Study on the Regulatory Mechanism of the PDK1-Mediated TGF-β/Smad Signaling Pathway in Hypoxia-Induced Yak Lungs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
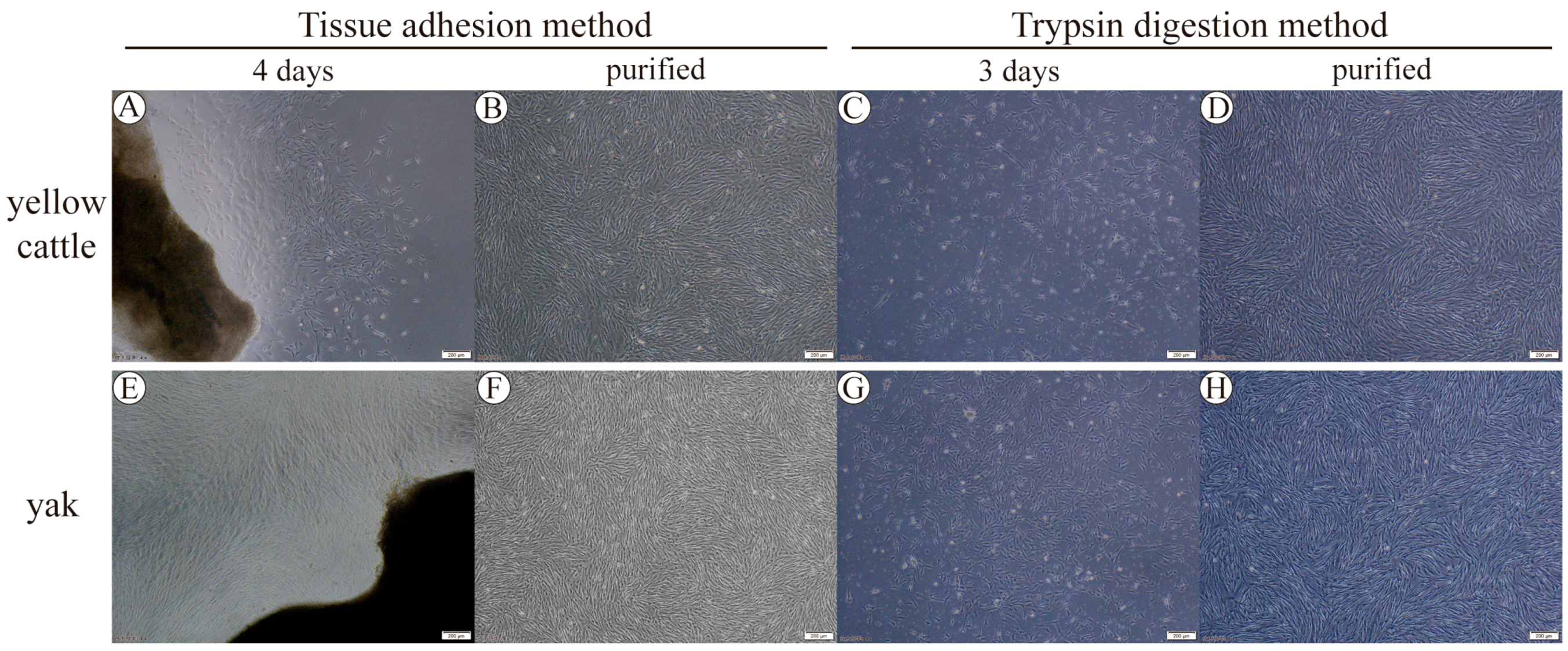
2.2. Cell Isolation and Culture
2.3. Cell Purification
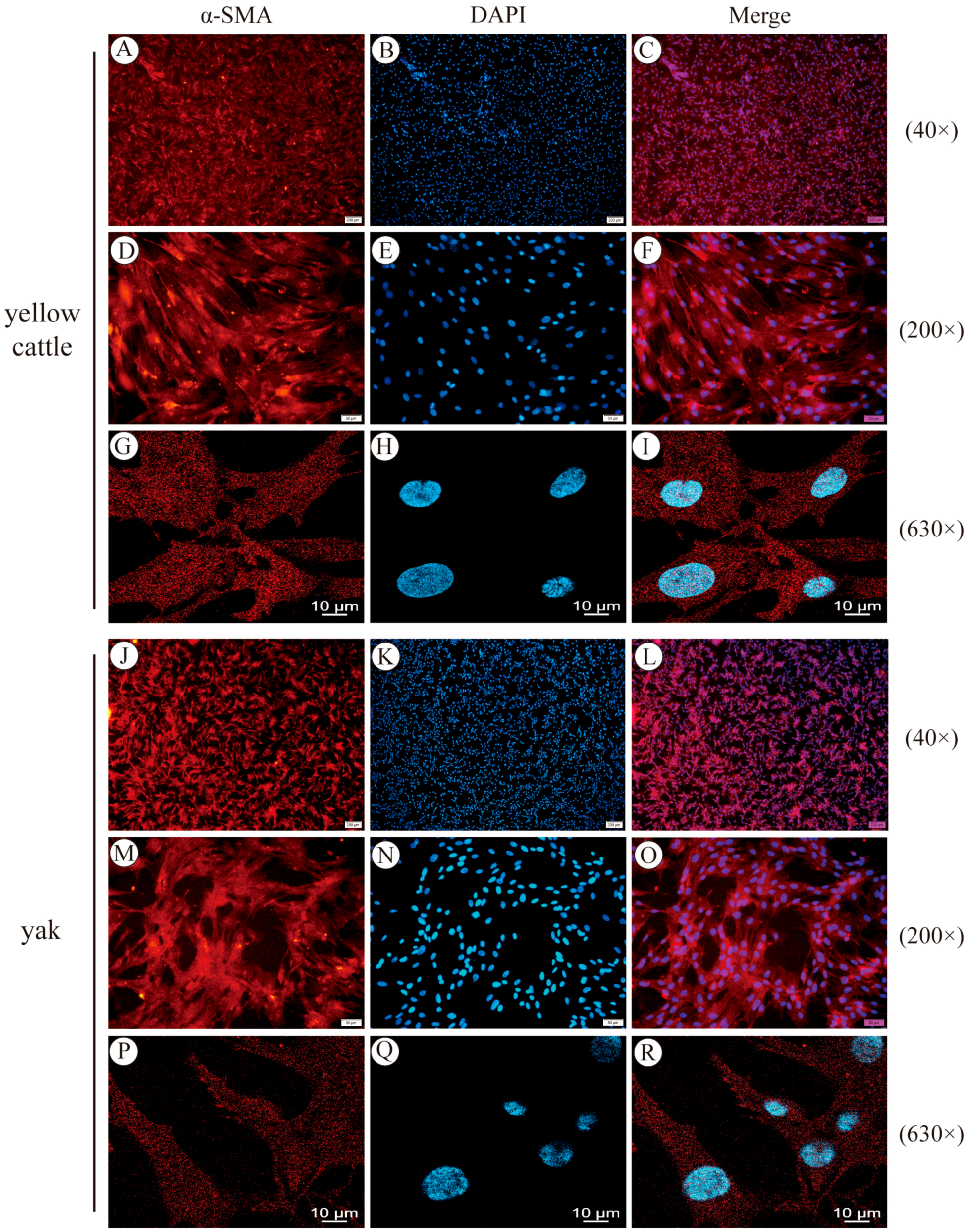
2.4. Immunofluorescence
2.5. Cell Processing and Grouping
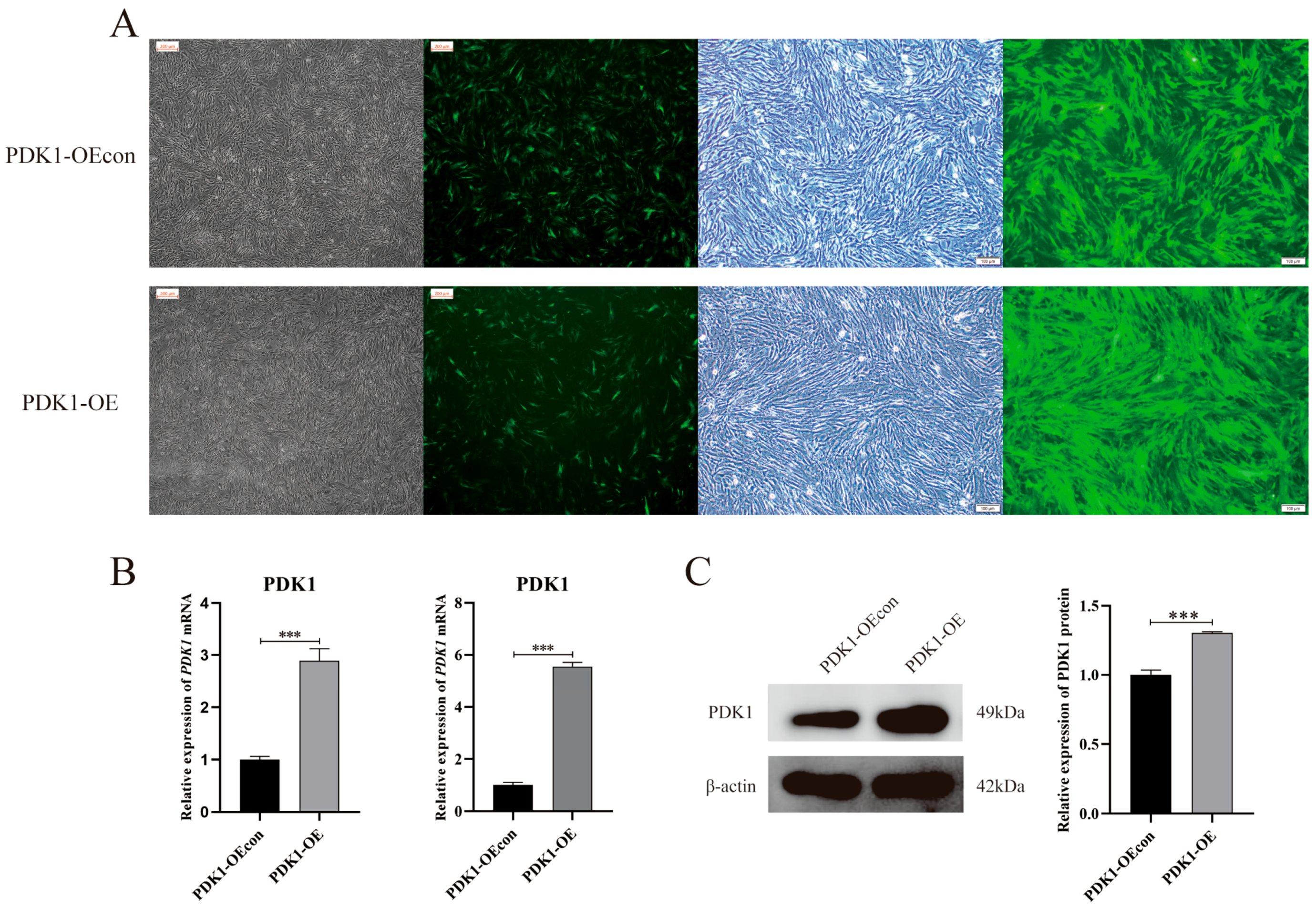
2.6. Construction of a Stable Transient Cell Line of PDK1-Overexpressing Yak PASMCs
2.6.1. Construction of PDK1 Overexpression Lentiviral Vector
2.6.2. Lentiviral Transfection and Screening
2.7. Cell Growth Curve
2.8. Apoptosis Analysis
2.9. ROS Analysis
2.10. Cell Scratching Analysis
2.11. Glucose and Lactic Acid Analysis
2.12. RT-qPCR Analysis
2.13. Western Blot Analysis
2.14. Statistics and Analysis
3. Results
3.1. Yellow Cattle and Yak Primary PASMC Isolation, Culture Purification, and Characterization
3.2. Construction of a Stable Transient Cell Line of PDK1-Overexpressing Yak PASMCs
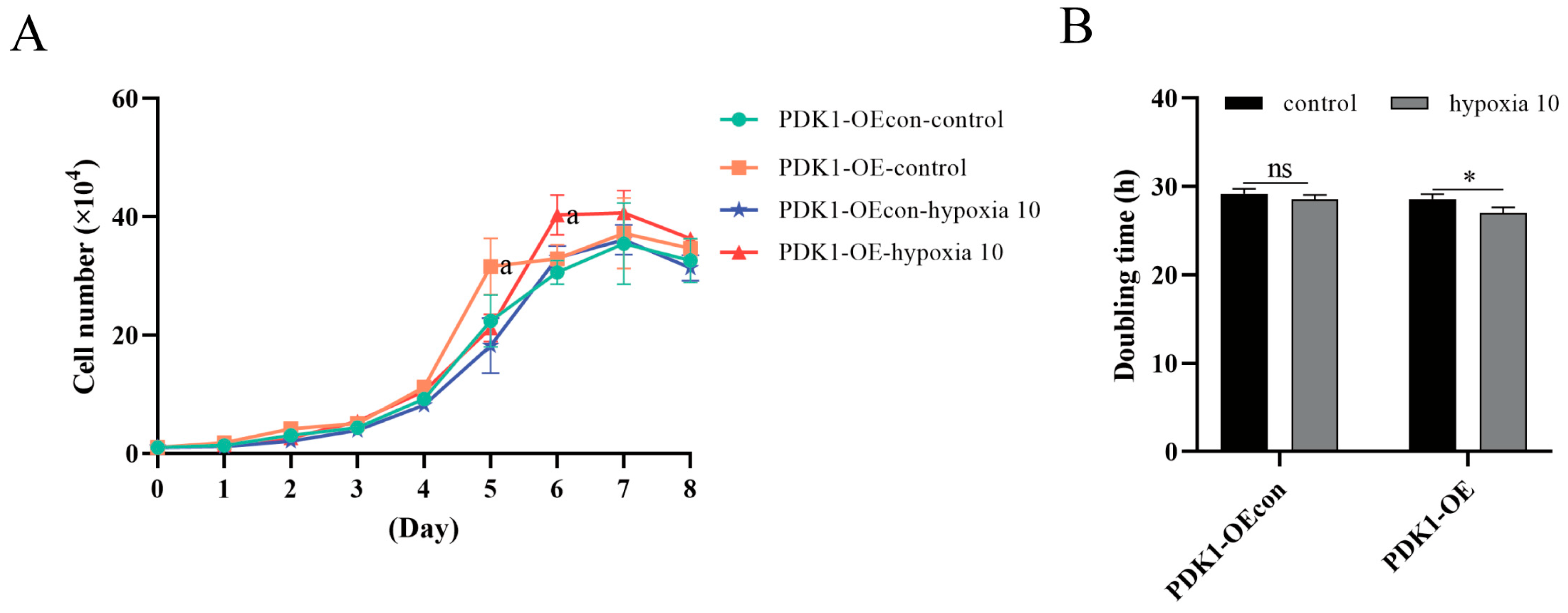
3.3. Effect of Hypoxia on Proliferation Levels of Yellow Cattle, Yak, and Their PDK1-Overexpressing PASMCs
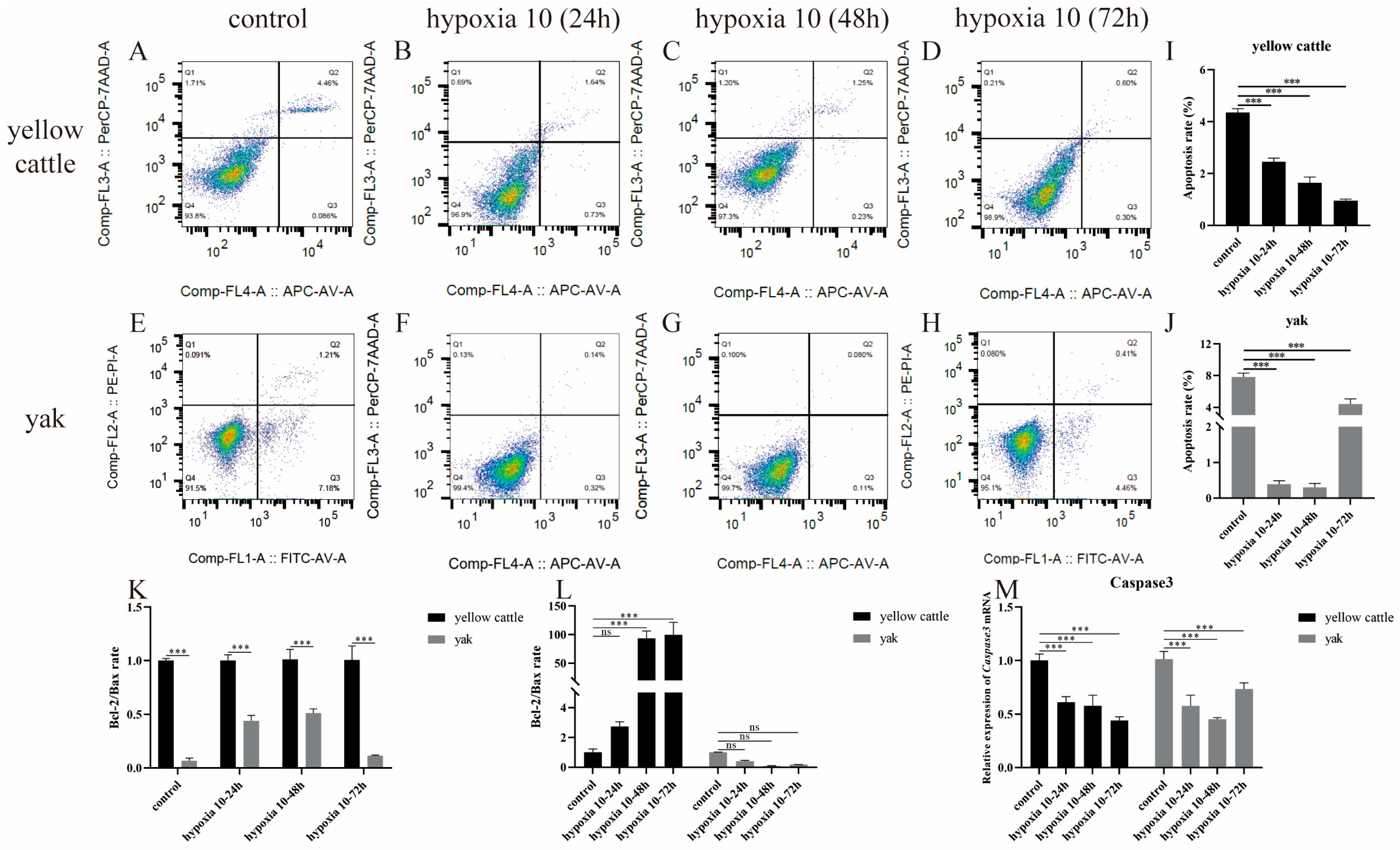
3.4. Effect of Hypoxia on Apoptosis Levels in Yellow Cattle, Yak, and Their PDK1-Overexpressing PASMCs
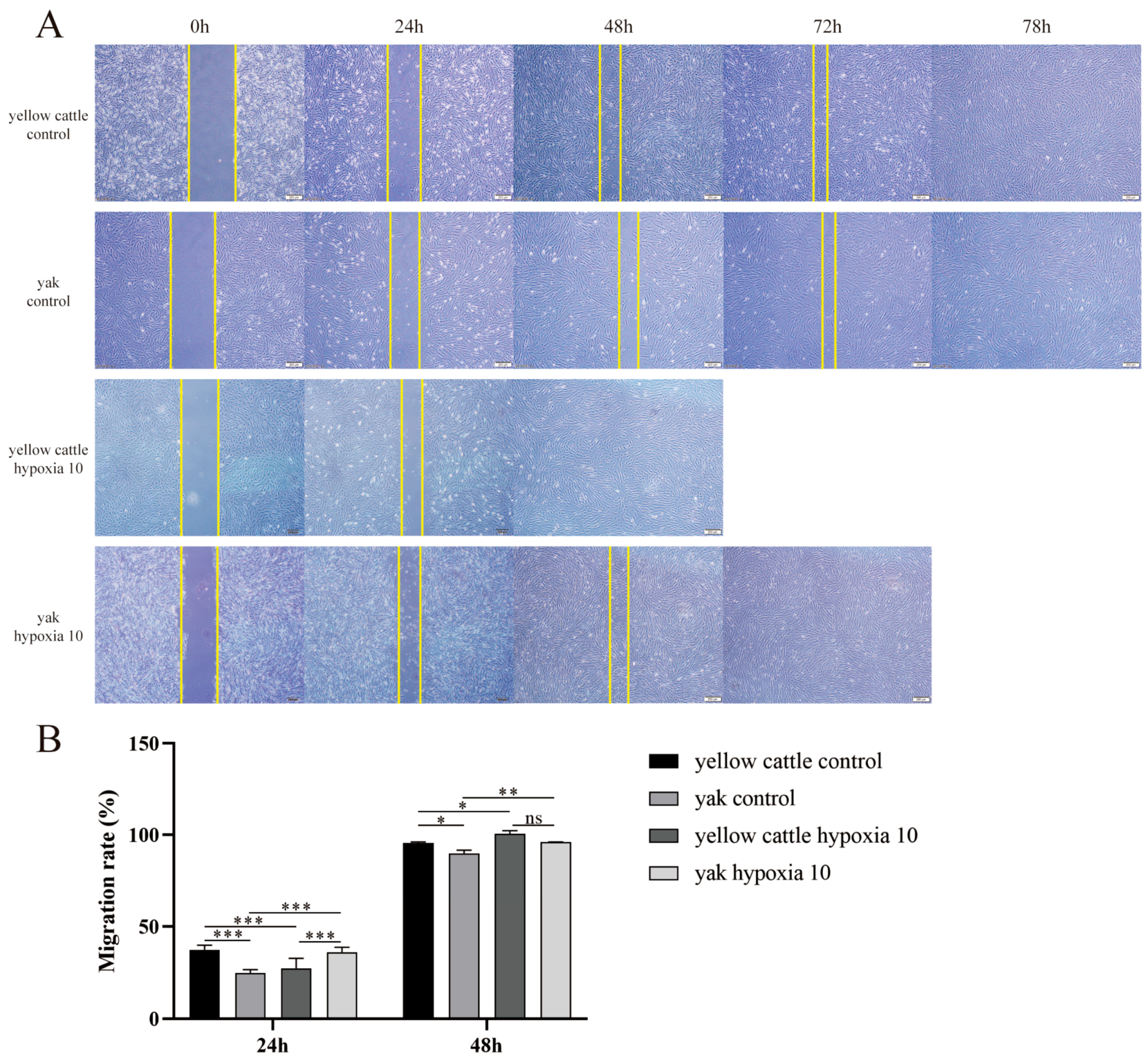
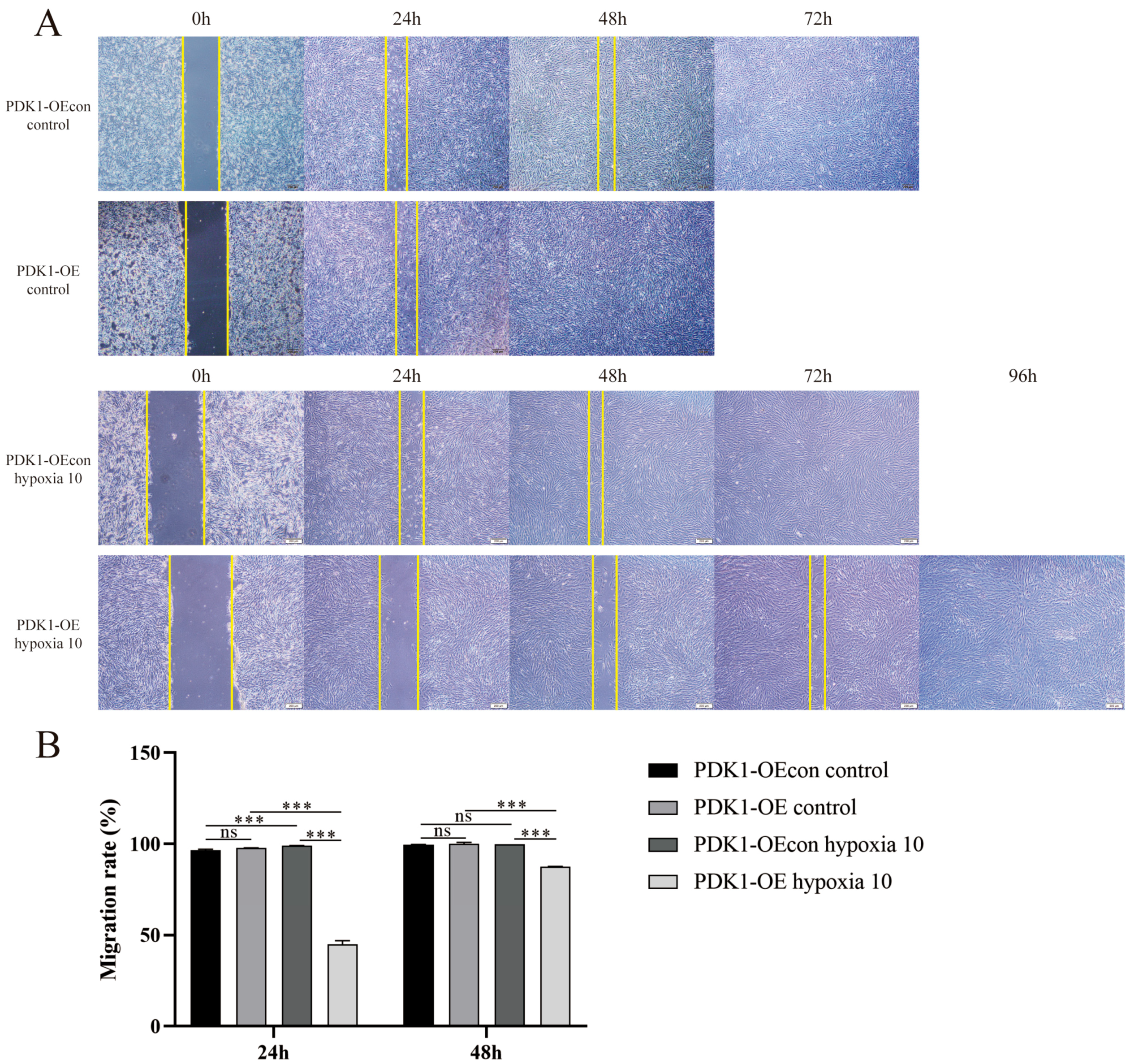
3.5. Effect of Hypoxia on Migration Levels of Yellow Cattle, Yak and Their PDK1-Overexpressing PASMCs
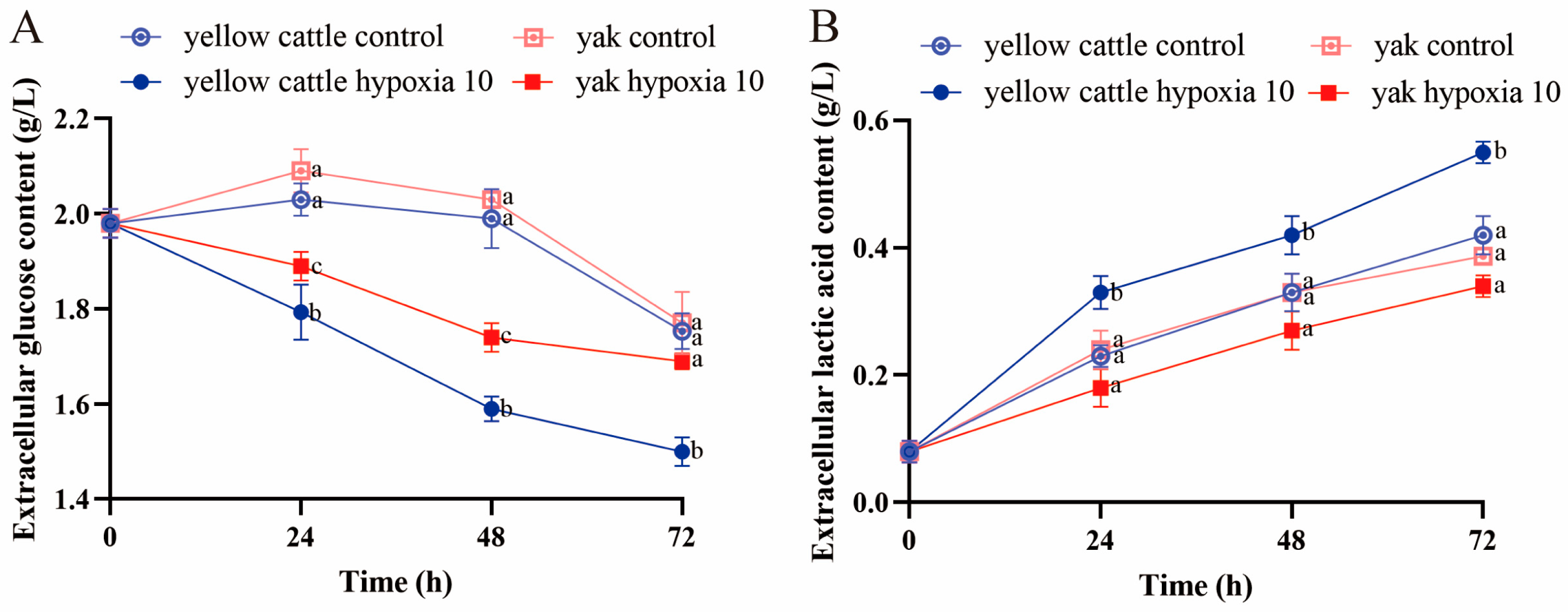
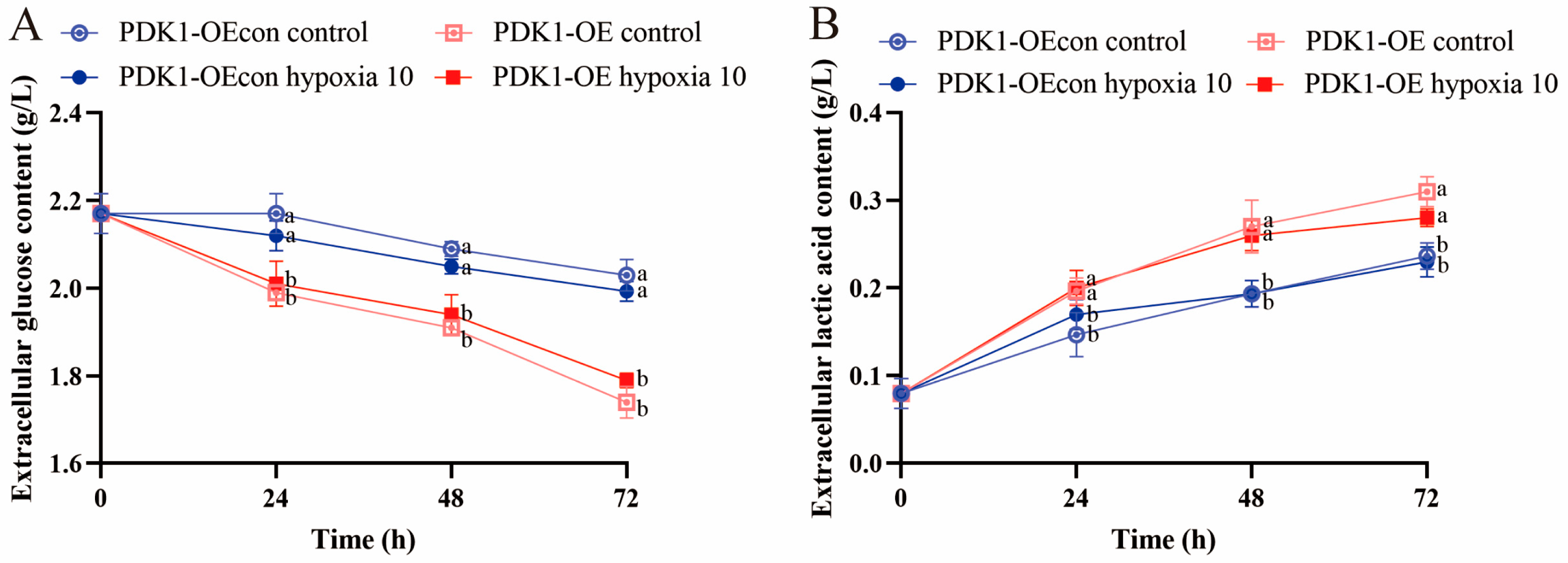
3.6. Effects of Hypoxia on Glucose Metabolism Function in Yellow Cattle, Yak, and Their PDK1-Overexpressing PASMCs
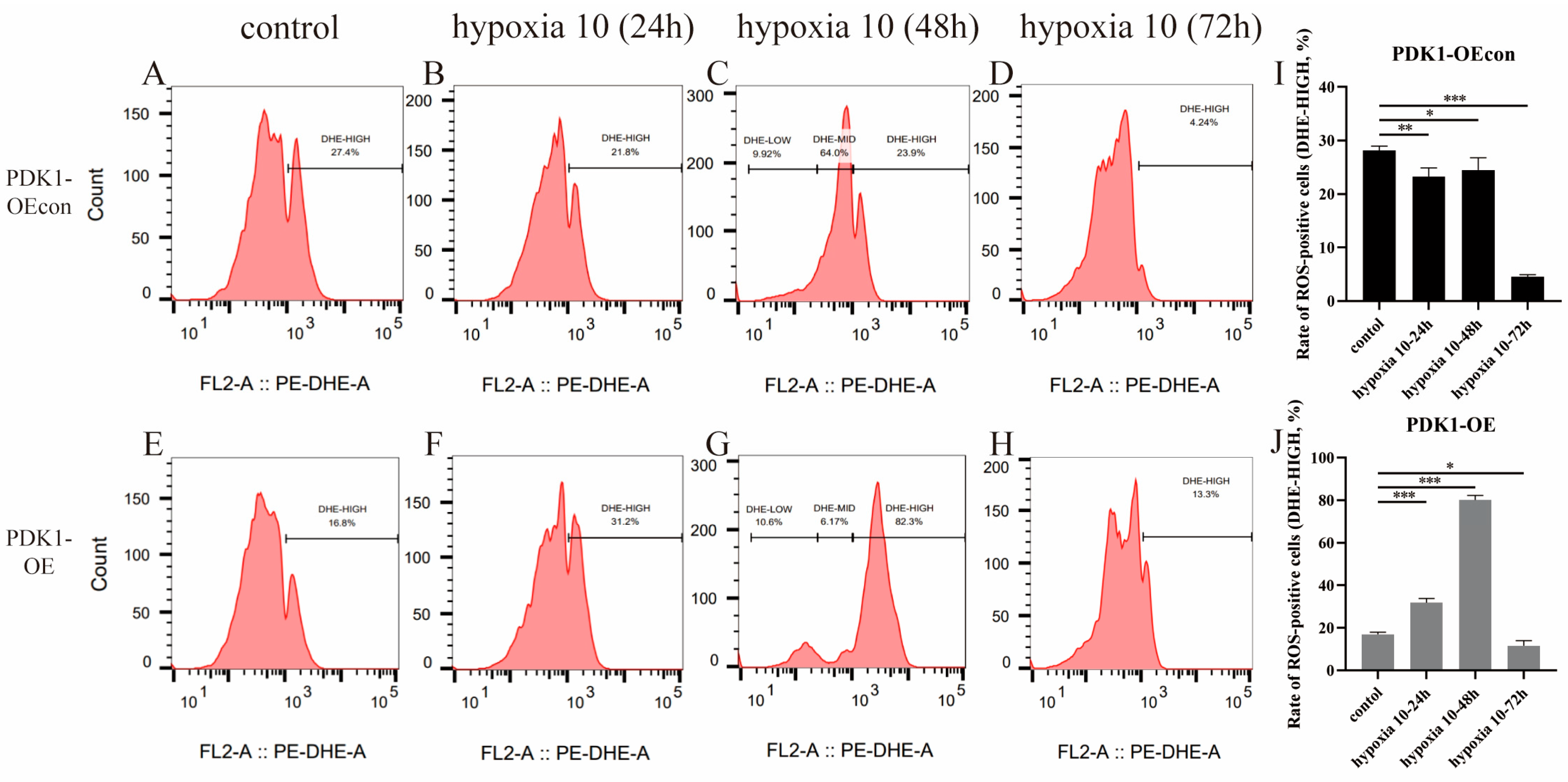
3.7. Effects of Hypoxia on ROS Levels in Yellow Cattle, Yak, and Their PDK1-Overexpressing PASMCs
