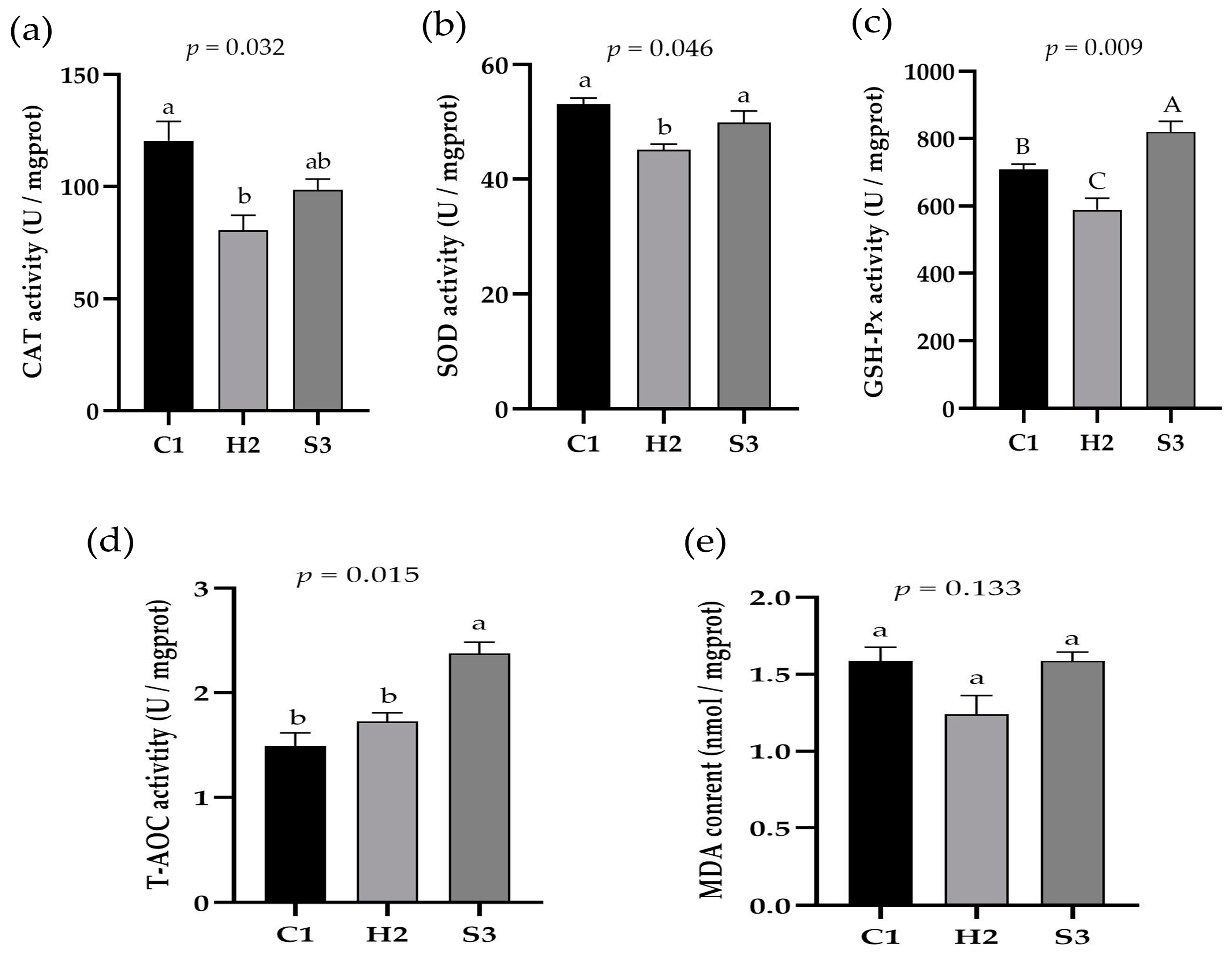
3.4. Effect of Adding SA to High-Concentrate Diets on the Liver Antioxidation of Sheep
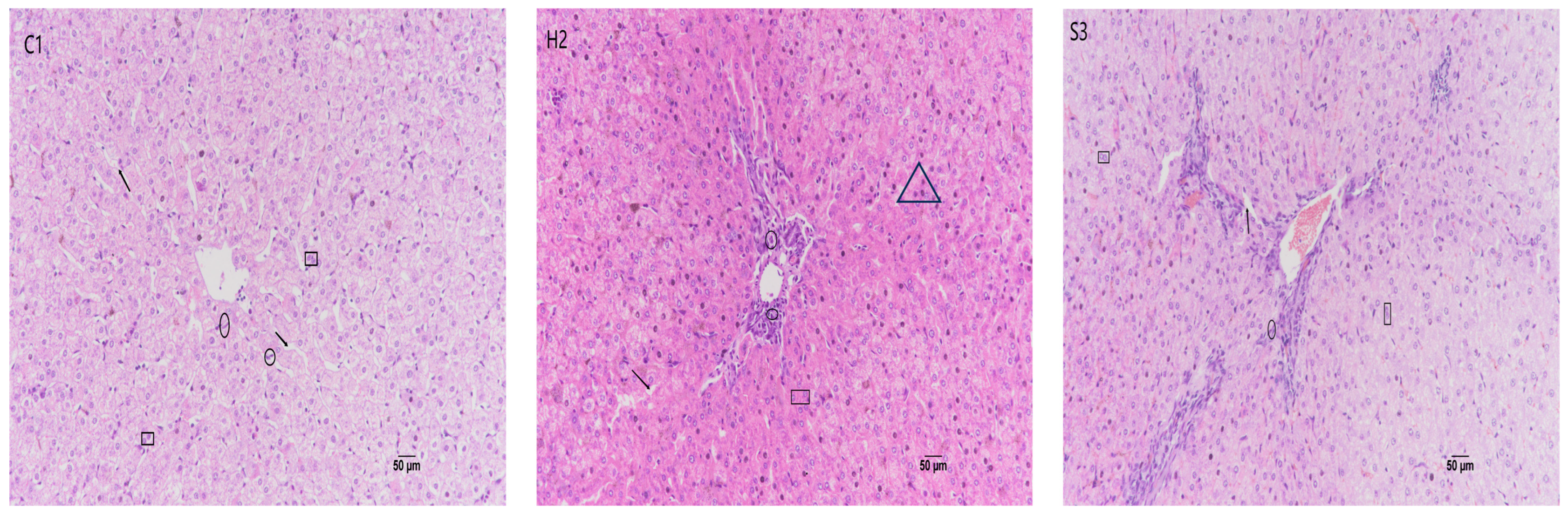
3.5. Effect of SA Addition to High-Concentrate Diets on Sheep Liver Tissue Sections
3.6. Effect of Adding SA to High-Concentrate Diets on the Sheep Liver Transcriptome
3.6.1. Quality Control of Liver Transcriptome Data
3.6.2. Sample Relationship Analysis
3.6.3. Differential Gene Analysis of the Three Groups
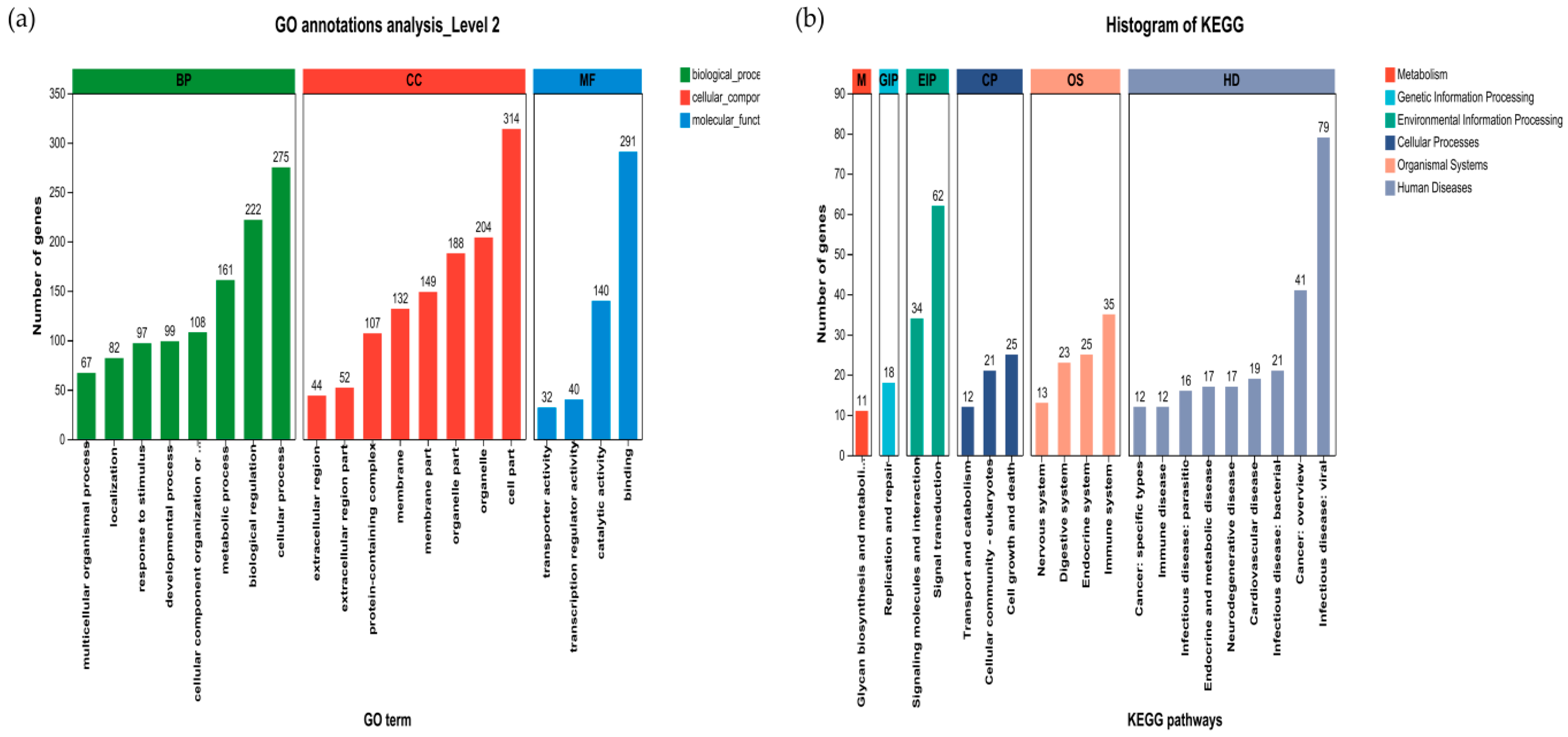
3.6.4. Effect of Liver Gene Function in Sheep
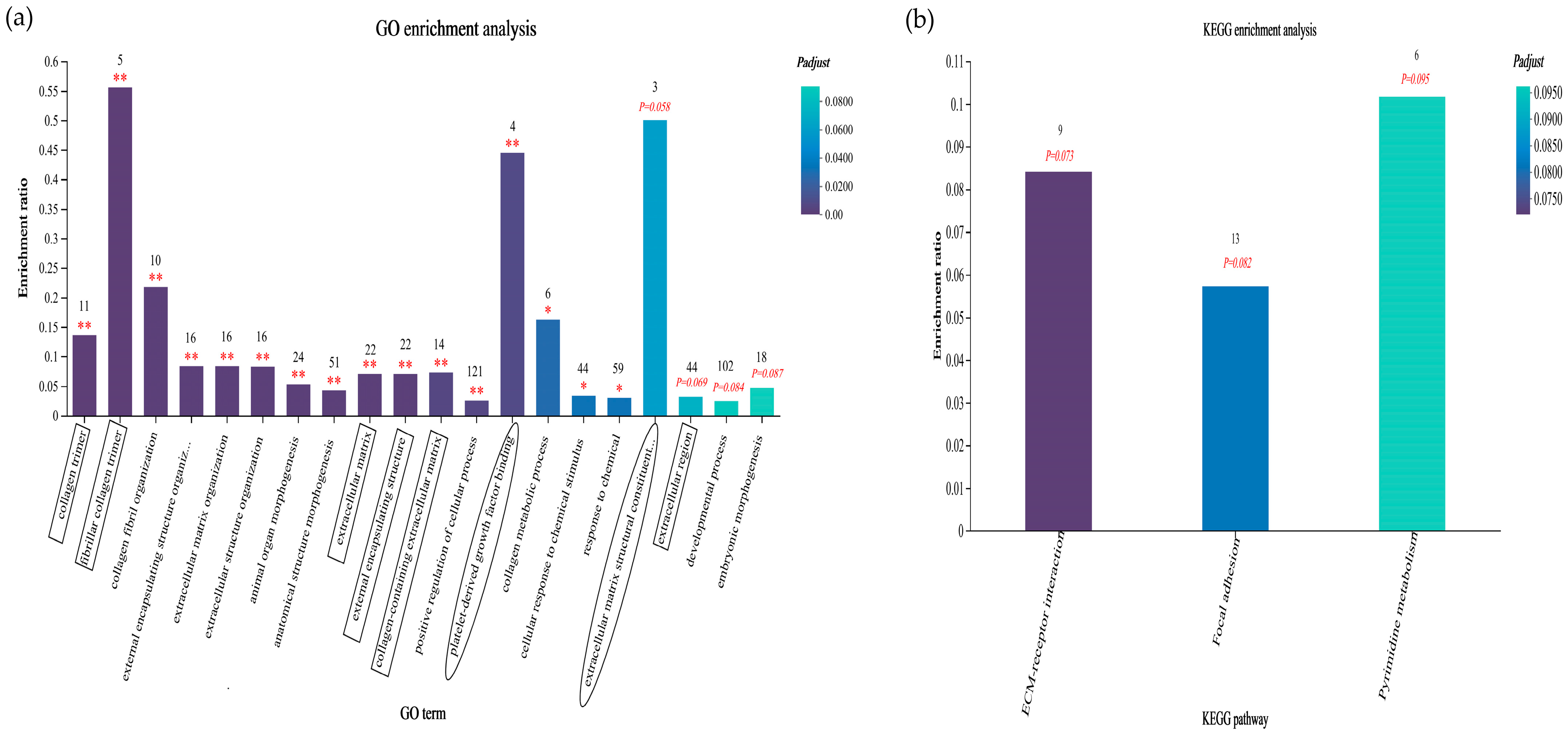
3.6.5. GO and KEGG Enrichment Analyses of Sheep Liver Data
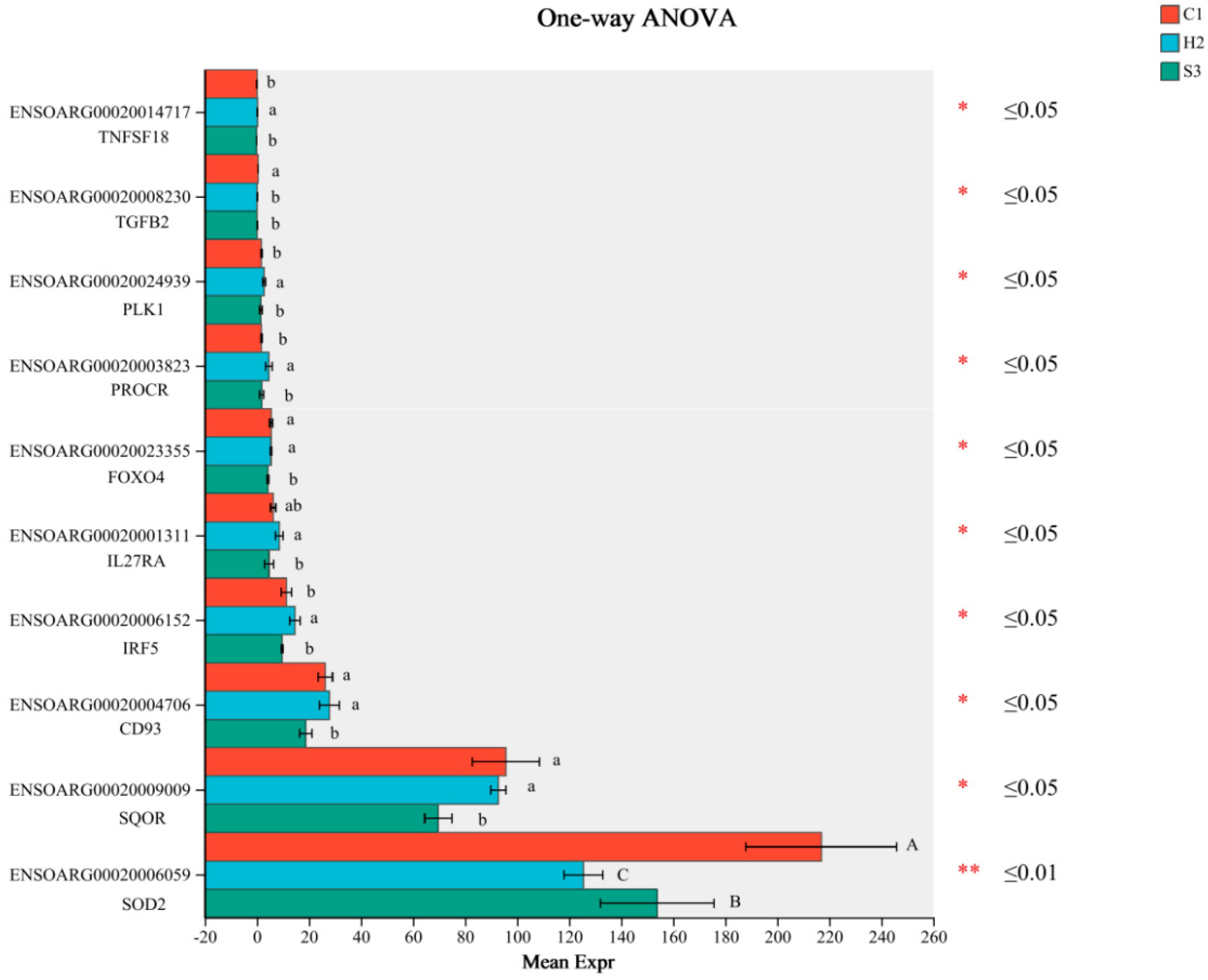
3.6.6. Analysis of Differentially Expressed Genes in Immune- and Antioxidant-Related Pathways in Sheep Liver
3.7. q-PCR Verification of Differential Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SA | Sophora alopecuroides | ALT | alanine aminotransferase |
| AST | astera aminotransferase | ALP | alkaline phosphatase |
| LDH | lactic dehydrogenase | TP | total protein |
| ALB | albumin | LPS | lipopolysaccharide |
| LBP | lipopolysaccharide binding protein | IL-1β | interleukin-1β |
| IL-6 | interleukin-6 | IL-10 | interleukin-10 |
| INF-γ | interferon-γ | TNF-α | tumor necrosis factor-α |
| CAT | catalase | SOD | superoxide dismutase |
| GSH-PX | glutathione peroxidase | T-AOC | total antioxidant capacity |
| MDA | malondialdehyde | SOD2 | superoxide dismutase 2 |
| SQOR | sulfide quinone oxidoreductase | CD93 | CD93 molecule |
| IRF5 | interferon regulatory factor 5 | IL27RA | interleukin 27 receptor subunit alpha |
| FOXO4 | forkhead box O4 | PROCR | protein C receptor |
| PLK1 | polo like kinase 1 | TGFB2 | transforming growth factor beta 2 |
| TNFSF18 | TNF superfamily member 18 | CRABP1 | cellular retinoic acid binding protein 1 |
| CCL17 | C-C motif chemokine ligand 17 | GDF2 | growth differentiation factor 2 |
| GO | Gene Ontology | KEGG | Kyoto Encyclopedia of Genes and Genomes |
| CC | cellular component | BP | biological process |
| MF | molecular function | MAPK | mitogen-activated protein kinase |
| SARA | subacute rumen acidosis | NF-κB | nuclear factor Kappa-B |
| ECM | extracellular matrix | FAs | focal adhesion pathways |
References
- Penner, G.B.; Oba, M.; Gäbel, G.; Aschenbach, J.R. A single mild episode of subacute ruminal acidosis does not affect ruminal barrier function in the short term. J. Dairy Sci. 2010, 93, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, U.K.; Khurram, A.; Patricia, R.J.; Gautam, S.; Sreenivas, P.S.; Reena, G.; Aditya, A. Lauric acid ameliorates lipopolysaccharide (LPS)-induced liver inflammation by mediating TLR4/MyD88 pathway in Sprague Dawley (SD) rats. Life Sci. 2021, 265, 118750. