Occurrence of Encephalitozoon cuniculi and Encephalitozoon hellem in European Wild Rabbits (Oryctolagus cuniculus) in Southern Germany (Bavaria)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
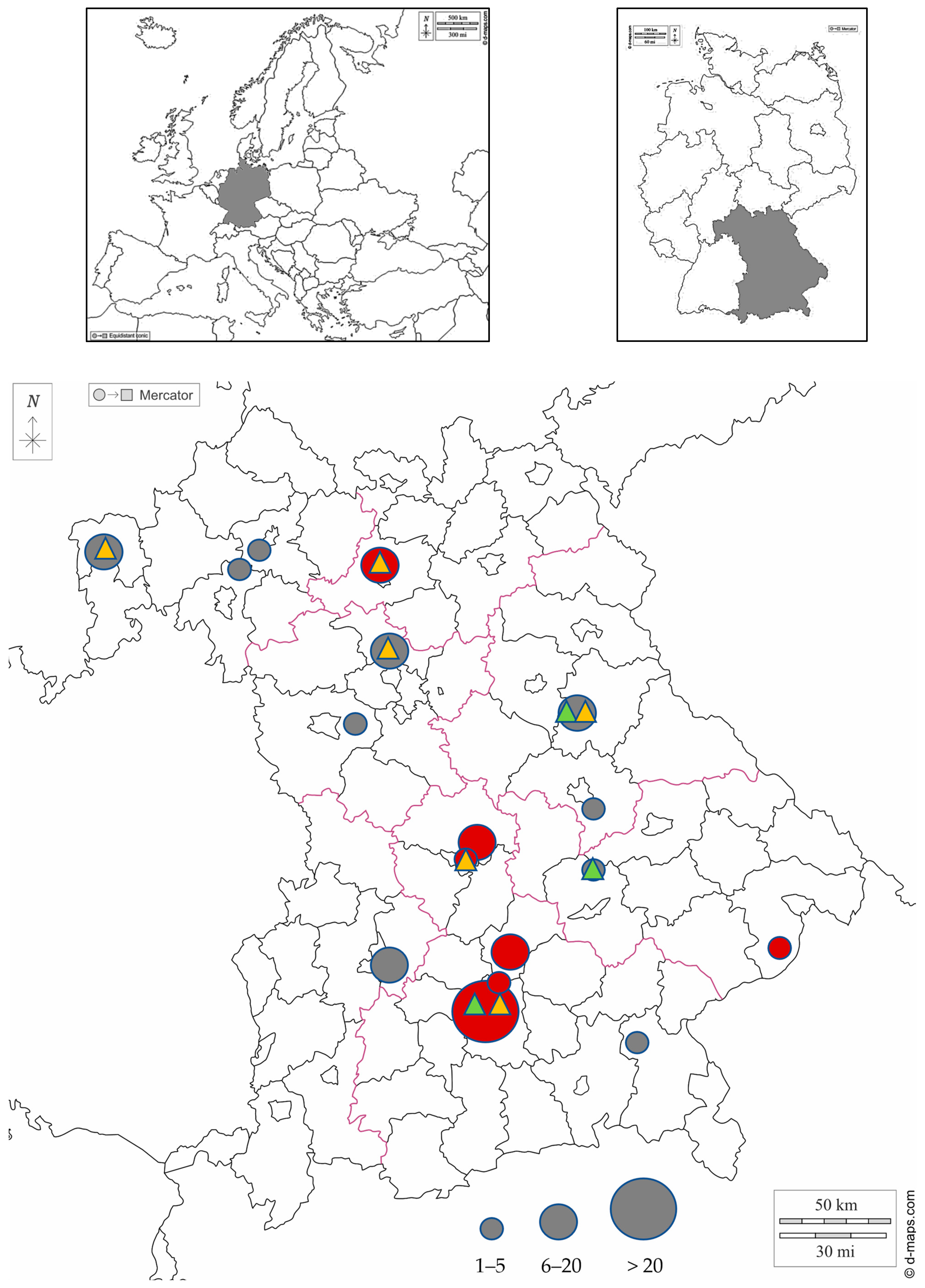
2.2. Study Area and Sample Collection
2.3. Indirect Immunofluorescence Antibody Test
2.4. DNA Extraction
2.5. Real-Time PCR
2.6. Nested PCR I
2.7. Nested PCR II
2.8. Sequencing
3. Results
3.1. Indirect Immunofluorescence Antibody Test for E. cuniculi
3.2. Genome detection by Real-Time PCR
3.3. Nested PCRs and Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, J.H.; Craighead, E.M. Infectious motor paralysis in young rabbits. J. Exp. Med. 1922, 36, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Deplazes, P.; Mathis, A.; Weber, R. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 2000, 6, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Weber, R.; Deplazes, P. Zoonotic potential of the microsporidia. Clin. Microbiol. Rev. 2005, 18, 423–445. [Google Scholar] [CrossRef]
- Hinney, B.; Sak, B.; Joachim, A.; Kváč, M. More than a rabbit’s tale—Encephalitozoon spp. in wild mammals and birds. Int. J. Parasitol. Parasites Wildl. 2016, 5, 76–87. [Google Scholar] [CrossRef]
- Didier, E.S.; Vossbrinck, C.R.; Baker, M.D.; Rogers, L.B.; Bertucci, C.D.; Shadduck, J.A. Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology 1995, 111, 411–421. [Google Scholar] [CrossRef]
- Talabani, H.; Sarfati, C.; Pillebout, E.; van Gool, T.; Derouin, F.; Menotti, J. Disseminated infection with a new genovar of Encephalitozoon cuniculi in a renal transplant recipient. J. Clin. Microbiol. 2010, 48, 2651–2653. [Google Scholar] [CrossRef] [PubMed]
- Valencakova, A.; Balent, P.; Ravaszova, P.; Horak, A.; Obornik, M.; Halanova, M.; Malcekova, B.; Novotny, F.; Goldova, M. Molecular identification and genotyping of Microsporidia in selected hosts. Parasitol. Res. 2012, 110, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Chai, Y.; Xiang, L.; Wang, W.; Zhou, Z.; Liu, H.; Zhong, Z.; Fu, H.; Peng, G. First identification and genotyping of Enterocytozoon bieneusi and Encephalitozoon spp. in pet rabbits in China. BMC Vet. Res. 2020, 16, 212. [Google Scholar] [CrossRef]
- Sokolova, O.I.; Demyanov, A.V.; Bowers, L.C.; Didier, E.S.; Yakovlev, A.V.; Skarlato, S.O.; Sokolova, Y.Y. Emerging microsporidian infections in Russian HIV-infected patients. J. Clin. Microbiol. 2011, 49, 2102–2108. [Google Scholar] [CrossRef]
- Cox, J.C.; Hamilton, R.C.; Attwood, H.D. An investigation of the route and progression of Encephalitozoon cuniculi infection in adult rabbits. J. Protozool. 1979, 26, 260–265. [Google Scholar] [CrossRef]
- Kimura, M.; Aoki, M.; Ichikawa-Seki, M.; Matsuo, K.; Yagita, K.; Itagaki, T. Detection and genotype of Encephalitozoon cuniculi DNA from urine and feces of pet rabbits in Japan. J. Vet. Med. Sci. 2013, 75, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in Humans. Clin. Microbiol. Rev. 2021, 34, e0001020. [Google Scholar] [CrossRef] [PubMed]
