The Relationship between the Infrared Eye Temperature of Beef Cattle and Associated Biological Responses at High Environmental Temperatures
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing
2.2. Animal Management
2.3. Animals and Treatments
- Thermoneutral conditions before hot conditions (TN; d 3–4)
- Transition period to HOT (TP1; d 5)
- Hot conditions (HOT; d 6–12)
- Transition period to recovery (TP2; d 13)
- Thermoneutral conditions after hot treatment (recovery; d 14–17)
2.4. Behavioural and Other Key Observations
| Item | Description |
|---|---|
| Standing | Animal standing with limb positioned upright |
| Lying | Animal resting on the floor with their limb laterally or sternally recumbent |
| Eating | Animal consuming feed at the trough |
| Rumination | Animal chewing the cud or regurgitating bolus |
| Respiration rate | Number of breaths in a 1 min period |
| Panting score | Animal visually scored for the extent of panting based on a 0 to 4.5 score scale |
| Grooming | Animal licking any part of the body or striking one part with another part of the body |
| Scratching | Animal rubbing or striking any part of the body against the fixture of the pen |
| Ear positions | |
| Ear raised | Both ears being held upright above the neck with the ear pinnae facing forwards or to the side |
| Ear forward | Both ears’ pinnae directed forwards in front of the focal animal and held horizontally |
| Ear backward | Both ears being held backwards on the focal animal’s head |
| Ear downward | Both ears being loosely hung downwards, falling perpendicular to the head |
| Ear specific | Both ear pinnae (right and left) being oriented in opposite directions, or perpendicular to head rump axis, thus failing to satisfy raised, forward, backward, or downward ear positions |
| Head positions | |
| Head raised | The head held upright above withers or body top-line |
| Head neutral | The head held horizontally at the level of withers or body top-line |
| Head downwards | The head held downwards below withers or body top-line |
| Stepping | |
| Front right (FR) limb | Animal raising a front right limb and replacing it forthwith on the surface of the pen |
| Front left (FL) limb | Animal raising a front left limb and replacing it forthwith on the surface of the pen |
| Back right (BR) limb | Animal raising a back right limb and replacing it forthwith on the surface of the pen |
| Back left (BL) limb | Animal raising a back left limb and replacing it forthwith on the surface of the pen |
| Tail positions | |
| Tail raised | Tail held in a fixed position, held at than 45 degrees from the vertical |
| Tail vertical | Tail hanging downward from the vertical line of body and relaxing with no movements |
| Tail swishing | Swift movement of the tail in any direction around the hind quarters from its base in a side-to-side flicking manner |
| Tail tucked | Tail held tightly pressed in a fixed position against the rump, with the tip of the tail tucked in behind the hind limb |