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Danesh, M.M.; Derakhshani, H.; Golder, H.; Khafipour, E.; Kleen, J.L.; Lean, I.; Loor, J.; Penner, G.; Zebeli, Q. Review: Enhancing gastrointestinal health in dairy cows. Animal 2018, 12, s399–s418. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, F.F.; Hou, F.Y.; Jin, Y.Q.; Zhang, X.Z.; Ma, Y.; Zhang, Y.; Fan, Y.T.; Yang, Z.Q.; Wang, H.R. Influences of naringin supplementation on ruminal fermentation, inflammatory response, antioxidant capacity and bacterial community in high-concentrate diet of fattening goats. Ital. J. Anim. Sci. 2022, 21, 1498–1507. [Google Scholar] [CrossRef]
- Chang, G.J.; Zhang, K.; Xu, T.L.; Jin, D.; Seyfert, H.M.; Shen, X.Z.; Zhuang, S. Feeding a high-grain diet reduces the percentage of LPS clearance and enhances immune gene expression in goat liver. BMC Vet. Res. 2015, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.O.; Plaizier, J.C.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Xie, W.; Xue, Y.; Zhang, H.Z.; Wang, Y.; Meng, M.J.; Chang, G.J.; Shen, X.Z. A high-concentrate diet provokes inflammatory responses by downregulating Forkhead box protein A2 (FOXA2) through epigenetic modifications in the liver of dairy cows. Gene 2022, 837, 146703. [Google Scholar] [CrossRef]
- Gane, E.J.; Crawford, D.G. The impact of research on haemochromatosis and liver transplantation in the Asia-Pacific region. Hepatol. Int. 2012, 6, 20–21. [Google Scholar] [CrossRef]
- Arumugam, R.; Sarikurkcu, C.; Mutlu, M.; Tepe, B. Sophora alopecuroides var. alopecuroides: Phytochemical composition, antioxidant and enzyme inhibitory activity of the methanolic extract of aerial parts, flowers, leaves, roots, and stems. S. Afr. J. Bot. 2020, 143, 282–290. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.W.; Huang, Q.Q.; Duan, H.; Zhao, G.D.; Liu, L.; Li, Y.X. Flavonoids from Sophora alopecuroides L. improve palmitate-induced insulin resistance by inhibiting PTP1B activity in vitro. Bioorg. Med. Chem. Lett. 2021, 35, 127775. [Google Scholar] [CrossRef]
- Guo, R.; Cao, N.N.; Wu, Y.; Wu, J.H. Optimized extraction and molecular characterization of polysaccharides from Sophora alopecuroides L. seeds. Int. J. Biol. Macromol. 2015, 82, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Balakrishnan, V. Role of cytokines in the pathogenesis of non-alcoholic fatty liver disease. Indian J. Clin. Biochem. 2011, 26, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Matsubara, T.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012, 56, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Song, C.Y.; Zeng, X.; Wang, Y.; Shi, J.; Qian, H.; Zhang, Y.; Fang, J.Q.; Sheng, X.; Zheng, J.M.; Chen, Y.X. Sophocarpine attenuates toll-like receptor 4 in steatotic hepatocytes to suppress pro-inflammatory cytokines synthesis (Article). J. Gastroenterol. Hepatol. 2015, 30, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Dong, J.N.; Xie, H.W.; Kang, S.S.; Feng, Z.A.; Gao, B.; Zhang, D. Protective effect of total alkaloid of Sophora alopecuroides on acute alcoholic liver injury in mice. J. Baotou Med. Coll. 2021, 37, 38–41. [Google Scholar] [CrossRef]
