Simple Summary
Fish meal (FM) is considered the primary protein source in fish feed, but the most expensive ingredient, which ultimately increases feed cost. Application of a substitute for FM in fish feed can lower feed cost, but it commonly leads to reduced palatability and lowered feed consumption of fish. Jack mackerel meal (JMM) has been reported to be an effective attractant and/or stimulant in the olive flounder diet. This study revealed that inclusion of 50% jack mackerel meal (JMM) at the expense of FM in the olive flounder (Paralichthys olivaceus) diet replacing 50% FM with duck by-product meal (DBM) lowered feed cost, but improved feed consumption and growth performance and economic return to the farmer. The results of this study will help olive flounder producers to improve their profitability.
Abstract
This experiment was performed to evaluate the inclusion impact of various levels of jack mackerel meal (JMM) in olive flounder (P. olivaceus) feeds substituting 50% FM by duck by-product meal (DBM) on growth, feed availability, and economic efficiency. Seven experimental diets were prepared. The control (Con) diet contained 60% FM. Fifty percent FM in the Con diet was substituted with DBM, and then the graded levels (0%, 10%, 20%, 30%, 40%, and 50%) of JMM were added instead of FM, named the DJ0, DJ10, DJ20, DJ30, DJ40, and DJ50 diets, respectively. All feeds were assigned to triplicate fish groups. At the end of 56 days’ feeding, fish fed the DJ40 and DJ50 diets exhibited comparable weight gain and specific growth rate to fish fed the Con diet. Higher feed consumption was observed in fish fed the Con, DJ40, and DJ50 diets compared to fish fed the DJ0 and DJ10 diets. Lower feed conversion ratio was observed in fish fed the Con diet compared to fish fed the DJ0, DJ10, DJ20, and DJ30 diets. Furthermore, the DJ50 diet led to the highest economic profit index (EPI). In conclusion, inclusion of 50% JMM in the olive flounder diet replacing 50% FM with DBM seems to be the most recommendable dietary treatment based on growth and feed consumption of olive flounder and EPI.
1. Introduction
The Republic of Korea is one of the leading aquacultural countries globally [1]. Marine finfish production in South Korea is dominated by olive flounder (Paralichthys olivaceus), and contributed to an annual production of 45,801 metric tons and an economic value of USD 386 million in 2022 [2]. In a land-based intensive olive flounder production system, the widespread use of raw fish-based moist pellets (MP) results in high production cost, nutrient loss, water pollution, disease outbreak, and mortality [3]. The utilization of formulated feed (FF) including extruded pellets has proven to be more environmentally-friend than MP, leading to elevated growth and nutrient utilization of fish [4]. However, FF for olive flounder relies heavily on fish meal (FM), containing up to 60% of the primary protein source [5]. The increasing demand and decreasing production of FM over time have contributed to rising its cost, prompting scientists to look for an alternative to FM in aquafeeds [6].
Various plant protein sources, including microalgae [7], macroalgae [8], dried grain from rice distillers [9], and soybean and cottonseed meal [10], have been explored as replacements for FM in olive flounder diets because of their sufficient protein content, affordable price, and year-round availability. However, challenges such as high fiber content, amino acid (AA) imbalances, and the existence of antinutritional factors commonly limit the extensive application of plant proteins in fish diets because of reduced palatability and feed consumption and compromised growth rate of fish [11,12]. Therefore, animal protein sources are favored over plant protein sources in fish diets due to their abundant AA and fatty acid (FA) profiles [13]. It has been reported that 10–50% FM can be replaced with alternative animal protein sources, such as silkworm pupae meal [14], chicken by-product meal [15], meat meal [16], meat and bone meal, and tuna by-product meal [17], without undesirable impacts on the growth or feed utilization of olive flounder.
Duck by-product meal (DBM) can be considered a prospective alternative for FM in fish feed. DBM is clean, dried, and ground duck tissue including skin, bone, head, feet, feathers, and blood sourced from duck processing plants where ducks are slaughtered for human consumption [18]. In 2021, global production of duck meat was 6.2 million metric tons. South Korea is considered one of the top duck meat producers, with production of 74,968 metric tons [19]. During duck processing, several thousand metric tons of organic by-products are being produced from the processing plant. DBM, an inexpensive ingredient but rich in protein and lipid, shows a high possibility for use as an FM replacement in the olive flounder diet. However, reduced feed palatability and feed consumption are the common concerns when FM is substituted with alternative protein sources in the olive flounder diet [16]. Therefore, inclusion of protein ingredient with feed attractant and stimulant effect in low-FM diets is one of the best methods to resolve those undesirable problems.