2.5. Body Surface Temperature
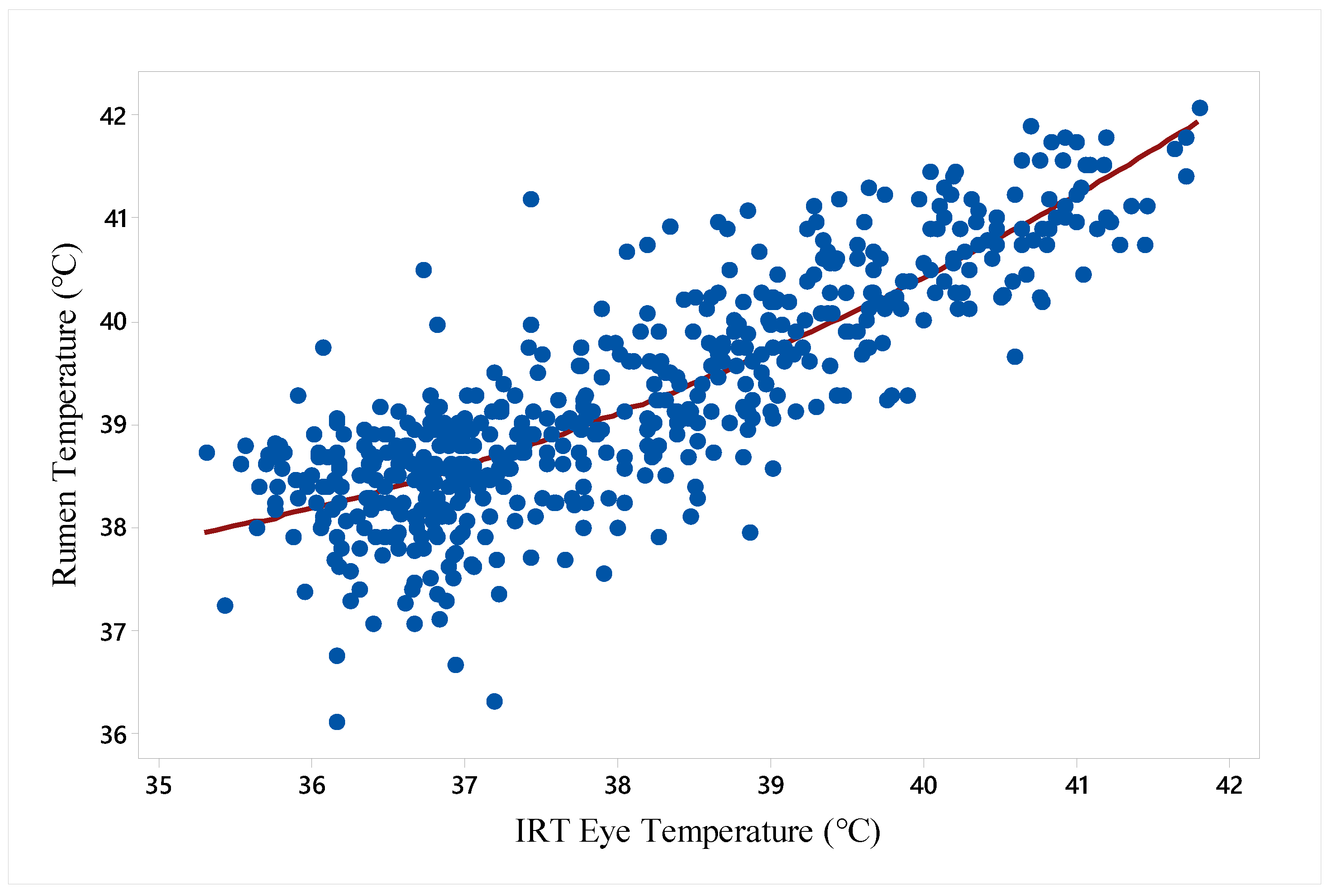
2.6. Rumen Temperature
2.7. Infrared Thermography (IRT)
2.8. Climatic Data
2.9. Statistical Analyses
3. Results
3.1. Initial Thermoneutral Period vs. Hot Period
3.2. Hot Period vs. Recovery Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-Invasive physiological indicators of heat stress in cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Sjaastad, O.V.; Hove, K.; Sand, O. Physiology of Domestic Animals; Scandinavian Veterinary Press: Oslo, Norway, 2010. [Google Scholar]
- Hempel, S.; Menz, C.; Pinto, S.; Galán, E.; Janke, D.; Estellés, F.; Müschner-Siemens, T.; Wang, X.; Heinicke, J.; Zhang, G.; et al. Heat stress risk in European dairy cattle husbandry under different climate change scenarios–uncertainties and potential impacts. Earth Syst. Dyn. 2019, 10, 859–884. [Google Scholar] [CrossRef]
- Yue, S.; Qian, J.; Du, J.; Liu, X.; Xu, H.; Liu, H.; Zhang, J.; Chen, X. Heat Stress Negatively Influence Mammary Blood Flow, Mammary Uptake of Amino Acids and Milk Amino Acids Profile of Lactating Holstein Dairy Cows. Pak. Vet. J. 2023, 43, 73–78. [Google Scholar]
- Li, J.B.; Wang, X.; Sun, A.; Li, H.B.; Luo, Y.; He, F.; Huan, C.; Zhou, X.; Li, C.J.; Zhang, B.Z.; et al. Comparative Transcriptomic Analysis of Spermatozoa from Xiangxi and Simmental Bulls under Heat Stress: Implications for Fertility Prediction. Pak. Vet. J. 2023, 43, 184–188. [Google Scholar]
- Hillman, P.; Gebremedhin, K.; Willard, S.; Lee, C.; Kennedy, A. Continuous measurements of vaginal temperature of female cattle using a data logger encased in a plastic anchor. Appl. Eng. Agric. 2009, 25, 291–296. [Google Scholar] [CrossRef]
- Reuter, R.; Carroll, J.; Hulbert, L.; Dailey, J.; Galyean, M. Development of a self-contained, indwelling rectal temperature probe for cattle research. J. Anim. Sci. 2010, 88, 3291–3295. [Google Scholar] [CrossRef]
- Lee, C.; Gebremedhin, K.; Parkhurst, A.; Hillman, P. Placement of temperature probe in bovine vagina for continuous measurement of core-body temperature. Int. J. Biometeorol. 2015, 59, 1201–1205. [Google Scholar] [CrossRef]
- Lees, A.; Lees, J.; Lisle, A.; Sullivan, M.; Gaughan, J. Effect of heat stress on rumen temperature of three breeds of cattle. Int. J. Biometeorol. 2018, 62, 207–215. [Google Scholar] [CrossRef]
- Lees, A.M.; Lees, J.; Sejian, V.; Wallage, A.; Gaughan, J. using infrared thermography as an in situ measure of core body temperature in lot-fed Angus steers. Int. J. Biometeorol. 2018, 62, 3–8. [Google Scholar] [CrossRef]
- Vogel, E.; Meyer, C.; Eckardt, R. Severe Heatwaves Show the Need to Adapt Livestock Management for Climate. The Conversation, 27 February 2017. Available online: www.theconversation.com/severe-heatwaves-show-the-need-to-adapt-livestock-management-for-climate-73447#:~:text=During%20the%20recent%20heatwave%20in%20New%20South%20Wales%2C,the%20likelihood%20of%20this%20kind%20of%20record-breaking%20heatwave (accessed on 1 February 2020).