- An, Y.W.; Yang, X.D.; Guo, S.Q.; Gao, Z.X.; Shi, C.X.; Gao, A.W.; Wang, H.R. Effects of adding Sophora alopecuroides to high grain diet on serum immune and antioxidant indexes of sheep. J. Yunnan Agric. Univ. 2022, 37, 804–811. [Google Scholar] [CrossRef]
- An, Y.W.; Wang, H.R.; Zong, Z.C.; Gao, Z.X.; Shi, C.X.; Li, S.F.; Khas-Erdene. Effects of adding Sophora alopecuroides to high concentrate diet on rumen fermentation parameters and microbial diversity of sheep. Front. Vet. Sci. 2023, 10, 1200272. [Google Scholar] [CrossRef]
- Daehwan, K.; Ben, L.; Steven, L.S. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Ringseis, R.; Zeitz, J.O.; Weber, A.; Koch, C.; Eder, K. Hepatic transcript profiling in early-lactation dairy cows fed rumen-protected niacin during the transition from late pregnancy to lactation. J. Dairy Sci. 2019, 102, 365–376. [Google Scholar] [CrossRef]
- Gao, D.; Ma, F.T.; Shan, Q.; Jin, Y.H.; Li, H.Y.; Chang, M.N.; Sun, P. Hepatic transcriptome analyses revealed molecular mechanism of lonicera japonica extract in relieving heat stress of dairy cows. Chin. J. Anim. Nutr. 2021, 33, 3309–3322. [Google Scholar]
- Ishikawa, M.; Ahi, S.T.; Murakami, Y.; Kimura, F.; Yamaguchi, M.; Abe, T.; Hashiguchi, A.; Sakamoto, M. Automatic segmentation of hepatocellular structure from HE-stained liver tissue. Digit. Pathol. 2013, 8676, 867611. [Google Scholar] [CrossRef]
- Abdela, N. Sub-acute ruminal acidosis (SARA) and its consequence in dairy cattle: A review of past and recent research at global prospective. Achieve Life Sci. 2016, 10, 187–196. [Google Scholar] [CrossRef]
- Plaizier, J.C. Replacing chopped alfalfa hay with alfalfa silage in barley grain and alfalfa-based total mixed rations for lactating dairy cows. J. Dairy Sci. 2005, 87, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Iglesias, C.; Devant, M. Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim. Feed Sci. Technol. 2007, 136, 146–153. [Google Scholar] [CrossRef]