- Harcourt-Brown, F. Encephalitozoon cuniculi infection in rabbits. Semin. Avian. Exot. Pet. Med. 2004, 13, 86–93. [Google Scholar] [CrossRef]
- Marcato, P.S.; Rosmini, R. Patologia del Coniglio e della Lepre: Atlante a Colori e Compendio; Società Editrice Esculapio: Bologna, Italy, 1986. [Google Scholar]
- Ozkan, O.; Karagoz, A.; Kocak, N. First molecular evidence of ocular transmission of Encephalitozoonosis during the intrauterine period in rabbits. Parasitol. Int. 2019, 71, 1–4. [Google Scholar] [CrossRef]
- Scharmann, W.; Reblin, L.; Griem, W. Untersuchungen über die Infektion von Kaninchen durch Enzephalitozoon cuniculi. Berl. Münch. Tierärztl. Wschr. 1986, 99, 20–24. [Google Scholar]
- Cox, J.C.; Gallichio, H.A. Serological and histological studies on adult rabbits with recent, naturally acquired encephalitozoonosis. Res. Vet. Sci. 1978, 24, 260–261. [Google Scholar] [CrossRef]
- Eröksüz, H.; Eröksüz, Y.; Metin, N.; Özer, H. Morphologic examinations of cases of naturally acquired encephalitozoonosis in a rabbit colony. Turk. J. Vet. Anim. Sci. 1999, 23, 191–196. [Google Scholar]
- Csokai, J.; Gruber, A.; Künzel, F.; Tichy, A.; Joachim, A. Encephalitozoonosis in pet rabbits (Oryctolagus cuniculus): Pathohistological findings in animals with latent infection versus clinical manifestation. Parasitol. Res. 2009, 104, 629–635. [Google Scholar] [CrossRef]
- Flatt, R.E.; Jackson, S.J. Renal nosematosis in young rabbits. Path. Vet. 1970, 7, 492–497. [Google Scholar]
- Rodríguez-Tovar, L.E.; Nevárez-Garza, A.M.; Trejo-Chávez, A.; Hernández-Martínez, C.A.; Hernández-Vidal, G.; Zarate-Ramos, J.J.; Castillo-Velázquez, U. Encephalitozoon cuniculi: Grading the histological lesions in brain, kidney, and liver during primoinfection outbreak in rabbits. J. Pathog. 2016, 2016, 5768428. [Google Scholar] [CrossRef]
- Harcourt-Brown, F.M.; Holloway, H.K.R. Encephalitozoon cuniculi in pet rabbits. Vet. Rec. 2003, 152, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Weigt, A.; Vercelli, A.; Rondena, M.; Grilli, G.; Giudice, C. Immunohistochemical identification of Encephalitozoon cuniculi in phacoclastic uveitis in four rabbits. Vet. Ophthalmol. 2005, 8, 271–275. [Google Scholar] [CrossRef]
- Künzel, F.; Gruber, A.; Tichy, A.; Edelhofer, R.; Nell, B.; Hassan, J.; Leschnik, M.; Thalhammer, J.G.; Joachim, A. Clinical symptoms and diagnosis of encephalitozoonosis in pet rabbits. Vet. Parasitol. 2008, 151, 115–124. [Google Scholar] [CrossRef]