3.8. Effects of Hypoxia on the Expression of Genes Related to the PDK1 and TGF-β/Smad Signaling Pathways in Yellow Cattle, Yak and Their PDK1-Overexpressing PASMCs
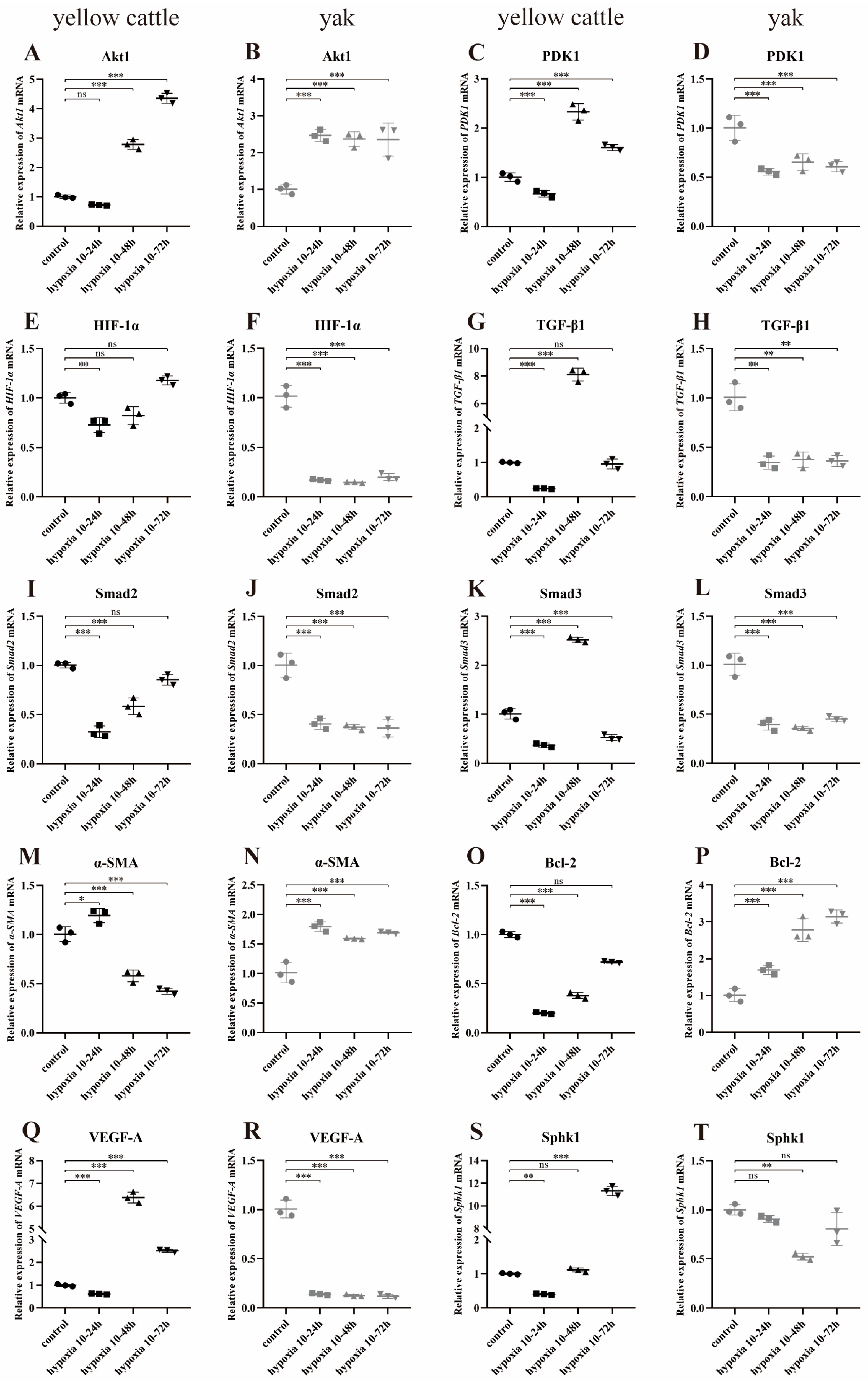
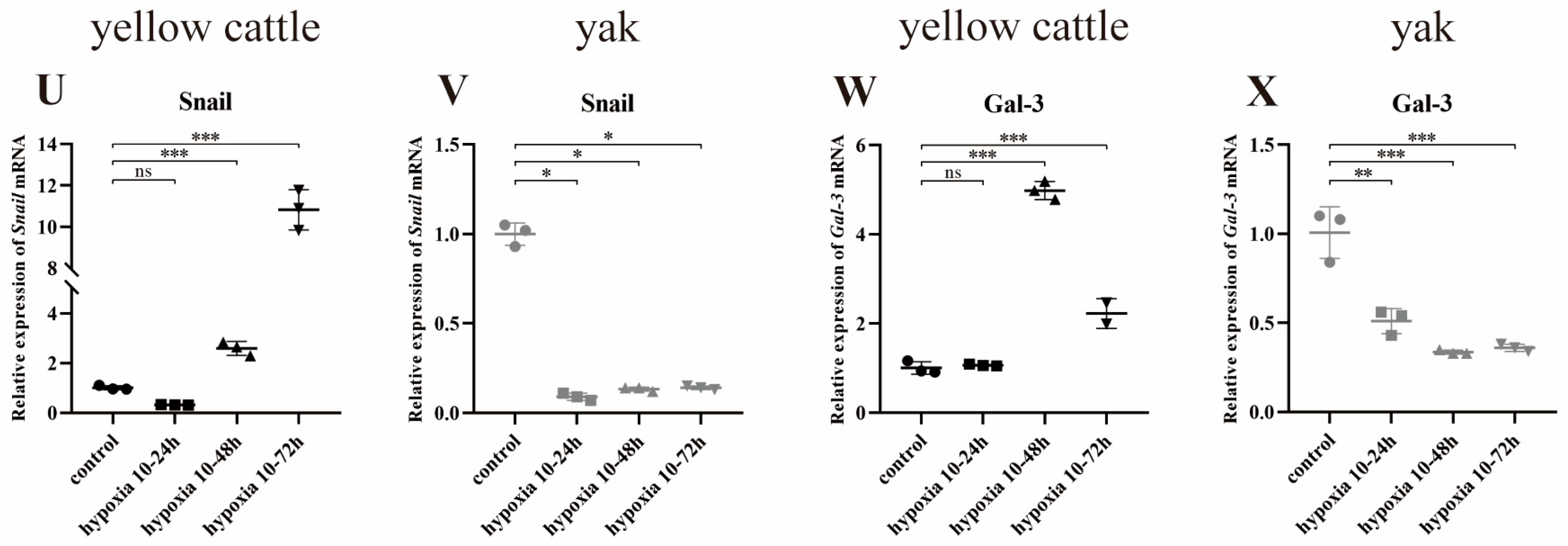
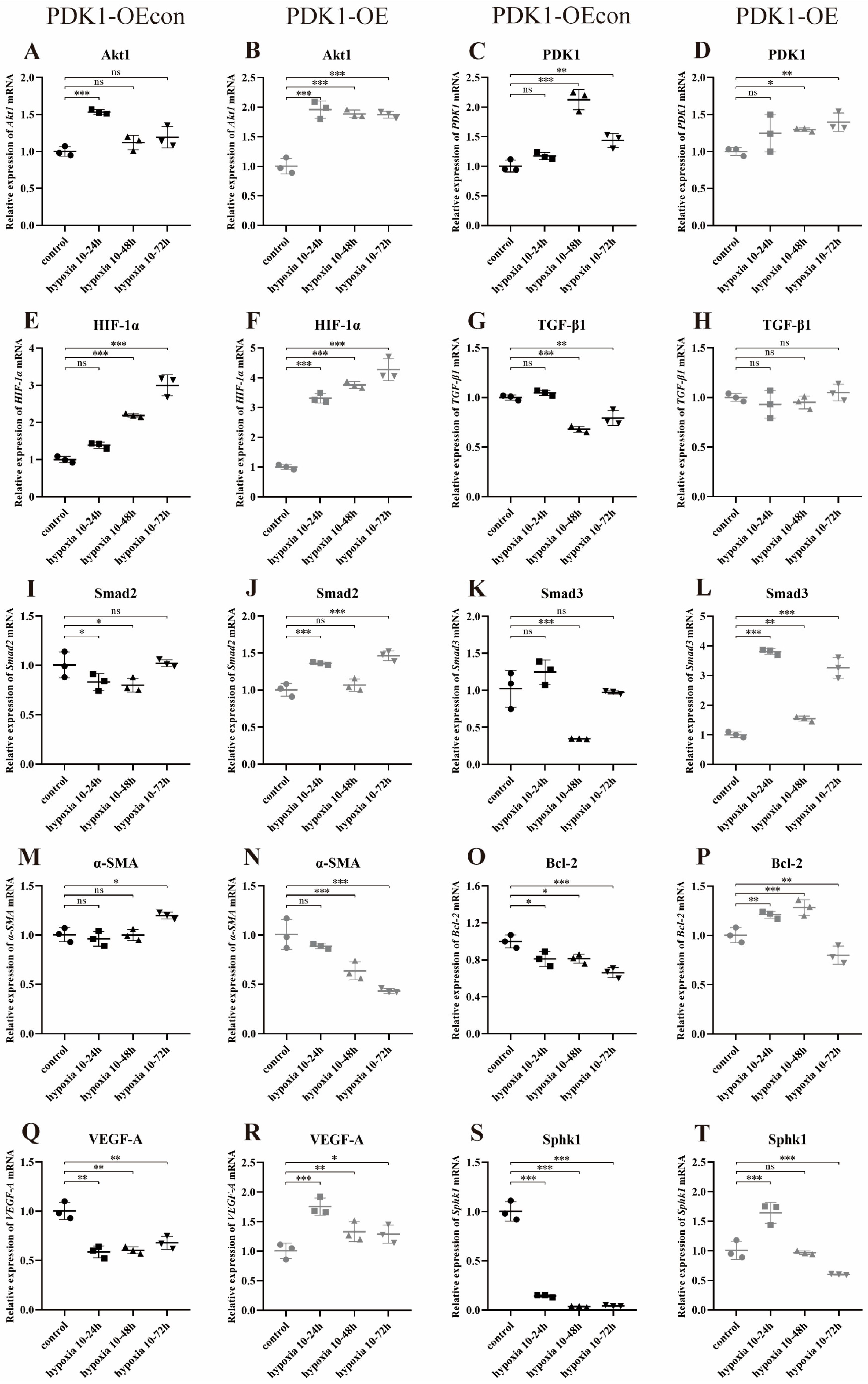
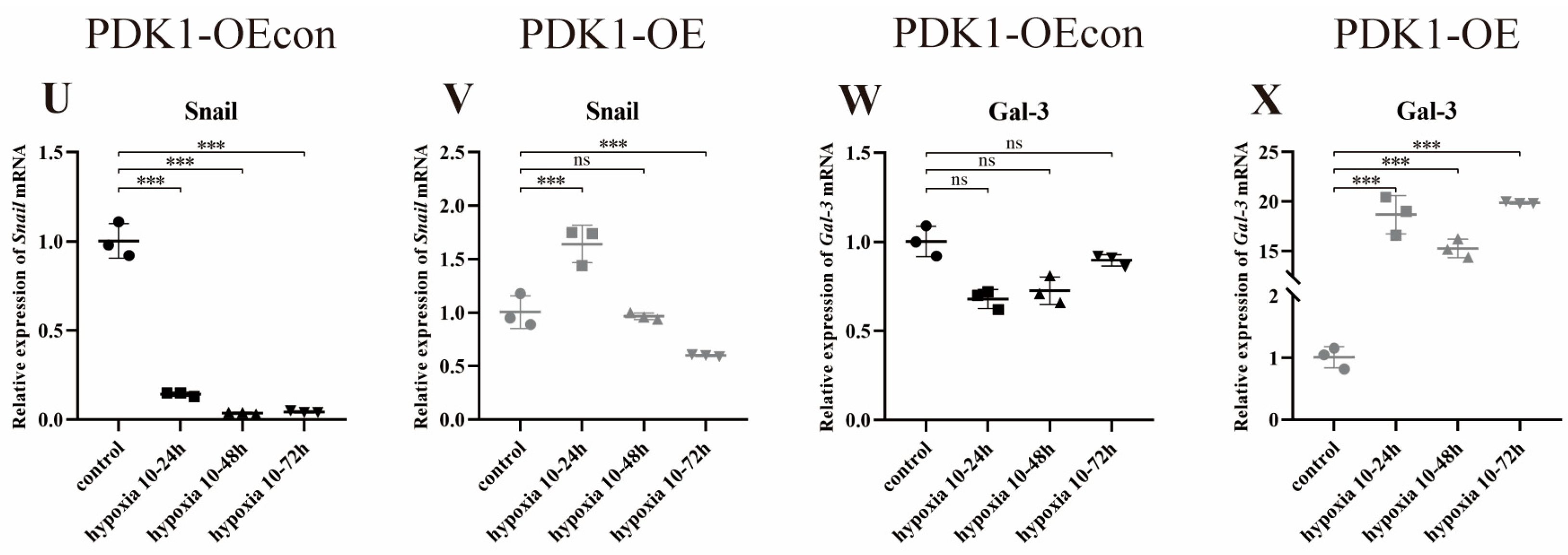
3.8.1. Effect of Hypoxia on the Expression of PDK1 and TGF-β/Smad Pathway-Related Genes in Yellow Cattle and Yak PASMCs
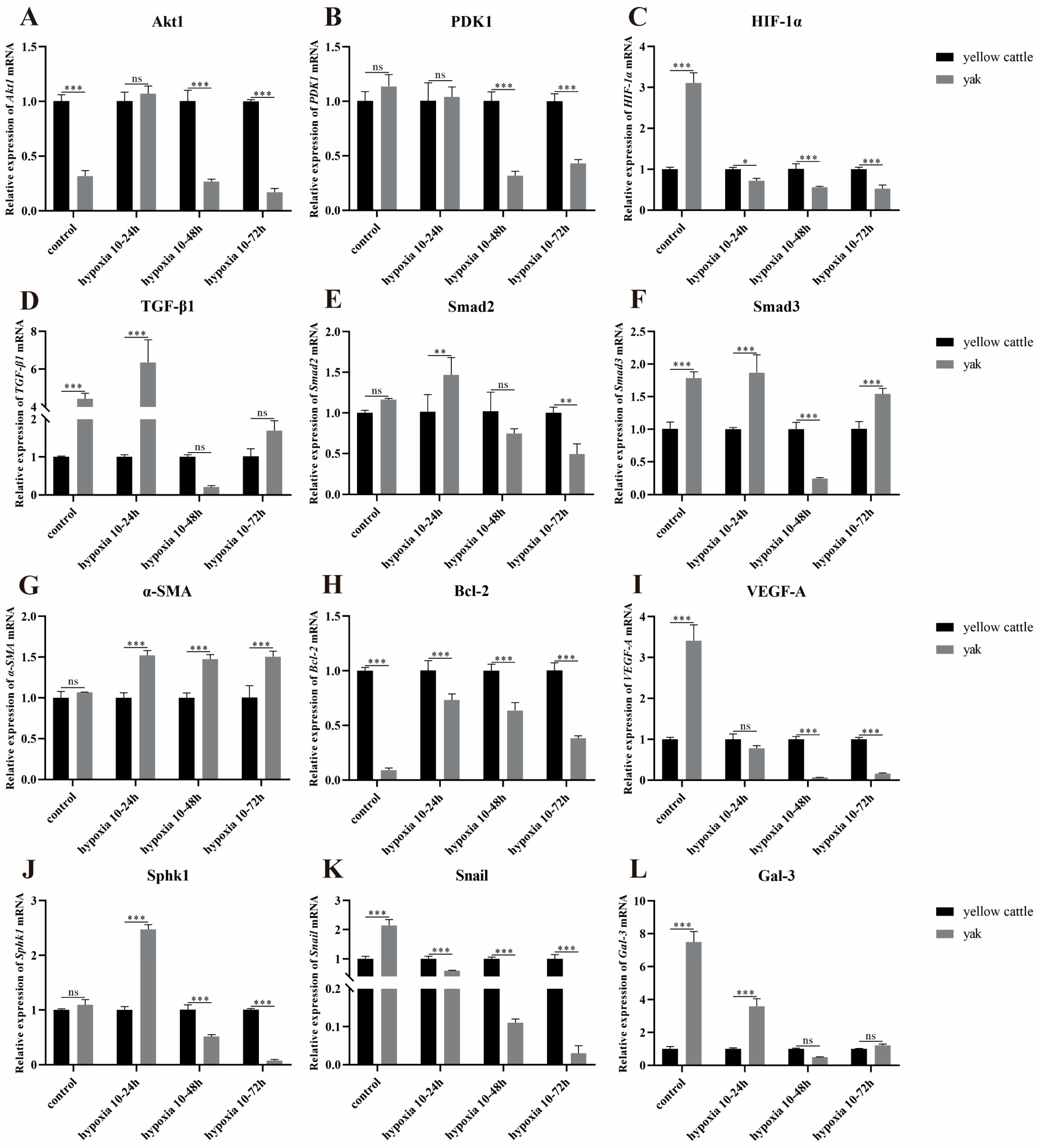
3.8.2. Effect of Hypoxia on the Expression of Genes Related to PDK1 and TGF-β/Smad Signaling Pathways in Yak PASMCs Overexpressing PDK1
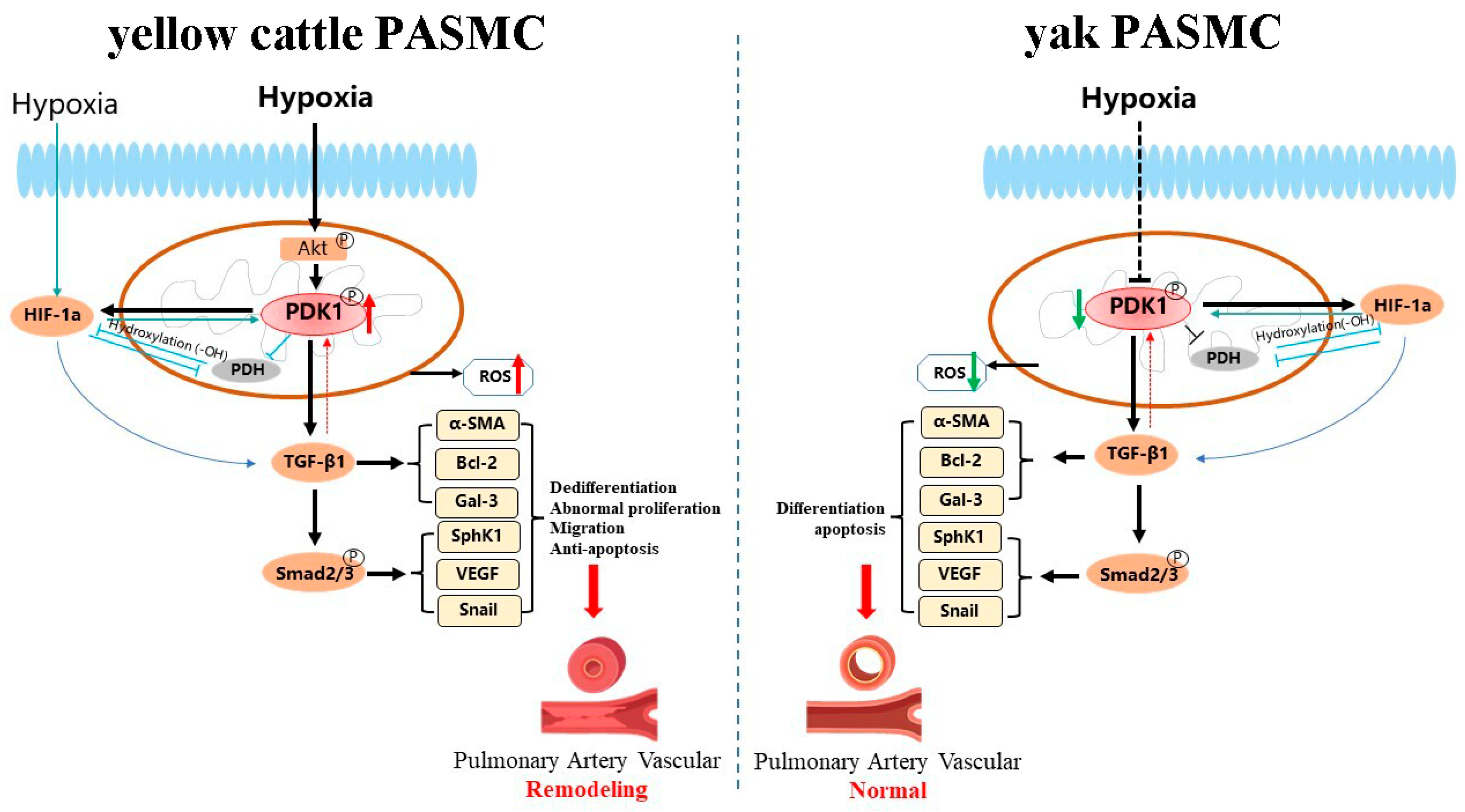
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Chen, S.Y.; Li, C.; Luo, Z.; Li, X.; Jia, X.; Lai, S.J. Favoring Expression of Yak Alleles in Interspecies F1 Hybrids of Cattle and Yak Under High-Altitude Environments. Front. Vet. Sci. 2022, 9, 892663. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Q.; He, Y.; Yang, L.; Zhang, X.; Shi, P.; Yang, L.; Liu, Z.; Zhang, F.; Liu, F.; et al. The Transcriptomic Landscape of Yaks Reveals Molecular Pathways for High Altitude Adaptation. Genome Biol. Evol. 2019, 11, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Durmowicz, A.G.; Hofmeister, S.; Kadyraliev, T.K.; Aldashev, A.A.; Stenmark, K.R. Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J. Appl. Physiol. (1985) 1993, 74, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Mizuno, S.; Sakai, A.; Matsukawa, S.; Kojonazarov, B.; Zamirbek, B.; Umeda, Y.; Morikawa, M.; Anzai, M.; Ishizuka, T.; et al. Blunted Activation of Rho-Kinase in Yak Pulmonary Circulation. BioMed Res. Int. 2015, 2015, 720250. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Fagan, K.A.; Frid, M.G. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ. Res. 2006, 99, 675–691. [Google Scholar] [CrossRef]