Feed attractant and stimulant are usually low-molecular-weight compounds, such as free AA, nucleosides, nucleotides, organic acids, and quaternary ammonium (NH4) bases, which are added to feed to enhance palatability [20]. Furthermore, incorporating feed attractants in diet facilitates faster feed ingestion and provides supplemental nutrients for protein and energy metabolism [21]. Both natural and synthetic stimulants are used in fish feed formulations. However, the absence of certain effective components in the synthetic stimulant renders them inferior to natural stimulant [22]. Carr et al. [23] emphasized that the tissue extracts of marine organisms contain natural stimulants and identified low-molecular-weight components from 30 species of marine fish, including jack mackerel (Trachurus japonicus), mollusks, and crustaceans. Jack mackerel meal (JMM) has been demonstrated to be a significant attractant and/or stimulant in various fish species, such as olive flounder [24], rockfish (Sebastes schlegeli) [25,26], and yellowtail (Seirola quinqueradiata) [27]. Furthermore, Ikeda et al. [28] and Takakuwa et al. [29] revealed that AA groups, particularly histidine and nucleotides, and inosine monophosphate (IMP) in the muscle extracts of jack mackerel showed the highest feed stimulant activity on olive flounder and greater amberjack (Seriola dumerili), respectively. Kim et al. [30] also revealed that among 16 protein ingredients, JMM showed the strongest feed attractiveness to rockfish. Inclusion of JMM in formulating low-FM diets can be a sustainable fish culture technique in increasing feed consumption and growth performance of fish.
Therefore, the present experiment was performed to elucidate the inclusion effect of graded levels of JMM in the low-FM diets of olive flounder replacing 50% FM with DBM on the growth and feed availability of olive flounder and to assess economic efficiency.
2. Materials and Methods
2.1. Experimental Diet Preparation
Seven diets with isonitrogenous content of 52.0% and isolipidic of 13.5% were prepared (Table 1). The primary protein sources in the control (Con) diet were FM (60%) and fermented soybean meal (10%). In addition, wheat flower (22.5%), and each of fish and soybean oil (2.5%) were used as the carbohydrate and lipid source, respectively, in the Con diet. Fifty percent FM in the Con diet was substituted with DBM and then the graded levels (0%, 10%, 20%, 30%, 40%, and 50%) of JMM were included at the coast of FM, referred as the DJ0, DJ10, DJ20, DJ30, DJ40, and DJ50 diets, respectively. After thoroughly mixing the ingredients of each diet, water was added at a ratio of 3:1 to form a dough. After considering the mouth size of olive flounder, the dough was then pelletized (4−6 mm in diameter) using a laboratory extruder. Finally, after drying at 30 °C in a forced-air oven for 48 h, all the experimental diets were stored at −20 °C until further use.

Table 1.
Ingredients and chemical composition of the experimental diets (%, dry matter basis).
2.2. Experimental Conditions
Healthy juvenile olive flounder of similar sizes were bought from a private fish farm and transported to the laboratory. Prior to the feeding experiment, all the fish were acclimatized to the rearing conditions for 2 weeks by providing a commercial pellet twice a day at a biomass ratio of 1.5–3%. Total of 525 juvenile fish (initial weight of 20.27 ± 0.03 g; mean ± SEM) were randomly distributed into 21 50 L flow-through tanks (53.4 × 34.0 × 27.4 cm) (25 fish/tank). Fifteen fish were stocked into each tank, and then the remaining ten fish were added to adjust to the same initial total weight of fish per tank. Each tank received sand-filtered seawater at a flow rate of 4.2 L/min and continuous aeration. A multifunctional water quality meter (AZ-8603, AZ Instrument, Taichung city, China) was used daily to monitor water quality. The temperature, dissolved oxygen, salinity, and pH ranged from 16.3 to 21.6 °C (19.7 °C ± 1.59 °C; mean ± SD), 7.3–8.0 mg/L (7.5 ± 0.22 mg/L), 30.8–32.2 g/L (31.3 ± 0.38 g/L), and 7.4–7.7 (7.5 ± 0.10), respectively.