- Didier, E.S.; Didier, P.J.; Friedberg, D.N.; Stenson, S.M.; Orenstein, J.M.; Yee, R.W.; Tio, F.O.; Davis, R.M.; Vossbrinck, C.; Millichamp, N. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J. Infect. Dis. 1991, 163, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Yee, R.W.; Tio, F.O.; Martinez, J.A.; Held, K.S.; Shadduck, J.A.; Didier, E.S. Resolution of microsporidial epithelial keratopathy in a patient with AIDS. Ophthalmology 1991, 98, 196–201. [Google Scholar] [CrossRef]
- de Bosschere, H.; Wang, Z.; Orlandi, P.A. First diagnosis of Encephalitozoon intestinalis and E. Hellem in a European brown hare (Lepus europaeus) with kidney lesions. Zoonoses Public Health 2007, 54, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mathis, A.; Tanner, I.; Weber, R.; Deplazes, P. Genetic and phenotypic intraspecific variation in the microsporidian Encephalitozoon hellem. Int. J. Parasitol. 1999, 29, 767–770. [Google Scholar] [CrossRef]
- Xiao, L.; Li, L.; Moura, H.; Sulaiman, I.; Lal, A.A.; Gatti, S.; Scaglia, M.; Didier, E.S.; Visvesvara, G.S. Genotyping Encephalitozoon hellem isolates by analysis of the polar tube protein gene. J. Clin. Microbiol. 2001, 39, 2191–2196. [Google Scholar] [CrossRef]
- Black, S.S.; Steinohrt, L.A.; Bertucci, D.C.; Rogers, L.B.; Didier, E.S. Encephalitozoon hellem in budgerigars (Melopsittacus undulatus). Vet. Pathol. 1997, 34, 189–198. [Google Scholar] [CrossRef]
- Phalen, D.N.; Logan, K.S.; Snowden, K.F. Encephalitozoon hellem infection as the cause of a unilateral chronic keratoconjunctivitis in an umbrella cockatoo (Cacatua alba). Vet. Ophthalmol. 2006, 9, 59–63. [Google Scholar] [CrossRef]
- Barton, C.E.; Phalen, D.N.; Snowden, K.F. Prevalence of Microsporidian Spores Shed by Asymptomatic Lovebirds: Evidence for a Potential Emerging Zoonosis. J. Avian Med. Surg. 2003, 17, 197–202. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, S.-S.; Lyoo, Y.S.; Park, H.-M. DNA detection and genotypic identification of potentially human-pathogenic microsporidia from asymptomatic pet parrots in South Korea as a risk factor for zoonotic emergence. Appl. Environ. Microbiol. 2011, 77, 8442–8444. [Google Scholar] [CrossRef] [PubMed]
- Snowden, K.; Phalen, D.N. Encephalitozoon infection in birds. Semin. Avian. Exot. Pet. Med. 2004, 13, 94–99. [Google Scholar] [CrossRef]
- Ozkan, O.; Ozkan, A.T.; Zafer, K. Encephalitozoonosis in New Zealand rabbits and potential transmission risk. Vet. Parasitol. 2011, 179, 234–237. [Google Scholar] [CrossRef]
- Berger Baldotto, S.; Cray, C.; Giannico, A.T.; Reifur, L.; Montiani-Ferreira, F. Seroprevalence of Encephalitozoon cuniculi Infection in Pet Rabbits in Brazil. J. Exot. Pet. Med. 2015, 24, 435–440. [Google Scholar] [CrossRef]
- Neuwirt, E. Ein Beitrag zur Diagnose der Encephalitozoonose (Nosematose) beim Kaninchen; Vergleich zwischen direkten und indirekten Nachweismethoden. Doctoral Thesis, Ludwig-Maximilians-Universität, München, Germany, 1988. [Google Scholar]