- Thompson, A.A.R.; Lawrie, A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol. Med. 2017, 23, 31–45. [Google Scholar] [CrossRef]
- Xin, J.W.; Chai, Z.X.; Zhang, C.F.; Zhang, Q.; Zhu, Y.; Cao, H.W.; YangJi, C.; Chen, X.Y.; Jiang, H.; Zhong, J.C.; et al. Differences in proteomic profiles between yak and three cattle strains provide insights into molecular mechanisms underlying high-altitude adaptation. J. Anim. Physiol. Anim. Nutr. 2022, 106, 485–493. [Google Scholar] [CrossRef]
- Gao, X.; Wang, S.; Wang, Y.F.; Li, S.; Wu, S.X.; Yan, R.G.; Zhang, Y.W.; Wan, R.D.; He, Z.; Song, R.D.; et al. Long read genome assemblies complemented by single cell RNA-sequencing reveal genetic and cellular mechanisms underlying the adaptive evolution of yak. Nat. Commun. 2022, 13, 4887. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, C.; Zhang, C.; Ma, M.; Zhang, F.; Zhang, L.; Chen, Y.; Cao, F.; Li, S.; Zhu, D. Reactive oxygen species effect PASMCs apoptosis via regulation of dynamin-related protein 1 in hypoxic pulmonary hypertension. Histochem. Cell Biol. 2016, 146, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Zhang, Y.; Liu, Q.; Sun, X.; He, X.; Yang, Q.; Yuan, P.; Zhou, X. Suppression of miR-4463 promotes phenotypic switching in VSMCs treated with Ox-LDL. Cell Tissue Res. 2021, 383, 1155–1165. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, N.Y.; Moonen, J.R.; Zhao, Z.; Shi, M.; Otsuki, S.; Wang, L.; Nguyen, T.; Yan, E.; Marciano, D.P.; et al. ALDH1A3 Coordinates Metabolism with Gene Regulation in Pulmonary Arterial Hypertension. Circulation 2021, 143, 2074–2090. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Zemskova, M.; Eccles, C.A.; Varghese, M.V.; Niihori, M.; Barker, N.K.; Luo, M.; Mandarino, L.J.; Langlais, P.R.; Rafikova, O.; et al. Single Mutation in the NFU1 Gene Metabolically Reprograms Pulmonary Artery Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 734–754. [Google Scholar] [CrossRef]