Each formulated diet was assigned to triplicated groups of fish. Throughout the 56-day feeding trial, olive flounder were carefully hand-fed to apparent satiation twice a day (08:30 and 17:30). The bottoms of the tanks were cleaned by siphoning daily after feeding in the morning, and the photoperiod followed the natural cycle. A daily feed supply to each tank was recorded and uneaten feed was not collected. Dead fish were removed immediately upon discovery and weighed. The feeding trial and subsequent handling and sampling of experimental fish were carried out as per the ethical guidelines of the Korea Maritime and Ocean University.
2.3. Measurement of the Biological Indices of Fish
After the 56-day feeding trial, all surviving fish were anesthetized with MS-222 at a concentration of 100 ppm, followed by 24 h starvation. All live fish from each tank were counted to calculate the survival rate and their collective weight was measured to determine weight gain. Ten randomly selected anesthetized fish from each tank were individually weighed, measured in total length, and then dissected to collect the viscera and liver for calculating the viscerosomatic index (VSI) and hepatosomatic index (HSI). The growth performance, feed utilization, and biological indices of olive flounder were calculated as the following [31]: specific growth rate (SGR, %/day) = (Ln final weight of fish − Ln initial weight of fish) × 100/days of feeding (56 days), feed conversion ratio (FCR) = feed supplied/weight gain of fish, protein efficiency ratio (PER) = weight gain of fish/protein supplied, protein retention (PR, %) = (final body protein − initial body protein) × 100/protein supplied, condition factor (K, g/cm3) = body weight of fish (g) × 100/total length of fish (cm)3, VSI (%) = viscera weight of fish × 100/body weight of fish, and HSI (%) = liver weight of fish × 100/body weight of fish.
2.4. Measurements of the Biochemical Composition of the Samples
Ten fish at the beginning of the trial and ten fish from each tank after the measurements of biological indices were homogenized and used for the proximate composition analysis. Chemical analyses for the experimental feeds and fish were performed according to the standard AOAC [32] method. Crude protein content was determined by a Kjeldahl apparatus (Kjeltec 2100 Distillation Unit, Foss Tecator, Hoganas, Sweden), and crude lipid content was determined by ether-extraction method (Soxtec TM 2043 Fat Extraction System, Foss Tecator, Hoganas, Sweden). Moisture content was determined by oven-drying at 105 °C for 24 h, and ash content was determined by using a muffle furnace at 550 °C for 4 h. To analyze AA, excluding methionine, cysteine, and tryptophan, the experimental feeds and whole-body fish were hydrolyzed with 6 N HCl for 24 h at 110 °C followed by ion exchange chromatography with an AA analyzer (L-8800 Auto-analyzer: Hitachi, Tokyo, Japan). To measure methionine and cysteine content, the samples were oxidized with performic acid at below 5 °C for 24 h to obtain methionine sulfone and cysteic acid, and they were then freeze-dried twice with deionized water. Then, the freeze-dried samples were hydrolysed and analyzed following similar process used for the other amino acids. Tryptophan analysis was conducted using high-performance liquid chromatography (S1125 HPLC pump system; Sykam GmbH, Eresing, Germany). Lipids for FA analyses in the feeds and whole-body fish were extracted using a mixture of chloroform and methanol (2:1 v/v), following the method of Folch et al. [33]. FA methyl esters were prepared by transesterification with 14% BF3-MeOH and analyzed by gas chromatography (Trace GC, Thermo, Waltham, MA, USA).
2.5. Analysis of Plasma and Serum Measurements of Fish
Blood was drawn from the caudal veins of five anesthetized fish from each tank using heparinized syringes. The plasma was then extracted and kept in separate aliquots in a freezer at −70 °C after centrifugation (2716× g at 4 °C) for 10 min. An automated chemistry system (Fuji Dri-Chem NX500i, Fujifilm, Tokyo, Japan) was utilized to analyze aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total bilirubin (T-BIL), total cholesterol (T-CHO), total protein (TP), triglyceride (TG), and albumin (ALB). Plasma samples of fish from each tank were pooled.
In addition, blood was drawn from five anesthetized fish from each tank using syringes. The serum was extracted and kept in separate aliquots in a freezer at −70 °C after centrifugation (2716× g at 4 °C) for 10 min. Serum lysozyme activity was measured using the turbidimetric assay as per Lange et al. [34], and superoxide dismutase (SOD) was measured using a commercial SOD Assay kit (Sigma MBS705758; Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions.