- Ma, P.; Hong, Y.F.; Liu, C.X.; Sun, Y.Q.; Liu, M.Z.; Yang, Z.G.; Ma, P.Y.; Wu, H.X.; Xue, F.G. Rumen microbiota responses to the enzymatic hydrolyzed cottonseed peptide supplement under high-concentrate diet feeding process. Front. Vet. Sci. 2022, 9, 984634. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.G.; Tory, R.; Jia, J.; Sivak, O.; Wasan, K.M. Discovery and development of toll-like receptor 4 (TLR4) antagonists: A new paradigm for treating sepsis and other diseases. Pharm. Res. 2008, 25, 1751–1761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dong, G.Z.; Ao, C.J.; Zhang, S.; Qiu, M.; Wang, X.; Wu, Y.X.; Erdene, K.; Jin, L.; Lei, C.L. Feeding a high-concentrate corn straw diet increased the release of endotoxin in the rumen and pro-inflammatory cytokines in the mammary gland of dairy cows. BMC Vet. Res. 2014, 10, 172. [Google Scholar] [CrossRef]
- Jiang, W.; Sun, R.; Wei, H.M.; Tian, Z.G. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc. Natl. Acad. Sci. USA 2005, 102, 17077–17082. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. Alfalfa pellet-induced subacute nuninal acidosis in dairy cows increases bacterial endotoxin in the rumen without causing inflammation. J. Dairy Sci. 2009, 92, 1712–1724. [Google Scholar] [CrossRef]
- Kiep, M.D.; Min-Kyoung, S.; Takeshi, K.; Nwet, N.W.; Prema, P.; Hien, M.N.; Yoshihiro, H.; Hiroyuki, M. Flavanols and flavanes from Crinum asiaticum and their effects on LPS signaling pathway through the inhibition of NF-κB activation. Planta Med. 2021, 88, 913–920. [Google Scholar] [CrossRef]
- Yang, Y.X.; Li, G.Y. Progression of lipopolysaccharide signal pathway. J. Cent. South Univ. (Med. Sci.) 2006, 31, 141–145. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, Y.; Liu, Q. Antiendotoxin effect of Sophora alopecuroides L. J. Chin. Med. Mat. 2006, 10, 1066–1069. [Google Scholar] [CrossRef]
- Wang, Z.J.; Song, Y.X.; Zhang, F.; Zhao, C.; Fu, S.X.; Xia, C.; Bai, Y.L. Early warning for inactive ovaries based on liver function index, serum MDA, IL-6, FGF21 and ANGPTL8 in dairy cows. Ital. J. Anim. Sci. 2022, 21, 113–122. [Google Scholar] [CrossRef]
- Dou, M.Z.; Xu, B.; Xiang, R.; Guo, D.Y.; Fan, Y.; Shi, X.Y.; Duan, L.F. The protective effect of matrine injection on acute alcoholic liver injury in mice. China J. Modern Med. 2021, 31, 13–18. [Google Scholar] [CrossRef]
- Zhang, H.C.; Xu, L.; Rui, H.M.; Cao, C.H.; Yang, F.M.; Yuan, B. Role of aloperine on ischemia-reperfusion-induced injury and inflammatory response in H9c2 cardiomyocytes. Chin. J. Pathophysiol. 2018, 34, 281–286. [Google Scholar] [CrossRef]
- Liu, W.; Cao, S.T.; Shi, D.; Yu, L.L.; Qiu, W.H.; Chen, W.H.; Wang, W. Single-chemical and mixture effects of multiple volatile organic compounds exposure on liver injury and risk of non-alcoholic fatty liver disease in a representative general adult population. Chemosphere 2023, 339, 139753. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Yuan, Y.J.; Wang, J.Q.; Yang, Y.X.; Bo, D.P.; Li, S.S.; Zhang, Y.D. Study on changes of plasma proteome of dairy cow injecting lipopolysaccharide into external pudic artery. Acta Vet. Zootech. Sin. 2011, 42, 1556–1561. [Google Scholar]