- Mccafferty, D.J. The value of infrared thermography for research on mammals: Previous applications and future directions. Mammal Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- Schaefer, A.; Cook, N.; Tessaro, S.; Deregt, D.; Desroches, G.; Dubeski, P.; Tong, A.; Godson, D. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Schaefer, A.; Cook, N.; Bench, C.; Chabot, J.; Colyn, J.; Liu, T.; Okine, E.; Stewart, M.; Webster, J. The non-invasive and automated detection of bovine respiratory disease onset in receiver calves using infrared thermography. Res. Vet. Sci. 2012, 93, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, J.B. Respiration Rate and Rectal Temperature Responses of Feedlot Cattle in Dynamic, Thermally Challenging Environments. Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Australia, 2002. [Google Scholar]
- Stewart, M.; Webster, J.; Verkerk, G.; Schaefer, A.; Colyn, J.; Stafford, K. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Stookey, J.; Stafford, K.; Tucker, C.; Rogers, A.; Dowling, S.; Verkerk, G.; Schaefer, A.; Webster, J. Effects of local anesthetic and a nonsteroidal antiinflammatory drug on pain responses of dairy calves to hot-iron dehorning. J. Dairy Sci. 2009, 92, 1512–1519. [Google Scholar] [CrossRef]
- Stewart, M.; Verkerk, G.; Stafford, K.; Schaefer, A.; Webster, J. Non-invasive assessment of autonomic activity for evaluation of pain in calves, using surgical castration as a model. J. Dairy Sci. 2010, 93, 3602–3609. [Google Scholar] [CrossRef]
- Gloster, J.; Ebert, K.; Gubbins, S.; Bashiruddin, J.; Paton, D.J. Normal variation in thermal radiated temperature in cattle: Implications for foot-and-mouth disease detection. BMC Vet. Res. 2011, 7, 73. [Google Scholar] [CrossRef]
- Uddin, J.; Phillips, C.J.C.; Goma, A.A.; McNeill, D.M. Relationships between infrared temperature and laterality. Appl. Anim. Behav. Sci. 2019, 220, 104855. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.; Schaefer, A.; Cook, N.; Scott, S. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welfare 2005, 14, 319–325. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.; Verkerk, G.; Colyn, J.; Schaefer, A. Infrared thermography as a non-invasive measure of stress in dairy cows. J. Anim. Sci. 2005, 83, 374. [Google Scholar]
- Mishra, M.; Martz, F.; Stanley, R.; Johnson, H.; Campbell, J.; Hilderbrand, E. Effect of diet and ambient temperature-humidity on ruminal pH, oxidation reduction potential, ammonia and lactic acid in lactating cows. J. Anim. Sci. 1970, 30, 1023–1028. [Google Scholar] [CrossRef]
- Shearer, J. Rumen acidosis, heat stress and laminitis. In Proceedings of the 4th Annual Arizona Dairy Production Conference, Tempe, AZ, USA, 11 October 2005. [Google Scholar]