2.6. Economic Analysis of the Study
The economic assessment of the experiment was performed by applying the formula proposed by Martínez-Llorens et al. [35]: economic conversion ratio (ECR, USD/kg) = feed consumption of fish (kg) × feed cost (USD/kg)/weight gain (kg), and economic profit index (EPI, USD/fish) = (final weight of fish (kg/fish) × selling price of fish (USD/kg)) − (feed consumption of fish (kg) × diet price (USD/kg)). The cost per kilogram (USD/kg) for each ingredient was as follows: FM = 2.23, DBM = 0.60, JMM = 2.67, fermented soybean meal = 0.70, wheat flour = 0.55, fish oil = 2.76, soybean oil = 1.79, vitamin premix = 8.28, mineral premix = 6.66, and choline = 1.30. The selling price of olive flounder was assumed as USD 12.44 /kg.
2.7. Statistical Analysis
Significant differences in means were examined using one-way ANOVA and Tukey’s post hoc test after the normality (Shapiro–Wilk) and homogeneity (Levene) tests on SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Percentage data underwent arcsine transformation prior to statistical analysis. Additionally, a follow-up trend analysis using orthogonal polynomial contrasts excluding the Con diet was conducted to evaluate whether the effect demonstrated linear, quadratic, or cubic trends. Statistical significance level was set at p < 0.05. Furthermore, regression analysis was undertaken to identify the best-fitting model.
3. Results
3.1. AA and FA Profiles of the Feeds
All essential AAs (EAAs) except for methionine and total content of EAA (∑EAA) in FM were relatively low compared to those in JMM, but higher than those in DBM, except for arginine content (Table 2). Leucine and lysine as well as aspartic acid and glutamic acid were the richest EAA and NEAA, respectively, in all FM, JMM, and DBM. Increased inclusion levels of JMM in the low-FM diets tended to increase all EAA content, except for phenylalanine.

Table 2.
Amino acid profiles (% of the diet) of feed ingredients and the experimental diets.
FM exhibited higher total content of saturated fatty acid (∑SFA), but lower total content of monounsaturated FA (∑MUFA) and lower total content of n-3 highly unsaturated FA (∑n-3 HUFA) including eicosapentaenoic acid (EPA, C20:5n-3) and docosahexaenoic acid (DHA, C22:6n-3) compared to JMM (Table 3). However, DBM exhibited higher ∑MUFA compared to both FM and JMM, but lower for EPA, DHA, and ∑n-3 HUFA (Table 3). Notably, elevated inclusion levels of JMM in the low-FM diets substituting 50% FM with DBM led to decreased ∑SFA, but increased ∑MUFA and ∑n-3 HUFA.

Table 3.
Fatty acid profiles (% of total fatty acids) of feed ingredients and the experimental diets.
3.2. Growth and Feed Availability of Fish
Survival of fish varied between 94.67% and 97.33% and was not significantly (p > 0.05) changed by dietary treatments (Table 4). Weight gain increased in fish fed the Con and DJ50 diets compared to fish fed the DJ0, DJ10, DJ20, and DJ30 diets, but was comparable to fish fed the DJ40 diet (Figure 1) (p < 0.001). Accordingly, SGR increased in fish fed the Con diet when compared to fish fed the DJ0, DJ10, DJ20, and DJ30 diets, but was comparable to fish fed the DJ40 and DJ50 diets (Figure 2) (p < 0.001). In orthogonal polynomial contrast, significant linear relationships (p = 0.001 for both) were observed in weight gain and SGR of olive flounder and inclusion levels of JMM in the low-FM diets. Regression analysis indicated linear relationships as the best-fit models between inclusion levels of JMM in the low-FM duets replacing 50% FM by DBM and weight gain (Y = 1.166689X + 48.4182, p < 0.001, R2 = 0.8524) and SGR (Y = 0.027709X + 2.1858, p < 0.001, R2 = 0.8237), respectively.

Table 4.
Survival (%), feed efficiency (FE), protein efficiency ratio (PER), protein retention (PR), condition factor (K), viscerosomatic index (VSI), and hepatosomatic index (HSI) of olive flounder fed the experimental diets for 56 days.