- Luo, D.; Tu, Z.C.; Yin, W.J.; Fan, C.L.; Chen, N.H.; Wu, Z.N.; Ding, W.L.; Li, Y.L.; Wang, G.C.; Zhang, Y.B. Uncommon bis-amide matrine-type alkaloids from Sophora alopecuroides with anti-inflammatory effects. Front. Chem. 2021, 9, 740421. [Google Scholar] [CrossRef]
- Luo, D.; Chen, N.H.; Wang, W.Z.; Zhang, J.H.; Li, C.J.; Zhuo, X.F.; Tu, Z.C.; Wu, Z.N.; Fan, C.L.; Zhang, H.P.; et al. Structurally diverse matrine-based alkaloids with anti-inflammatory effects from Sophora alopecuroides. Chin. J. Chem. 2021, 39, 3339–3346. [Google Scholar] [CrossRef]
- Liang, J.P.; Xie, J.N.; Zhou, Y. Effects of sophoridine on the serum inflammatory factors in mice with endotoxemia. Guide China Med. 2012, 10, 1–2. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020, 32, 101500. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Xu, J.L.; Jiang, N.; Sheng, G.G. Effect of endotoxin on hepatocyte mitochondrial transmembrane potential in rats and the intervention action of astragalus membranacenus. Chin. J. Clin. Transl. Hepatol. 2005, 15, 357–358+361. [Google Scholar] [CrossRef]
- Protective effects of matrine on immunological liver injury model in BALB/c mice. J. Ningxia Med. Univ. 2009, 31, 11–12+15. [CrossRef]
- Qiu, M.N.; Liu, J.; Feng, P.; Su, Y.X.; Guo, R.; Shi, F.Y.; Wang, S.; Zhao, B.Y. Cytochrome P450s regulates aloperine-induced pathological changes in mouse liver and kidney. Res. Vet. Sci. 2020, 132, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Zhou, T.T.; Wang, C.G.; Wang, W.; Chan, S.M. Comparative transcriptomics reveals eyestalk ablation induced responses of the neuroendocrine-immune system in the Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 106, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Golbert, D.C.; Correa-de-Santana, E.; Ribeiro-Alves, M.; de Vasconcelos, A.T.; Savino, W. ITGA6 gene silencing by RNA interference modulates the expression of a large number of cell migration-related genes in human thymic epithelial cells. BMC Genom. 2013, 14, S3. [Google Scholar] [CrossRef]
- Zhang, H.J.; Tao, J.; Sheng, L.; Hu, X.; Rong, R.M.; Xu, M.; Zhu, T.Y. Twist2 promotes kidney cancer cell proliferation and invasion via regulating ITGA6 and CD44 expression in the ECM-Receptor-Interaction pathway. Biomed. Pharmacother. 2016, 81, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; McMurray, J.L.; Mohammed, F.; Bicknell, R. C-type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019, 286, 3299–3332. [Google Scholar] [CrossRef]
- Borah, S.; Vasudevan, D.; Swain, R.K. C-type lectin family XIV members and angiogenesis. Oncol. Lett. 2019, 18, 3954–3962. [Google Scholar] [CrossRef]
- Tossetta, G.; Piani, F.; Borghi, C.; Marzioni, D. Role of CD93 in health and disease. Cells 2023, 12, 1778. [Google Scholar] [CrossRef]