- Conte, G.; Ciampolini, R.; Cassandro, M.; Lasagna, E.; Calamari, L.; Bernabucci, U.; Abeni, F. Feeding and nutrition management of heat-stressed dairy ruminants. Ital. J. Anim. Sci. 2018, 17, 604–620. [Google Scholar] [CrossRef]
- Erdman, R.A. Dietary buffering requirements of the lactating dairy cow: A review. J. Dairy Sci. 1988, 71, 3246–3266. [Google Scholar] [CrossRef]
- Brown-Brandl, T.M.; Nienaber, J.A.; Eigenberg, R.A.; Mader, T.L.; Morrow, J.; Dailey, J. Comparison of heat tolerance of feedlot heifers of different breeds. Livest. Sci. 2006, 105, 19–26. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.; Brown-Brandl, T. Environmental factors influencing heat stress in feedlot cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Lisle, A. A new heat load index for feedlot cattle. J. Anim. Sci. 2008, 86, 226–234. [Google Scholar] [CrossRef]
- Idris, M. Behavioural and Physiological Responses of Beef Cattle to Hot Environmental Conditions. Ph.D. Thesis, The University of Queensland, Gatton Campus, QLD, Australia, 2020. [Google Scholar]
- Idris, M.M.; Gaughan, J.B.; Phillips, C.J.C. Behavioural responses of beef cattle to hot conditions. Animals 2024, 14, 2444. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Idris, M.; Gay, C.C.; Woods, I.G.; Sullivan, M.; Gaughan, J.B.; Phillips, C.J.C. Automated quantification of the behaviour of beef cattle exposed to heat load conditions. Animals 2023, 13, 1125. [Google Scholar] [CrossRef]
- Thom, E.C. The discomfort index. Weatherwise 1959, 12, 57–61. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Pereira, A.M.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Avalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical applications and factors involved in validating thermal windows used in infrared thermography in cattle and river buffalo to assess health and productivity. Animals 2021, 11, 2247. [Google Scholar] [CrossRef]
- Macmillan, K.; Colazo, M.G.; Cook, N.J. Evaluation of infrared thermography compared to rectal temperature to identify illness in early postpartum dairy cows. Res. Vet. Sci. 2019, 125, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Brown–Brandl, T.M.; Yanagi, T.; Xin, H.; Gates, R.S.; Bucklin, R.A.; Ross, G.S. A new telemetry system for measuring core body temperature in livestock and poultry. Appl. Eng. Agric. 2003, 19, 583. [Google Scholar]
- Lees, A.M. Biological Responses of Feedlot Cattle to Heat Load. Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Australia, 2016. [Google Scholar]
- Stewart, M.; Webster, J.; Schaefer, A.; Stafford, K. Infrared thermography and heart rate variability for non-invasive assessment of animal welfare. ANZCCART Hum. Sci. News 2008, 21, 1–4. [Google Scholar]