- Meyer-Breckwoldt, A. Epidemiologische und klinische Untersuchungen zur Enzephalitozoonose bei Zwergkaninchen. Doctoral Thesis, Tierärztliche Hochschule Hannover, Hannover, Germany, 1996. [Google Scholar]
- Fa, J.E.; Sharples, C.M.; Bell, D.J.; DeAngelis, D. An individual-based model of rabbit viral haemorrhagic disease in European wild rabbits (Oryctolagus cuniculus). Ecol. Model. 2001, 144, 121–138. [Google Scholar] [CrossRef]
- Chalupský, J.; Vávra, J.; Bedrník, P. Detection of antibodies to Encephalitozoon cuniculi in rabbits by the indirect immunofluorescent antibody test. Folia Parasitol. 1973, 20, 281–284. [Google Scholar]
- Leipig, M.; Matiasek, K.; Rinder, H.; Janik, D.; Emrich, D.; Baiker, K.; Hermanns, W. Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J. Vet. Diagn. Investig. 2013, 25, 16–26. [Google Scholar] [CrossRef]
- Katzwinkel-Wladarsch, S.; Lieb, M.; Helse, W.; Löscher, T.; Rinder, H. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1996, 1, 373–378. [Google Scholar] [CrossRef]
- Asakura, T.; Nakamura, S.; Ohta, M.; Une, Y.; Furuya, K. Genetically unique microsporidian Encephalitozoon cuniculi strain type III isolated from squirrel monkeys. Parasitol. Int. 2006, 55, 159–162. [Google Scholar] [CrossRef]
- Jeklova, E.; Leva, L.; Kovarcik, K.; Matiasovic, J.; Kummer, V.; Maskova, J.; Skoric, M.; Faldyna, M. Experimental oral and ocular Encephalitozoon cuniculi infection in rabbits. Parasitology 2010, 137, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Csokai, J.; Joachim, A.; Gruber, A.; Tichy, A.; Pakozdy, A.; Künzel, F. Diagnostic markers for encephalitozoonosis in pet rabbits. Vet. Parasitol. 2009, 163, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.; Flock, U.; Sauter-Louis, C.; Hartmann, K. Encephalitozoon cuniculi in rabbits in Germany: Prevalence and sensitivity of antibody testing. Vet. Rec. 2014, 174, 350. [Google Scholar] [CrossRef] [PubMed]
- Zanet, S.; Palese, V.; Trisciuoglio, A.; Cantón Alonso, C.; Ferroglio, E. Encephalitozoon cuniculi, Toxoplasma gondii and Neospora caninum infection in invasive Eastern Cottontail Rabbits Sylvilagus floridanus in Northwestern Italy. Vet. Parasitol. 2013, 197, 682–684. [Google Scholar] [CrossRef]
- Baz-González, E.; Martin-Carrillo, N.; García-Livia, K.; Abreu-Acosta, N.; Foronda, P. Molecular Detection of Microsporidia in Rabbits (Oryctolagus cuniculus) in Tenerife, Canary Islands, Spain. Biology 2022, 11, 1796. [Google Scholar] [CrossRef]
- Martínez-Padilla, A.; Caballero-Gómez, J.; Magnet, Á.; Gómez-Guillamón, F.; Izquierdo, F.; Camacho-Sillero, L.; Jiménez-Ruiz, S.; Del Águila, C.; García-Bocanegra, I. Zoonotic Microsporidia in Wild Lagomorphs in Southern Spain. Animals 2020, 10, 2218. [Google Scholar] [CrossRef]
- Rego, L.; Castro-Scholten, S.; Cano, C.; Jiménez-Martín, D.; Köster, P.C.; Caballero-Gómez, J.; Bailo, B.; Dashti, A.; Hernández-Castro, C.; Cano-Terriza, D.; et al. Iberian wild leporidae as hosts of zoonotic enteroparasites in Mediterranean ecosystems of Southern Spain. Zoonoses Public Health 2023, 70, 223–237. [Google Scholar] [CrossRef]