- Li, S.F.; Ma, T.; Yang, J.L.; An, Y.W.; Zhang, Y.; Yang, X.D.; Wang, H.R. Effects of Sophora alopecuroides L. on c-Jun N-terminal kinase/p38 mitogen-activated protein kinase signaling pathway and tight junction protein expression in rumen epithelium of fattening lambs fed high concentrate diet. Chin. J. Anim. Nutr. 2022, 34, 3940–3952. [Google Scholar]
- Marjolein, P.B.; Renata, M.C.B.; Diana, A.P.; Julian, D.D.K.; Kasper, W.J.D.; Benjamin, R.M.B.; Sarah, S.; Yvonne, R.; Hester, V.W.; Danny, A.F.; et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017, 169, 132. [Google Scholar] [CrossRef]
- Tsubouchi, K.; Araya, J.; Minagawa, S.; Hara, H.; Ichikawa, A.; Saito, S.; Kadota, T.; Sato, N.; Yoshida, M.; Kurita, Y.; et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy 2017, 13, 1420–1434. [Google Scholar] [CrossRef] [PubMed]
- Han, X.D.; Yuan, T.; Zhang, J.L.; Shi, Y.G.; Li, D.G.; Dong, Y.P.; Fan, S.J. FOXO4 peptide targets myofibroblast ameliorates bleomycin-induced pulmonary fibrosis in mice through ECM-receptor interaction pathway. J. Cell. Mol. Med. 2022, 26, 3269–3280. [Google Scholar] [CrossRef]
- Kumar, R.; Sanawar, R.; Li, X.D.; Li, F. Structure, biochemistry, and biology of PAK kinases (Review). Gene 2017, 605, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.G.; Ning, K.; Li, F. Group II p21-activated kinases as therapeutic targets in gastrointestinal cancer. World J. Gastroenterol. 2016, 22, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Tomar, A.; Schlaepfer, D.D. A PAK-activated linker for EGFR and FAK. Dev. Cell 2010, 18, 170–172. [Google Scholar] [CrossRef]
- Gabarra-Niecko, V.; Schaller, M.D.; Dunty, J.M. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003, 22, 359–374. [Google Scholar] [CrossRef]
- Kim, J.S.; Steve Lim, S.T. LED light-induced ROS differentially regulates focal adhesion kinase activity in HaCaT cell viability. Curr. Issues Mol. Biol. 2022, 44, 1235–1246. [Google Scholar] [CrossRef]
- Mehta, D. Focal adhesion kinase regulation of endothelial barrier function apoptosis, and neovascularization. Microvasc. Res. 2012, 83, 1–2. [Google Scholar] [CrossRef]
- Lim, S.T.; Chen, X.L.; Lim, Y. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol. Cell 2008, 29, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Lang, A.; Lindquist, J.N.; Yata, Y.; Gabele, E.; Scanga, A.; Brenner, D.A.; Rippe, R.A. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt signaling in hepatic stellate cell proliferation and type I collagen expression. J. Biol. Chem. 2003, 278, 8083–8090. [Google Scholar] [CrossRef] [PubMed]


| Ingredients | Composition, % | Items | Nutrient Levels | ||
|---|---|---|---|---|---|
| C1 | H2 | C1 | H2 | ||