- Owens, G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol. Rev. 1995, 75, 487–517. [Google Scholar] [CrossRef]
- Ruan, C.; Lu, J.; Wang, H.; Ge, Z.; Zhang, C.; Xu, M. miR-26b-5p regulates hypoxia-induced phenotypic switching of vascular smooth muscle cells via the TGF-β/Smad4 signaling pathway. Mol. Med. Rep. 2017, 15, 4185–4190. [Google Scholar] [CrossRef]
- Xu, M.; Pang, Q.; Xu, S.; Ye, C.; Lei, R.; Shen, Y.; Xu, J. Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget 2017, 9, 3188–3197. [Google Scholar] [CrossRef]
- Wang, K.; Wen, D.; Cui, Y.; Zhang, Q.; He, J. Expression Analysis of TGF-B1/Smad Signaling Pathway Related Factors During Lung Hypoxia Adaptation in Yak (Bos grunniens). J. Agric. Biotechnol. 2023, 31, 105–114. [Google Scholar] [CrossRef]
- Wang, X.; Shen, X.; Yan, Y.; Li, H. Pyruvate dehydrogenase kinases (PDKs): An overview toward clinical applications. Biosci. Rep. 2021, 41, BSR20204402. [Google Scholar] [CrossRef]
- McMurtry, M.S.; Bonnet, S.; Wu, X.; Dyck, J.R.; Haromy, A.; Hashimoto, K.; Michelakis, E.D. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ. Res. 2004, 95, 830–840. [Google Scholar] [CrossRef]
- Michelakis, E.D.; McMurtry, M.S.; Wu, X.C.; Dyck, J.R.; Moudgil, R.; Hopkins, T.A.; Lopaschuk, G.D.; Puttagunta, L.; Waite, R.; Archer, S.L. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: Role of increased expression and activity of voltage-gated potassium channels. Circulation 2002, 105, 244–250. [Google Scholar] [CrossRef]