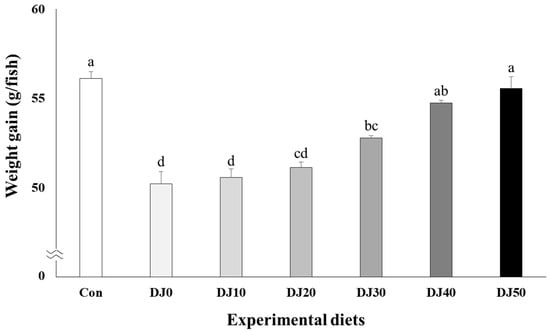
Figure 1.
Weight gain (g/fish) of olive flounder (Paralichthys olivaceus) fed the experimental diets for 56 days (mean of triplicate ± SE) (p < 0.001). Con: 60% FM; DJ0–DJ50: 50% DBM with 0% to 50% JMM. (Orthogonal polynomial contrast (linear; p = 0.001, quadratic; p = 0.080, cubic; p = 0.156); Y = 1.166689X + 48.4182, p < 0.001, R2 = 0.8524).
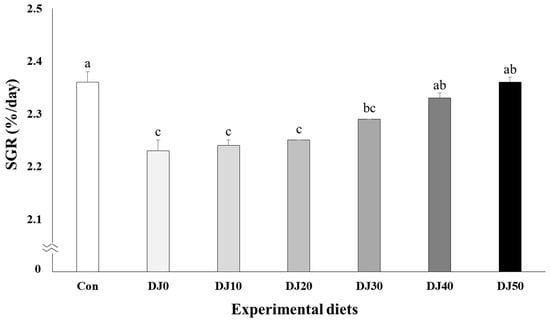
Figure 2.
Specific growth rate (SGR, %/day) of olive flounder (Paralichthys olivaceus) fed the experimental diets for 56 days (mean of triplicate ± SE) (p < 0.001). Con: 60% FM; DJ0–DJ50: 50% DBM with 0% to 50% JMM. (Orthogonal polynomial contrast (linear; p = 0.001, quadratic; p = 0.177, cubic; p = 0.334); Y = 0.027709X + 2.1858, p < 0.001, R2 = 0.8237).
Higher feed consumption was observed in fish fed the Con, DJ40, and DJ50 diets compared to olive flounder fed the DJ0 and DJ10 diets, but comparable to fish fed the DJ20 and DJ30 diets (Figure 3) (p < 0.001). In orthogonal polynomial contrast, a significant linear relationship (p = 0.001) was observed in feed consumption and inclusion levels of JMM in the low-FM feeds. Regression analysis indicated the linear model was the best fit between inclusion levels of JMM in the low-FM feeds and feed consumption of fish (Y = 0.929921X + 49.2673, p < 0.0001, R2 = 0.7368).
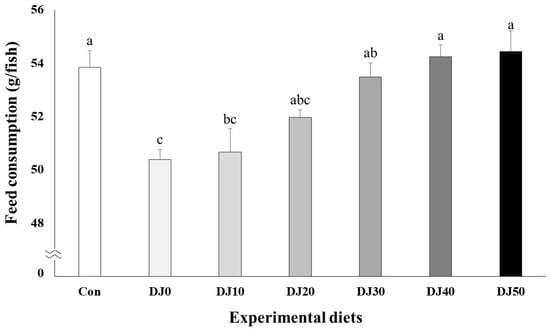
Figure 3.
Feed consumption (g/fish) of olive flounder (Paralichthys olivaceus) fed the experimental diets for 56 days (mean of triplicate ± SE) (p < 0.001). Con: 60% FM; DJ0–DJ50: 50% DBM with 0% to 50% JMM. (Orthogonal polynomial contrast (linear; p = 0.001, quadratic; p = 0.638, cubic; p = 0.195); Y = 0.929921X + 49.2673, p < 0.001, R2 = 0.7368).