| Mixed hay | 50.00 | 30.00 | Metabolizable energy (2), MJ/kg | 9.53 | 10.20 |
| Corn | 36.10 | 52.00 | Crude protein, % | 14.17 | 14.45 |
| Soybean meal | 12.00 | 15.20 | Ether extract, % | 2.84 | 3.72 |
| Limestone | 0.60 | 1.00 | Neutral detergent fiber, % | 33.18 | 25.03 |
| CaHPO4 | 0.10 | 0.10 | Acid detergent fiber, % | 22.36 | 15.37 |
| NaCl | 0.50 | 0.50 | Nonstructural carbohydrate, % | 43.6 | 49.9 |
| Premix (1) | 0.20 | 0.20 | Ca, % | 0.77 | 0.71 |
| NaHCO3 | 0.50 | 1.00 | P, % | 0.38 | 0.37 |
| Total | 100.00 | 100.00 | |||
| NCBI Reference | Gene | Primer Sequence (5′-3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|---|
| NM_001009465.2 | IL-1β | IL-1β F: CAGCCGTGCAGTCAGTAA IL-1β R: TGTGAGAGGAGGTGGAGAG | 100 bp | 57 |
| NM_001009392.1 | IL-6 | IL-6 F: GGGTAAAGAACGCAAAGGT IL-6 R: TGACCAGAGGAGGGAATG | 136 bp | 55 |
| NM_001145185.2 | SOD2 | SOD2 F: GCTTCGAGGCAAAGGGAGAT SOD2 R: AACTGATGGACGTGGAACCC | 88 bp | 60 |
| NM_001009803.1 | IFN-γ | IFN-γ F: CAGGAGCTACCGATTTCAAC IFN-γ R: AAACCCAAAAGCACACAGA | 100 bp | 56 |
| NM_001024860.1 | TNF-α | TNF-α F: ACACCATGAGCACCAAAAGC TNF-α R: AGGCACCAGCAACTTCTGGA | 103 bp | 60 |
| NM_001161888.1 | CD93 | CD93 F: CTTGGGGAAGACACGGGAAA CD93 R: GAGGTAGCCTCAGGGTTGTC | 89 bp | 59 |
| XM_004018462.5 | GPX1 | GPX1 F: AACGTAGCATCGCTCTGAGG GPX1 R: CAAACTGGTTGCACGGGAAG | 115 bp | 57 |
| XM_027971670.2 | PAK6 | PAK6 F: CGCTGCCTGAGGATTTAATGGA PAK6 R: CTGTCAAAGGGAGTGCGGC | 145 bp | 61 |
| XM_027962288.2 | VCL | VCL F: ATGCTGTTGGGTTCCCTGTC VCL R: GACCACTTGGTAGCTTCCCG | 139 bp | 60 |
| XM_042246630.1 | ITGA5 | ITGA5 F: TGCTGTGAACCAGAGTCGTC ITGA5 R: TGGGACGAGGAGAGACTGAG | 159 bp | 59 |
| XM_004013602.4 | TGFB2 | TGFB2 F: TTACCCTCGGAAACTGTCTGC TGFB2 R: GGCATCAAGGTACCCACAGAA | 102 bp | 60 |
| NM_001009784.3 | β-actin | ACTB F: CATCGTCCACCGCAAAT ACTB R: GCCATGCCAATCTCATCTC | 103 bp | 56 |
| Sample | Raw Reads | Clean Reads | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) | Uniquely Mapped (%) |
|---|---|---|---|---|---|---|---|
| C1-1 | 48,466,846 | 47,954,082 | 0.0250 | 98.04 | 94.12 | 48.97 | 84.97 |
| C1-2 | 56,038,432 | 55,523,466 | 0.0249 | 98.08 | 94.24 | 49.67 | 84.88 |
| C1-3 | 48,483,846 | 48,016,986 | 0.0249 | 98.07 | 94.24 | 50.16 | 86.77 |
| H2-1 | 50,097,052 | 49,600,336 | 0.0248 | 98.13 | 94.39 | 50.03 | 85.92 |
| H2-2 | 52,449,996 | 51,964,618 | 0.0249 | 98.10 | 94.27 | 49.28 | 84.83 |
| H2-3 | 48,366,524 | 47,724,756 | 0.0254 | 97.87 | 93.75 | 49.91 | 85.56 |
| S3-1 | 49,668,102 | 49,066,542 | 0.0254 | 97.88 | 93.74 | 48.76 | 84.85 |
| S3-2 | 50,354,892 | 49,889,566 | 0.0248 | 98.13 | 94.35 | 49.04 | 86.76 |
| S3-3 | 44,943,236 | 44,425,012 | 0.0265 | 97.48 | 92.72 | 51.77 | 85.03 |
| Gene | Gene Description | FC | p-Value | ||
|---|---|---|---|---|---|
| C1/H2 | S3/H2 | S3/C1 | |||