- Hassoun, P.M.; Mouthon, L.; Barberà, J.A.; Eddahibi, S.; Flores, S.C.; Grimminger, F.; Jones, P.L.; Maitland, M.L.; Michelakis, E.D.; Morrell, N.W.; et al. Inflammation, growth factors, and pulmonary vascular remodeling. J. Am. Coll. Cardiol. 2009, 54 (Suppl. S1), S10–S19. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, T.; Wu, W.; Ge, X.; Xiong, X.; Zhang, Z.; Hu, C. LOX-1 mediated phenotypic switching of pulmonary arterial smooth muscle cells contributes to hypoxic pulmonary hypertension. Eur. J. Pharmacol. 2018, 818, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tong, X. Advances in molecular mechanism of vascular remodeling in pulmonary arterial hypertension. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019, 48, 102–110. [Google Scholar] [CrossRef]
- Mao, J.; Liu, C.; Zhang, Y.; Zhang, Q.; Liu, H.; Ma, L. Salidroside inhibits phenotypic transformation of rat pulmonary artery smooth muscle cells induced by hypoxia. China J. Chin. Mater. Medica 2022, 47, 1024–1030. [Google Scholar] [CrossRef]
- Lee, D.C.; Sohn, H.A.; Park, Z.Y.; Oh, S.; Kang, Y.K.; Lee, K.M.; Kang, M.; Jang, Y.J.; Yang, S.J.; Hong, Y.K.; et al. A lactate-induced response to hypoxia. Cell 2015, 161, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, J.; Zheng, L.; Meissl, K.; Chaneton, B.; Selivanov, V.A.; Mackay, G.; van der Burg, S.H.; Verdegaal, E.M.; Cascante, M.; Shlomi, T.; et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 2013, 498, 109–112. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, L.; Zhang, W.; Shi, J.; Lin, X.; Lv, Z.; Zhang, W.; Liang, R.; Jiang, S. MiR-23b controls TGF-β1 induced airway smooth muscle cell proliferation via TGFβR2/p-Smad3 signals. Mol. Immunol. 2016, 70, 84–93. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, W.; Dong, Y.; Hu, J.; Wei, K.; Yang, S.; Lai, X.; Tang, H. Hypoxia induces tumor cell growth and angiogenesis in non-small cell lung carcinoma via the Akt-PDK1-HIF1α-YKL-40 pathway. Transl. Cancer Res. 2020, 9, 2904–2918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mu, Y.; Ding, H.; Du, B.; Zhou, M.; Li, Q.; Gong, S.; Zhang, F.; Geng, D.; Wang, Y. 1α, 25-Dihydroxyvitamin D3 Promotes Angiogenesis After Cerebral Ischemia Injury in Rats by Upregulating the TGF-β/Smad2/3 Signaling Pathway. Front. Cardiovasc. Med. 2022, 9, 769717. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Liu, H.; Liu, J.; Cao, X.; Wu, Q.; Wang, S. Effect of hypoxia on the expression of bFGF, VEGF and the ultrastructural changes of pulmonary artery in rats. J. Chin. High. Alt. Med. Biol. 2017, 038, 164–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M.; Liang, Y.; Li, R.; Zhang, L.; Chen, S.; Yang, K.; Ding, H.; Tan, X.; Zhang, Q.; et al. Study of Transcriptomic Analysis of Yak (Bos grunniens) and Cattle (Bos taurus) Pulmonary Artery Smooth Muscle Cells under Oxygen Concentration Gradients and Differences in Their Lung Histology and Expression of Pyruvate Dehydrogenase Kinase 1-Related Factors. Animals 2023, 13, 3450. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Mi, R.; Liu, F. Progress in the differential study of signaling proteins Smad2 and Smad3 in the TGFβ/Smads pathway. Chin. J. Clin. 2013, 7, 6579–6581. [Google Scholar] [CrossRef]
- Haddadi, N.; Lin, Y.; Simpson, A.M.; Nassif, N.T.; McGowan, E.M. “Dicing and Splicing” Sphingosine Kinase and Relevance to Cancer. Int. J. Mol. Sci. 2017, 18, 1891. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, D.; Shi, H.; Qian, H.; Chen, H.; Zeng, Q.; Jin, F.; Ye, Y.; Ou, Z.; Guo, M.; et al. PDK1 promotes breast cancer progression by enhancing the stability and transcriptional activity of HIF-1α. Genes Dis. 2023, 11, 101041. [Google Scholar] [CrossRef]