Significantly a greater FCR was found in fish fed the Con diet compared to olive flounder fed the DJ0, DJ10, DJ20, and DJ30 diets (p < 0.003), but comparable to olive flounder fed the DJ40 and DJ50 diets. In orthogonal polynomial contrast, significant linear (p = 0.050) and quadratic (p = 0.026) relationships were observed in FCR and inclusion levels of JMM in the low-FM feeds. Regression analysis indicated the quadratic model (p < 0.012 and adjusted R2 = 0.368) to be the best fit between inclusion levels of JMM in the low-FM feeds and FCR. PER of olive flounder fed the DJ50 diet was significantly (p < 0.005) higher than that of olive flounder fed the DJ0 and DJ10 diets, but not significantly (p > 0.05) different from that of olive flounder fed the Con, DJ20, DJ30, or DJ40 diets. In orthogonal polynomial contrast, a significant linear relationship (p = 0.001) was observed in PER and inclusion levels of JMM in the low-FM feeds. Regression analysis indicated a linear model (p < 0.001 and adjusted R2 = 0.533) to be the best fit between inclusion levels of JMM in the low-FM feeds and PER. PR of fish was not significantly (p > 0.142) changed by dietary treatments.
However, K of fish varied from 0.84 to 0.91 g/cm3, VSI varied from 4.14% to 4.63%, and HIS varied from 1.09% to 1.43%, but these values were not significantly (p > 0.05 for all) affected by dietary treatments.
3.3. Proximate Composition of the Whole-Body Fish
Moisture, crude protein, crude lipid, and ash content of the whole-body fish ranged from 74.63% to 75.63%, 16.10% to 17.47%, 2.80% to 3.70%, and 3.27% to 4.47%, respectively (Table 5). None of these measurements was significantly (p > 0.05 for all) influenced by dietary treatments.

Table 5.
Chemical composition (%, wet weight) of whole-body olive flounder at the end of the 56-day feeding trial.
3.4. Plasma and Serum Measurements of Fish
Plasma AST, ALT, ALP, T-BIL, T-CHO, TG, TP, and ALB level of fish varied from 8.33 to 10.67 U/L, 4.67 to 6.00 U/L, 70.00 to 73.67 U/L, 0.13 to 0.23 mg/dL, 179.00 to 181.67 mg/dL, 438.3 to 443.33 mg/dL, 1.73 to 2.33 g/dL, and 0.43 to 0.63 g/dL, respectively (Table 6). The experimental diets did not (p > 0.05 for all) alter these parameters. Serum SOD ranged from 2.57 to 3.12 ng/L and lysozyme activity of fish ranged from 296.67 to 546.67 U/mL. The experimental diets did not significantly (p > 0.815 and p > 0.878, respectively) affect the serum SOD or lysozyme activity of fish.

Table 6.
Plasma and serum parameters of olive flounder at the end of the 56-day feeding trial.
3.5. AA and FA Profiles of the Whole-Body Fish
The experimental diets did not show any significant impact on the AA profiles of the whole-body olive flounder (Table 7) (p > 0.05).

Table 7.
Amino acid profiles (% of wet weight) of the whole-body of olive flounder fed the experimental diets for 56 days.
However, significantly higher ∑SFA, EPA, DHA, and ∑n-3 HUFA content were found in the whole-body olive flounder fed the Con feed than those of olive flounder fed all other feeds (Table 8) (p < 0.001 for all). In orthogonal polynomial contrast, significant linear (p = 0.002 and p = 0.001, respectively) relationships were observed in the ∑SFA and ∑MUFA in the whole-body olive flounder and inclusion levels of JMM in the low-FM diets. However, significant linear (p = 0.000), quadratic (p = 0.001), and cubic (p = 0.001) relationships were observed in the ∑n-3 HUFA in the whole-body of olive fish and inclusion levels of JMM in the low-FM diets. In regression analysis, the linear model (p < 0.001; adjusted R2 = 0.927 and p < 0.003; adjusted R2 = 0.891, respectively) was found to be the best fit model between inclusion levels of JMM in the low-FM diets and the ∑SFA and ∑MUFA of olive flounder and. However, the cubic (p < 0.001 and adjusted R2 = 0.964) model was found to be the best fit model between inclusion levels of JMM in the low-FM diets and the ∑n-3 HUFA of fish in regression analysis.

Table 8.
Fatty acid profiles (% of total fatty acids) of the whole body of olive flounder fed the experimental diets for 56 days.