- Espinosa, J.; Ferreras, M.C.; Benavides, J.; Cuesta, N.; Pérez, C.; García Iglesias, M.J.; García Marín, J.F.; Pérez, V. Causes of Mortality and Disease in Rabbits and Hares: A Retrospective Study. Animals 2020, 10, 158. [Google Scholar] [CrossRef]
- Lamalle, A.; Haverson, V.A.; Hughes, K. Renal pathology in wild European rabbits. Vet. Rec. 2023, 193, e2948. [Google Scholar] [CrossRef]
- Latney, L.V.; Bradley, C.W.; Wyre, N.R. Encephalitozoon cuniculi in pet rabbits: Diagnosis and optimal management. Vet. Med. Res. Rep. 2014, 5, 169–180. [Google Scholar] [CrossRef]
- Kunstýř, I.; Lev, L.; Naumann, S. Humoral antibody response of rabbits to experimental infection with Encephalitozoon cuniculi. Vet. Parasitol. 1986, 21, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Jeklova, E.; Jekl, V.; Kovarcik, K.; Hauptman, K.; Koudela, B.; Neumayerova, H.; Knotek, Z.; Faldyna, M. Usefulness of detection of specific IgM and IgG antibodies for diagnosis of clinical encephalitozoonosis in pet rabbits. Vet. Parasitol. 2010, 170, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M. Encephalitozoon cuniculi in wild European rabbits and a fox. Res. Vet. Sci. 1979, 26, 114. [Google Scholar] [CrossRef]
- Chalupský, J.; Vávrá, J.; Gaudin, J.C.; Vandewalle, P.; Arthur, C.P.; Guenezan, M.; Launay, H. Serological evidence of the occurrence of encephalitozoonosis and toxoplasmosis in the European wild rabbit (Oryctolagus cuniculus) in France. Bull. Soc. fr. parasitol. 1990, 8, 91–95. [Google Scholar]
- Thomas, C.; Finn, M.; Twigg, L.; Deplazes, P.; Thompson, R.C. Microsporidia (Encephalitozoon cuniculi) in wild rabbits in Australia. Aust. Vet. J. 1997, 75, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Balent, P.; Halanova, M.; Sedlakova, T.; Valencakova, A.; Cislakova, L. Encephalitozoon cuniculi infection in rabbits and laboratory mice in Eastern Slovakia. Bull. Vet. Inst. Pulawy 2004, 48, 113–116. [Google Scholar]
- Cox, J.C.; Pye, D.; Edmonds, J.W.; Shepherd, R. An investigation of Encephalitozoon cuniculi in the wild rabbit Oryctolagus cuniculus in Victoria, Australia. Epidemiol. Infect. 1980, 84, 295–300. [Google Scholar] [CrossRef]
- Cox, J.C.; Ross, J. A serological survey of Encephalitozoon cuniculi infection in the wild rabbit in England and Scotland. Res. Vet. Sci. 1980, 28, 396. [Google Scholar] [CrossRef]
- Blevins, M. Prevalence of Encephalitozoon cuniculi in a population of wild rabbits in Norfolk, England. Zoomed 2007, 7, 28–36. [Google Scholar]
- Bose, H.M.; Woodhouse, M.A.; Powell, R. Absence of Encephalitozoon cuniculi antibodies in wild rabbits in England. Vet. Rec. 2015, 177, 48. [Google Scholar] [CrossRef]