- He, G.; Jiang, Y.; Zhang, B.; Wu, G. The effect of HIF-1α on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac. J. Clin. Nutr. 2014, 23, 174–180. [Google Scholar] [CrossRef]
- Gan, D.; Yue, S.; Jiang, Y.; Zhang, D.; Shi, H.; Qian, H.; Zhou, T.; Fang, W.; Yao, M.; Zuo, G.; et al. Nucleus-located PDK1 regulates growth, invasion and migration of breast cancer cells. Life Sci. 2020, 253, 117722. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, H.; Li, Z.; Zhuang, Y.; Liu, Y.; Zhou, S.; Xiao, Y.; Xie, C.; Zhou, F.; Zhou, Y. 2-Methoxyestradiol enhances radiosensitivity in radioresistant melanoma MDA-MB-435R cells by regulating glycolysis via HIF-1α/PDK1 axis. Int. J. Oncol. 2017, 50, 1531–1540. [Google Scholar] [CrossRef]
- Marsboom, G.; Toth, P.T.; Ryan, J.J.; Hong, Z.; Wu, X.; Fang, Y.H.; Thenappan, T.; Piao, L.; Zhang, H.J.; Pogoriler, J.; et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012, 110, 1484–1497. [Google Scholar] [CrossRef]
- Piao, L.; Sidhu, V.K.; Fang, Y.H.; Ryan, J.J.; Parikh, K.S.; Hong, Z.; Toth, P.T.; Morrow, E.; Kutty, S.; Lopaschuk, G.D.; et al. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: Therapeutic benefits of dichloroacetate. J. Mol. Med. 2013, 91, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Kurrey, N.K.; Jalgaonkar, S.P.; Joglekar, A.V.; Ghanate, A.D.; Chaskar, P.D.; Doiphode, R.Y.; Bapat, S.A. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 2009, 27, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Tang, X.; Gao, R.; Kong, L.; Zhang, J.; Qin, W.; Hu, N.; Zhang, A.; Ma, K.; Li, L.; et al. Galectin-3 participates in PASMC migration and proliferation by interacting with TGF-β1. Life Sci. 2021, 274, 119347. [Google Scholar] [CrossRef]