3.6. Economic Analysis of the Experiment
The prices of low-FM diets increased with JMM inclusion levels, but their prices were all still much lower than that of the Con diet (Table 9). The ECR of the Con diet was significantly (p < 0.001) higher than that of all FM-replaced feeds. However, no significant (p > 0.05) difference in ECR was found among the DJ20, DJ30, DJ40, and DJ50 diets. In orthogonal polynomial contrast, significant linear (p = 0.001) and quadratic (p = 0.030) relationships were observed in ECR and inclusion levels of JMM in the low-FM feeds. In regression analysis, the quadratic model (p < 0.001 and adjusted R2 = 0.732) was found to be the best fit between inclusion levels of JMM in the low-FM feeds and ECR. Superior (p < 0.001) EPI was obtained in the DJ40 and TJ50 diets compared to the DJ0, DJ10, DJ20, and DJ30 diets, but comparable to the Con diet. In orthogonal polynomial contrast, significant linear (p = 0.000) and quadratic (p = 0.045) relationships were observed in EPI and inclusion levels of JMM in the low-FM feeds. In regression analysis, the linear model (P < 0.001 and adjusted R2 = 0.812) was found to be the best fit between inclusion levels of JMM in the low-FM feeds and EPI.

Table 9.
Effect of dietary replacement of fish meal (FM) with duck by-product meal (DBM) supplemented with the graded levels of jack mackerel meal (JMM) on economic parameters of the study.
4. Discussion
No significant differences in weight gain or SGR of olive flounder fed the DJ40 and DJ50 diets compared to fish fed the Con diet in this experiment implied that 50% of FM could be replaced with DBM without negatively affecting the growth performance of fish, as long as 40–50% of JMM is included at the expense of FM in a 60% FM-based diet. Nevertheless, linear increase in weight gain and SGR of fish with increased JMM inclusion levels in low-FM diets in regression analysis indicated that the DJ50 diet appeared to be the most recommendable feeding strategy according to the growth performance of olive flounder. Furthermore, inferior ECR in all DJ diets compared to the 60% FM-based diet appeared to be more feasible than the Con diet in this experiment because of the lower price of DBM than FM. In particular, the highest EPI, representing the greatest economic return to the farmer, was observed in the DJ50 diet based on the economic analysis (Table 9). This also supports the finding of this study that the DJ50 diet was the most desirable dietary treatment based on the results of weight gain and SGR of fish in regression analysis. However, inferior weight gain and SGR of olive flounder fed the DJ0 diet in contrast to olive flounder fed the Con diet implied that 50% FM substitution with DBM in a diet without JMM inclusion could not catch up with the growth of olive flounder fed the 60% FM-basal diet. However, the gradual improvement in growth performance of fish fed the low-FM diets replacing 50% FM by DBM with increased JMM inclusion levels proved that inclusion of JMM in low-FM diets effectively boosted the growth performance.
Enhanced growth performance of fish appeared to be proportional to enhanced feed consumption in all DJ diets in this study. Linear increases in feed consumption of olive flounder fed the low-FM diets with increased JMM inclusion levels were probably because of the feed-enhancing effect of JMM, indicating that 50% JMM inclusion is the most recommended feeding strategy in low-FM diets substituting 50% FM with DBM. This desirable effect might be attributed to the relatively high levels of EAA, except for methionine, and NEAA present in JMM over FM. Likewise, previous studies have also reported an increase in feed consumption of rockfish (Sebastes schlegeli) and olive flounder when JMM was introduced as the feed enhancer and/or stimulant in low-FM diets [26,31]. The feeding response of fish is influenced by two primary chemoreception channels: olfaction, being responsible for smell and location, and gustation, being responsible for taste or consumption [41,42]. Some AAs, including lysine, methionine, glycine, alanine, and proline, are the major classes of olfactory and gustatory feeding stimulants for fish [12,20]. Furthermore, the studies performed by Takakuwa et al. [29] and Ikeda et al. [28] pointed out that muscle extracts of jack mackerel are an abundant source of AAs and nucleotides, which exhibit a feeding-stimulatory effect on fish. The AAs and nucleotides possess potent chemosensory capabilities and contribute significant flavor and taste in fish diets [43]. The incorporation of attractants into feed not only enables quicker access to feed but also creates conditions for faster ingestion [20].