| COL1A1 | collagen type I alpha 1 chain | 0.52 ** | 0.35 ** | 0.68 | 0.004 |
| COL1A2 | collagen type I alpha 2 chain | 0.53 ** | 0.39 ** | 0.74 | 0.004 |
| COL6A2 | collagen type VI alpha 2 chain | 0.68 * | 0.56 * | 0.82 | 0.021 |
| COL6A3 | collagen type VI alpha 3 chain | 0.37 ** | 0.34 ** | 0.90 | 0.003 |
| COL6A5 | collagen type VI alpha 5 chain | 0.65 | 0.33 * | 0.51 | 0.039 |
| PAK6 | p21 (RAC1)-activated kinase 6 | 0.24 * | 0.30 * | 1.25 | 0.034 |
| ARHGAP35 | Rho GTPase-activating protein 35 | 1.08 | 0.88 * | 0.81 * | 0.024 |
| PDGFB | platelet-derived growth factor subunit B | 0.74 * | 0.50 ** | 0.67 * | 0.006 |
| ITGA5 | integrin subunit alpha 5 | 0.63 * | 0.99 | 1.59 * | 0.014 |
| LAMC1 | laminin subunit gamma 1 | 0.84 | 0.68 * | 0.81 | 0.038 |
| VCL | vinculin | 0.89 | 0.71 * | 0.79 * | 0.019 |
| Gene | Group | p-Value | ||
|---|---|---|---|---|
| C1 | H2 | S3 | ||
| IL-6 | 0.62 ± 0.05 B | 1.00 ± 0.02 A | 1.04 ± 0.13 A | <0.001 |
| INF-γ | 0.98 ± 0.13 | 1.00 ± 0.04 | 0.95 ± 0.04 | 0.506 |
| IL-1β | 1.02 ± 0.10 | 1.00 ± 0.03 | 1.08 ± 0.06 | 0.706 |
| TNF-α | 0.89 ± 0.09 ab | 1.00 ± 0.03 a | 0.78 ± 0.06 b | 0.018 |
| CD93 | 0.73 ± 0.11 B | 1.00 ± 0.05 A | 0.64 ± 0.07 B | 0.004 |
| GPX1 | 1.04 ± 0.04 | 1.00 ± 0.03 | 1.07 ± 0.06 | 0.167 |
| SOD2 | 1.41 ± 0.02 A | 1.00 ± 0.05 C | 1.30 ± 0.02 B | <0.001 |
| PAK6 | 0.81 ± 0.05 b | 1.00 ± 0.04 a | 0.85 ± 0.11 b | 0.025 |
| ITGA5 | 0.68 ± 0.03 B | 1.00 ± 0.04 A | 0.93 ± 0.13 A | <0.001 |
| TGFB2 | 0.55 ± 0.02 B | 1.00 ± 0.04 A | 0.99 ± 0.16 A | 0.002 |
| VCL | 0.98 ± 0.04 A | 1.00 ± 0.06 A | 0.82 ± 0.03 B | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Wang, H.; Gao, A.; Li, S.; Yang, J.; Li, B.; Lu, H. Effects of Sophora alopecuroides in a High-Concentrate Diet on the Liver Immunity and Antioxidant Function of Lambs According to Transcriptome Analysis. Animals 2024, 14, 182. https://doi.org/10.3390/ani14020182
An Y, Wang H, Gao A, Li S, Yang J, Li B, Lu H. Effects of Sophora alopecuroides in a High-Concentrate Diet on the Liver Immunity and Antioxidant Function of Lambs According to Transcriptome Analysis. Animals. 2024; 14(2):182. https://doi.org/10.3390/ani14020182
Chicago/Turabian StyleAn, Yawen, Hairong Wang, Aiwu Gao, Shufang Li, Jinli Yang, Boyang Li, and Henan Lu. 2024. "Effects of Sophora alopecuroides in a High-Concentrate Diet on the Liver Immunity and Antioxidant Function of Lambs According to Transcriptome Analysis" Animals 14, no. 2: 182. https://doi.org/10.3390/ani14020182
APA StyleAn, Y., Wang, H., Gao, A., Li, S., Yang, J., Li, B., & Lu, H. (2024). Effects of Sophora alopecuroides in a High-Concentrate Diet on the Liver Immunity and Antioxidant Function of Lambs According to Transcriptome Analysis. Animals, 14(2), 182. https://doi.org/10.3390/ani14020182