- Wang, J.; Feng, W.; Li, F.; Shi, W.; Zhai, C.; Li, S.; Zhu, Y.; Yan, X.; Wang, Q.; Liu, L.; et al. SphK1/S1P mediates TGF-β1-induced proliferation of pulmonary artery smooth muscle cells and its potential mechanisms. Pulm. Circ. 2019, 9, 2045894018816977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, Y.; Qin, A.; Li, R.; Zhang, Y.; Chen, S.; Xu, J.; Qiao, Z.; Yang, K. Proteomic Analysis for Yaks PASMCs under Hypoxia and Normoxia Conditions Based on TMT technique. Acta Vet. Zootech. Sin. 2022, 53, 2182–2193. [Google Scholar] [CrossRef]




| Component Name | System (μL) |
|---|---|
| virus volume | (MOI × cell number)/viral titer |
| HitranG P Infection Enhancement Solution | 20 |
| complete medium (10% FBS DME/F12) | - |
| Total | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, J.; Zhang, M.; Li, X.; Zhang, F.; Zhou, M.; Yang, K.; Chen, W.; Ding, H.; Tan, X.; et al. Study on the Regulatory Mechanism of the PDK1-Mediated TGF-β/Smad Signaling Pathway in Hypoxia-Induced Yak Lungs. Animals 2024, 14, 2422. https://doi.org/10.3390/ani14162422
Zhang Y, Wang J, Zhang M, Li X, Zhang F, Zhou M, Yang K, Chen W, Ding H, Tan X, et al. Study on the Regulatory Mechanism of the PDK1-Mediated TGF-β/Smad Signaling Pathway in Hypoxia-Induced Yak Lungs. Animals. 2024; 14(16):2422. https://doi.org/10.3390/ani14162422
Chicago/Turabian StyleZhang, Yiyang, Jun Wang, Meng Zhang, Xiaoyun Li, Fan Zhang, Manlin Zhou, Kun Yang, Weiji Chen, Haie Ding, Xiao Tan, and et al. 2024. "Study on the Regulatory Mechanism of the PDK1-Mediated TGF-β/Smad Signaling Pathway in Hypoxia-Induced Yak Lungs" Animals 14, no. 16: 2422. https://doi.org/10.3390/ani14162422
APA StyleZhang, Y., Wang, J., Zhang, M., Li, X., Zhang, F., Zhou, M., Yang, K., Chen, W., Ding, H., Tan, X., Zhang, Q., & Qiao, Z. (2024). Study on the Regulatory Mechanism of the PDK1-Mediated TGF-β/Smad Signaling Pathway in Hypoxia-Induced Yak Lungs. Animals, 14(16), 2422. https://doi.org/10.3390/ani14162422