- Magalhães, T.R.; Pinto, F.F.; Queiroga, F.L. A multidisciplinary review about Encephalitozoon cuniculi in a One Health perspective. Parasitol. Res. 2022, 121, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Keeble, E.J.; Shaw, D.J. Seroprevalence of antibodies to Encephalitozoon cuniculi in domestic rabbits in the United Kingdom. Vet. Rec. 2006, 158, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Gannon, J. A survey of Encephalitozoon cuniculi in laboratory animal colonies in the United Kingdom. Lab. Anim. 1980, 14, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Frölich, K.; Thiede, S.; Kozikowski, T.; Jakob, W. A review of mutual transmission of important infectious diseases between livestock and wildlife in Europe. Ann. N. Y. Acad. Sci. 2002, 969, 4–13. [Google Scholar] [CrossRef] [PubMed]
| Number of Rabbits | |
|---|---|
| Age | |
| Juvenil | 36 |
| Adult | 122 |
| Sex | |
| Male | 88 |
| Female | 70 |
| Sampling year | |
| 2021 | 7 |
| 2022 | 126 |
| 2023 | 25 |
| Rabbit No. | Locations (Administrative Districts) | IgG Titre | IgM Titre |
|---|---|---|---|
| 2 | Munich | 1:80 | - |
| 3 | Munich | - | 1:80 |
| 9 | Munich | >1:1280 | 1:320 |
| 13 | Freising | 1:640 | 1:80 |
| 17 | Munich | 1:80 | - |
| 34 | Bamberg | 1:160 | - |
| 37 | Bamberg | 1:80 | - |
| 45 | Passau | 1:80 | 1:80 |
| 46 | Passau | - | 1:80 |
| 52 | Munich | 1:1280 | - |
| 53 | Munich | 1:320 | 1:80 |
| 56 | Munich | 1:80 | 1:80 |
| 62 | Munich | 1:80 | 1:80 |
| 63 | Munich | 1:80 | - |
| 97 | Munich | 1:80 | - |
| 102 | Munich | 1:80 | 1:80 |
| 103 | Munich | 1:80 | 1:80 |
| 106 | Munich | 1:80 | - |
| 109 | Munich | 1:80 | - |
| 121 | Munich | 1:80 | 1:80 |
| 133 | Ingolstadt | 1:80 | 1:80 |
| 138 | Eichstätt | 1:80 | 1:80 |
| 145 | Eichstätt | 1:80 | 1:80 |
| 148 | Eichstätt | 1:80 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breuninger, K.; Rinder, M.; Korbel, R. Occurrence of Encephalitozoon cuniculi and Encephalitozoon hellem in European Wild Rabbits (Oryctolagus cuniculus) in Southern Germany (Bavaria). Animals 2024, 14, 2880. https://doi.org/10.3390/ani14192880
Breuninger K, Rinder M, Korbel R. Occurrence of Encephalitozoon cuniculi and Encephalitozoon hellem in European Wild Rabbits (Oryctolagus cuniculus) in Southern Germany (Bavaria). Animals. 2024; 14(19):2880. https://doi.org/10.3390/ani14192880
Chicago/Turabian StyleBreuninger, Katharina, Monika Rinder, and Rüdiger Korbel. 2024. "Occurrence of Encephalitozoon cuniculi and Encephalitozoon hellem in European Wild Rabbits (Oryctolagus cuniculus) in Southern Germany (Bavaria)" Animals 14, no. 19: 2880. https://doi.org/10.3390/ani14192880
APA StyleBreuninger, K., Rinder, M., & Korbel, R. (2024). Occurrence of Encephalitozoon cuniculi and Encephalitozoon hellem in European Wild Rabbits (Oryctolagus cuniculus) in Southern Germany (Bavaria). Animals, 14(19), 2880. https://doi.org/10.3390/ani14192880