- Church, J.S.; Hegadoren, P.; Paetkau, M.; Miller, C.; Regev-Shoshani, G.; Schaefer, A.; Schwartzkopf-Genswein, K. Influence of environmental factors on infrared eye temperature measurements in cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef]
- Shu, H.; Li, Y.; Fang, T.; Xing, M.; Sun, F.; Chen, X.; Bindelle, J.; Wang, W.; Guo, L. Evaluation of the best region for measuring eye temperature in dairy cows exposed to heat stress. Front. Vet. Sci. 2022, 9, 857777. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Schumacker, P.; Rowland, J.; Saltz, S. Glossary of terms for thermal physiology Third Edition revised by The Commission for Thermal Physiology of the International Union of Physiological Sciences (IUPS Thermal Commission). JPN J. Physiol. 2001, 51, 245–280. [Google Scholar]
- Robins, A.; Phillips, C. Lateralised visual processing in domestic cattle herds responding to novel and familiar stimuli. Laterality 2010, 15, 514–534. [Google Scholar] [CrossRef]
- Kappel, S.; Mendl, M.T.; Barrett, D.C.; Murrell, J.C.; Whay, H.R. Lateralized behaviour as indicator of affective state in dairy cows. PLoS ONE 2017, 12, e0184933. [Google Scholar] [CrossRef]
- Vermunt, J.J.; Tranter, B.P. Heat stress in dairy cattle–A review, and some of the potential risks associated with the nutritional management of this condition. In Review of AVA QLD Division Conference 25-27/3/10; Australian Veterinary Association: St Leonards, Australia, 2011; pp. 212–221. [Google Scholar]
- Brown-Brandl, T.; Eigenberg, R.; Nienaber, J.; Hahn, G.L. Dynamic response indicators of heat stress in shaded and non-shaded feedlot cattle, Part 1: Analyses of indicators. Biosyst. Eng. 2005, 90, 451–462. [Google Scholar] [CrossRef]
- Fogsgaard, K.K.; Røntved, C.M.; Sørensen, P.; Herskin, M.S. Sickness behavior in dairy cows during Escherichia coli mastitis. J. Dairy Sci. 2012, 95, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Ghorbani, G.; Kargar, S.; Drackley, J.K. Late-gestation heat stress abatement on performance and behavior of Holstein dairy cows. J. Dairy Sci. 2015, 98, 6865–6875. [Google Scholar] [CrossRef]
- Alam, M.; Hashem, M.; Rahman, M.; Hossain, M.; Haque, M.; Sobhan, Z.; Islam, M. Effect of heat stress on behavior, physiological and blood parameters of goat. Progress. Agric. 2011, 22, 37–45. [Google Scholar] [CrossRef]
- Soriani, N.; Panella, G.; Calamari, L. Rumination time during the summer season and its relationships with metabolic conditions and milk production. J. Dairy Sci. 2013, 96, 5082–5094. [Google Scholar] [CrossRef] [PubMed]
- Robins, A.; Berthoux, G.; Santurtun, E.; Navarro, G.; Phillips, C.J. Sheep Quickstep while the Floor Rock and Rolls: Visuomotor Lateralization during Simulated Sea Travel. Animals 2019, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Rousing, T.; Bonde, M.; Badsberg, J.H.; Sørensen, J.T. Stepping and kicking behaviour during milking in relation to response in human–animal interaction test and clinical health in loose housed dairy cows. Livest. Prod. Sci. 2004, 88, 1–8. [Google Scholar] [CrossRef]
- Goma, A.A.; Pearce, G.P.; Uddin, J.; Rimon, E.; Davies, H.; Phillips, C.J.C. A forced lateralisation test for dairy cows and its relation to their behaviour. Appl. Anim. Behav. Sci. 2018, 207, 8–19. [Google Scholar] [CrossRef]
- Mandel, R.; Whay, H.R.; Nicol, C.J.; Klement, E. The effect of food location, heat load, and intrusive medical procedures on brushing activity in dairy cows. J. Dairy Sci. 2013, 96, 6506–6513. [Google Scholar] [CrossRef]
- Mader, T.L.; Griffin, D. Management of cattle exposed to adverse environmental conditions. Vet. Clin. N. Am.-Food Anim. Prac. 2015, 31, 247–258. [Google Scholar] [CrossRef]
- de Oliveira, D.; Keeling, L.J. Routine activities and emotion in the life of dairy cows: Integrating body language into an affective state framework. PLoS ONE 2018, 13, e0195674. [Google Scholar] [CrossRef]
- Duke, J. Temperature Disturbances. In Anesthesia Secrets, 4th ed.; Mosby Elsevier: Philadelphia, PA, USA, 2011; pp. 217–221. ISBN 9780323065245. [Google Scholar] [CrossRef]
- Nienaber, J.; Hahn, G. Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 2007, 52, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, P.H.; Rice, M.; Karlen, M.G.; Calleja, L.; Barnett, J.L.; Nash, J.; Coleman, G.J. Human–animal interactions at abattoirs: Relationships between handling and animal stress in sheep and cattle. Appl. Anim. Behav. Sci. 2011, 135, 24–33. [Google Scholar] [CrossRef]
- Boissy, A.; Aubert, A.; Désiré, L.; Greiveldinger, L.; Delval, E.; Veissier, I. Cognitive sciences to relate ear postures to emotions in sheep. Anim. Welfare 2011, 20, 47. [Google Scholar] [CrossRef]
- Gleerup, K.B.; Andersen, P.H.; Munksgaard, L.; Forkman, B. Pain evaluation in dairy cattle. Appl. Anim. Behav. Sci. 2015, 171, 25–32. [Google Scholar] [CrossRef]
- Kluever, B.M.; Breck, S.W.; Howery, L.D.; Krausman, P.R.; Bergman, D.L. Vigilance in cattle: The influence of predation, social interactions, and environmental factors. Rangeland Ecol. Manag. 2008, 61, 321–328. [Google Scholar] [CrossRef]
- de Oliveira, F.A.; Luna, S.P.L.; do Amaral, J.B.; Rodrigues, K.A.; SantAnna, A.C.; Daolio, M.; Brondani, J.T. Validation of the UNESP-Botucatu unidimensional composite pain scale for assessing postoperative pain in cattle. BMC Vet. Res. 2014, 10, 200. [Google Scholar] [CrossRef]
- Reefmann, N.; Bütikofer Kaszàs, F.; Wechsler, B.; Gygax, L. Ear and tail postures as indicators of emotional valence in sheep. Appl. Anim. Behav. Sci. 2009, 118, 199–207. [Google Scholar] [CrossRef]
- Jaddoa, M.A.; Al-Jumaily, A.; Gonzalez, L.; Cuthbertson, H. Automatic eyes localization in thermal images for temperature measurement in cattle. In Proceedings of the 12th International Conference on Intelligent Systems and Knowledge Engineering (ISKE), Nanjing, China, 24–26 November 2017; pp. 1–6. [Google Scholar]
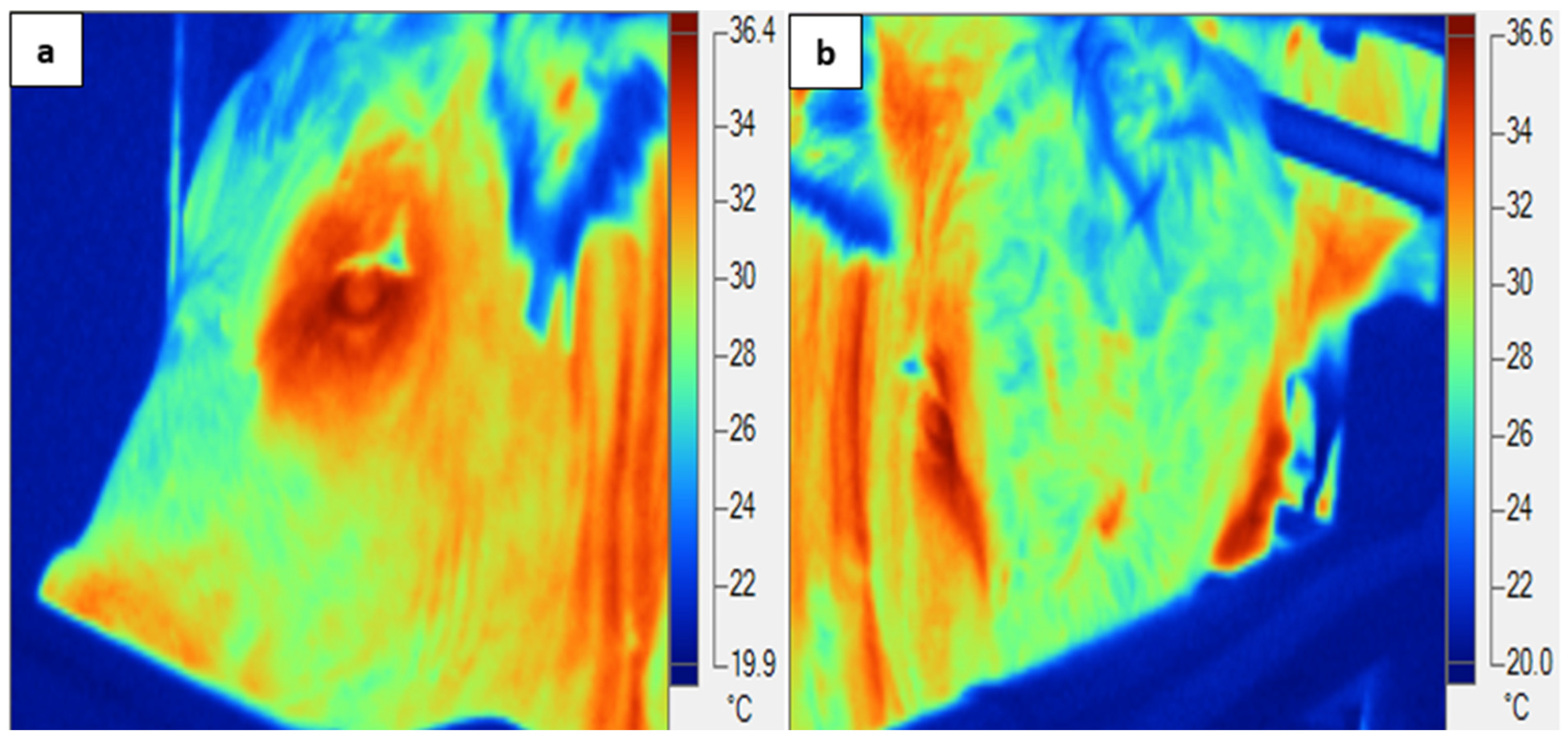

| Day | Treatment Phase | Min TA (°C) | Max TA (°C) | Mean TA (°C) | Min RH (%) | Max RH (%) | Mean RH (%) | Min THI | Max THI | Mean THI |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | ACC | 19.7 | 21.0 | 20.1 | 60.9 | 90.6 | 66.2 | 65.5 | 69.1 | 66.3 |
| 1 | ACC | 19.7 | 21.0 | 20.1 | 60.9 | 90.6 | 66.2 | 65.5 | 69.1 | 66.3 |
| 2 | ACC | 19.5 | 21.6 | 20.0 | 60.0 | 89.3 | 67.1 | 65.3 | 68.9 | 66.2 |
| 3 | TN | 19.5 | 20.8 | 19.9 | 61.3 | 90.1 | 67.9 | 65.3 | 68.8 | 66.1 |
| 4 | TN | 19.6 | 24.0 | 20.2 | 60.0 | 89.1 | 68.2 | 65.5 | 72.0 | 66.4 |
| 5 | TP1 | 19.9 | 40.5 | 33.2 | 42.9 | 88.4 | 66.1 | 66.3 | 92.6 | 84.9 |
| Finisher dietary cohort—transition to 30 °C from 00.00 h on day 5 Substituted dietary cohort—transition to 30 °C from 21.00 h on day 5 | ||||||||||
| 6 | HOT | 28.4 | 40.2 | 33.0 | 43.3 | 82.8 | 65.8 | 80.5 | 91.8 | 84.8 |
| 7 | HOT | 28.4 | 38.1 | 32.1 | 42.3 | 84.2 | 63.7 | 78.3 | 89.1 | 83.0 |
| 8 | HOT | 24.9 | 34.3 | 28.7 | 44.3 | 82.0 | 65.9 | 73.6 | 85.0 | 78.4 |
| 9 | HOT | 22.6 | 34.4 | 28.0 | 45.81 | 79.5 | 66.2 | 69.9 | 85.7 | 77.5 |
| 10 | HOT | 20.6 | 30.3 | 24.3 | 54.4 | 80.5 | 66.7 | 67.2 | 80.0 | 72.3 |
| 11 | HOT | 20.4 | 30.4 | 24.2 | 45.3 | 80.6 | 65.8 | 67.1 | 79.2 | 72.0 |
| 12 | HOT | 19.7 | 21.3 | 20.3 | 50.0 | 90.5 | 64.6 | 65.8 | 68.8 | 66.4 |
| 13 | TP2 | 19.7 | 20.7 | 20.1 | 56.4 | 91.3 | 65.5 | 65.6 | 68.6 | 66.2 |
| 14 | Recovery | 19.7 | 21.4 | 20.1 | 58.1 | 89.0 | 66.7 | 65.6 | 69.6 | 66.2 |
| 15 | Recovery | 19.6 | 20.5 | 19.9 | 58.4 | 90.3 | 66.4 | 65.6 | 68.2 | 66.0 |
| 16 | Recovery | 19.4 | 25.0 | 20.5 | 57.8 | 93.5 | 66.4 | 65.2 | 73.2 | 66.8 |
| 17 | Recovery | 19.3 | 23.7 | 21.1 | 58.1 | 69.0 | 61.9 | 64.9 | 71.1 | 67.5 |
| Item | Starter | Intermediate | Finisher | Substituted |
|---|---|---|---|---|
| Ingredients, % of diet | ||||
| Grain-based mix * | 62.1 | 74.5 | 86.8 | 78.7 |
| Whole cottonseed | 9.0 | 16.5 | 9.0 | 9.0 |
| Lucerne hay | 28.9 | 9.0 | 4.2 | 12.3 |
| Nutrient composition | ||||
| DM, g/kg fresh weight | 880 | 893 | 887 | 886 |
| ADF, g/kg DM | 263 | 257 | 119 | 177 |
| NDF, g/kg DM | 404 | 375 | 229 | 253 |
| NEg, MJ/kg DM | 29 | 29 | 30 | 30 |
| ME, MJ/kg DM | 116 | 119 | 132 | 131 |
| DE, MJ/kg DM | 143 | 147 | 163 | 162 |
| Crude fibre, g/kg DM | 218 | 197 | 87 | 124 |
| Nitrogen-free extract, g/kg DM | 503 | 548 | 678 | 685 |
| Fat, g/kg DM | 46 | 43 | 46 | 43 |
| Feed digestibility, g/kg DM | 768 | 791 | 861 | 868 |
| Digestible DM, g/kg DM | 676 | 707 | 763 | 769 |
| Digestible protein g/kg DM | 133 | 125 | 130 | 131 |
| Starch, g/kg DM | 229 | 218 | 432 | 432 |
| Parameters | Finisher Diet | Substituted Diet | SED | F-Value (1, 18; d.f. †) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| TN | HOT | TN | HOT | Period (P) | Diet (D) | D × P | |||
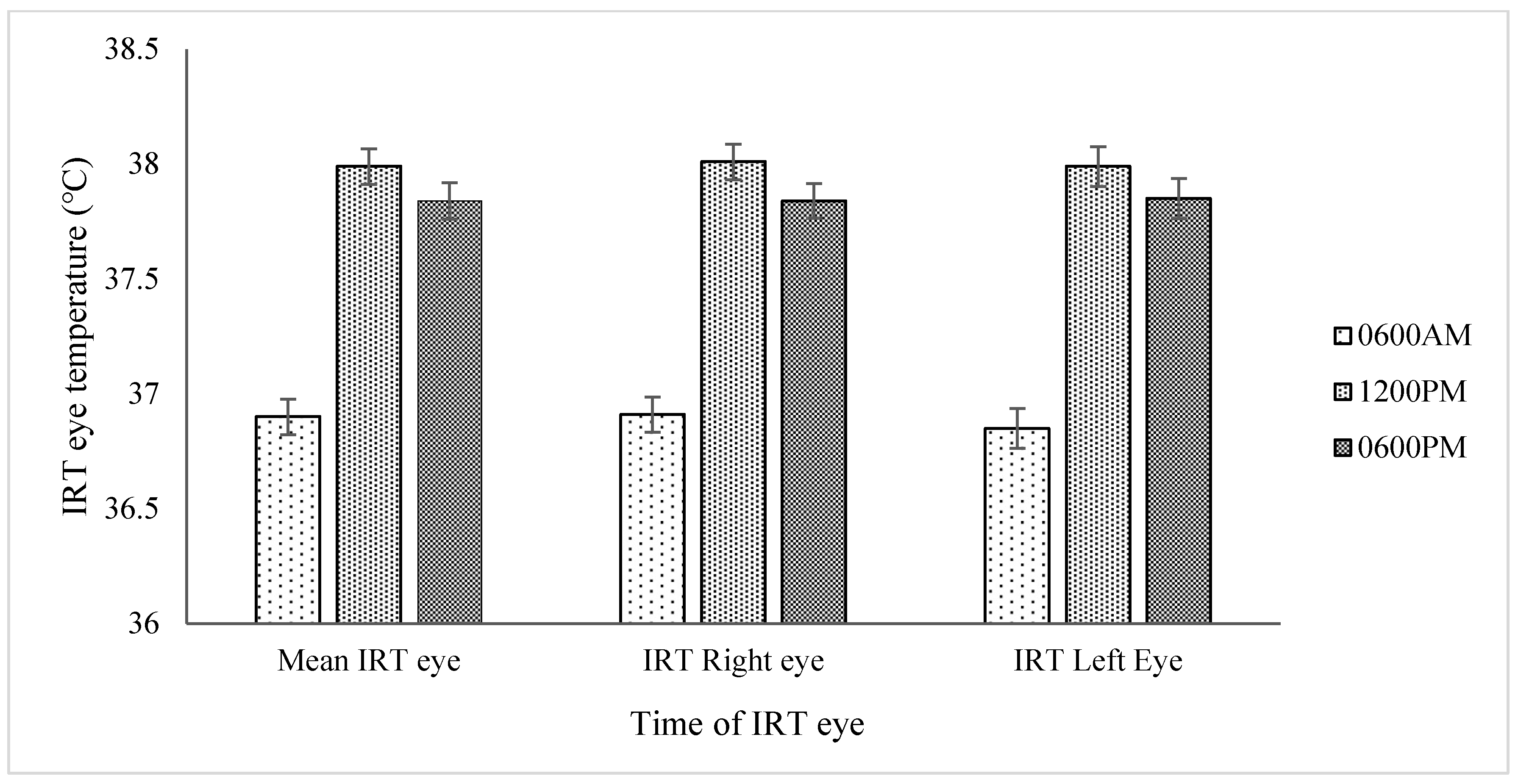
| Mean eye temperature (°C) | 36.75 b | 38.73 a | 36.91 b | 38.52 a | 0.123 | 429 | ≤0.001 | 0.800 | 0.050 |
| Right eye temperature (°C) | 36.73 b | 38.72 a | 36.91 b | 38.50 a | 0.114 | 492 | ≤0.001 | 0.900 | 0.020 |
| Left eye temperature (°C) | 36.76 | 38.74 | 36.93 | 38.51 | 0.149 | 287 | ≤0.001 | 0.800 | 0.070 |
| Right/left eye ratio (°C) | 0.999 | 0.999 | 0.999 | 1.00 | 0.0016 | 0.11 | 0.800 | 0.800 | 0.970 |
| Parameters | Finisher Diet | Substituted Diet | SED | F-Value (d.f. †) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HOT | Recovery | HOT | Recovery | Period (P) | Diet (D) | Time (t) | D × P | D × d | |||
| Mean Eye Temperature (°C) | 38.73 | 36.58 | 38.52 | 36.49 | 0.241 | 1758 (1, 540) | ≤0.001 | 0.300 | ≤0.001 | 0.200 | 0.900 |
| Right Eye Temperature (°C) | 38.73 | 36.59 | 38.50 | 36.52 | 0.247 | 1545 (1, 511) | ≤0.001 | 0.300 | ≤0.001 | 0.100 | 0.700 |
| Left Eye Temperature (°C) | 38.72 | 36.57 | 38.52 | 36.43 | 0.242 | 1486 (1, 484) | ≤0.001 | 0.300 | ≤0.001 | 0.600 | 0.900 |
| Right/Left Eye ratio (°C) | 1.00 | 1.00 | 1.00 | 1.00 | 0.0023 | 2.09 (1, 459) | 0.149 | 0.782 | 0.366 | 0.993 | 0.580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, M.; Sullivan, M.; Gaughan, J.B.; Phillips, C.J.C. The Relationship between the Infrared Eye Temperature of Beef Cattle and Associated Biological Responses at High Environmental Temperatures. Animals 2024, 14, 2898. https://doi.org/10.3390/ani14192898
Idris M, Sullivan M, Gaughan JB, Phillips CJC. The Relationship between the Infrared Eye Temperature of Beef Cattle and Associated Biological Responses at High Environmental Temperatures. Animals. 2024; 14(19):2898. https://doi.org/10.3390/ani14192898
Chicago/Turabian StyleIdris, Musadiq, Megan Sullivan, John B. Gaughan, and Clive J. C. Phillips. 2024. "The Relationship between the Infrared Eye Temperature of Beef Cattle and Associated Biological Responses at High Environmental Temperatures" Animals 14, no. 19: 2898. https://doi.org/10.3390/ani14192898
APA StyleIdris, M., Sullivan, M., Gaughan, J. B., & Phillips, C. J. C. (2024). The Relationship between the Infrared Eye Temperature of Beef Cattle and Associated Biological Responses at High Environmental Temperatures. Animals, 14(19), 2898. https://doi.org/10.3390/ani14192898