FCR tended to decrease however, PER of olive flounder tended to improve with increasing JMM inclusion levels in low-FM diets in this experiment. This finding aligns with Kikuchi’s [44] study, in which the maximum weight gain, FE, and PER were reported in olive flounder fed a low-FM diet supplemented with 5% blue mussel meat as a feed stimulant in a 75% FM-based diet. Tharaka et al. [45] and Khosravi et al. [46] also reported improvements in the growth rate, feed consumption, and PER of olive flounder fed diets incorporated with protein hydrolysates (tilapia, shrimp, and krill hydrolysates) and a low-FM diet supplemented with krill meal, respectively. Contrary to this study, however, increased JMM inclusion as feed stimulant up to 100% in the low-FM diets did not change PER of olive flounder [24,31]. This discrepancy in the impact of JMM inclusion on feed utilization of olive flounder could potentially be attributed to differences in feed formulation, protein sources, nutritional profiles including AAs, and the types and doses of stimulants used.
Somatic indices, such as K, HIS, and VSI, are used to evaluate the health condition of fish [47]. In this experiment, these indices of fish were not changed by dietary treatments. This agrees with Kim et al.’s [48] report, where dietary FM replacement with different animal by-products did not influence these indices in olive flounder. Moreover, supplementation with crude attractants (10% fish soluble, 5% squid meal, 5% krill meal, and their mixture) and marine protein hydrolysates as feed enhancers in low-FM diets had no impacts on K, VSI, or HSI of red sea bream (Pagrus major) [49,50].
AAs are essential precursors of vital biomolecules (antibodies, enzymes, hormones, and nucleotides), and deficiencies in EAAs might affect fish growth, feed utilization, immunity, survivability, and many other physiological process [39]. Therefore, the AA profiles of a diet are highly crucial in preparing low-FM feeds. The requirements for arginine (2.04–2.10%), lysine (1.55–1.97%), and threonine (1.03%) for olive flounder were met in all formulated feeds in the present experiment [36,37,39]. However, the methionine content in all experimental feeds including the Con diet seemed to be slightly lower than the requirement (1.44–1.49%) in the presence of 0.06% cysteine for olive flounder [38]. Since cysteine can spare the methionine requirement in fish diets, which has been reported to be 50% and 60% in the diets of red drum (Sciaenops ocellatus) and channel catfish (Ictalurus punctatus), respectively [51,52], it is assumed that the growth of olive flounder was not negatively affected by slightly low methionine content in the experimental feeds due to the presence of high amounts of cysteine (0.59–0.67%) in this experiment.
Long-chain n-3 HUFA including DHA and EPA are considered indispensable FAs for appropriate growth and development of olive flounder [53]. They must be supplied through diets because farmed fish have limited or no capacity to synthesize them in their bodies [54]. The ∑n-3 HUFA in the Con, DJ40, and DJ50 diets met ∑n-3 HUFA requirements in the feed of olive flounder (5.80–7.25% of total FA) [40]. This likely explains why the fish fed DJ0, DJ10, DJ20, and DJ30 diets showed reduced growth performance compared to fish fed the Con diet in this experiment. Higher ∑SFA, and ∑n-3 HUFA, but lower ∑MUFA content in the whole body of fish fed the Con diet were attained based on FA profiles of the experimental feeds in this experiment. These findings are supported by other studies [16,48] showing that dietary FA profiles were mirrored in the whole-body FA profiles of fish.
Plasma measurements are strongly correlated with the health, nutritional status, and environmental condition of fish and can reveal the physiological and metabolic status of fish [55]. No distinctive changes in plasma parameters in this study indicates that olive flounder were in similar nutritional and physiological conditions. Similarly, incorporated protein hydrolysates in feeds did not affect the plasma parameters of red sea bream [56] or olive flounder, except for ALT [46].
Serum SOD and lysozyme are important defense enzymes that play significant roles in detoxifying free radicals during oxidative stress conditions and in lysing the bacterial cell wall during bacterial invasion, respectively [57]. In this experiment, no significant differences in serum SOD or lysozyme activity of fish were found. This is consistent with previous studies, where no significant difference in serum SOD or lysozyme activity in olive flounder were observed following dietary replacement of fermented tuna by-product meal [58], chicken by-product meal [15], and meat meal [16] for FM. However, in contradiction to this study, Tharaka et al. [45] observed an improvement in serum SOD and lysozyme activity in olive flounder fed low-FM diets supplemented with Antarctic krill (Euphausia superba) meal, probably due to the presence of chitin, phospholipid, and astaxanthin, which have an immunostimulatory effect.
The chemical composition and AA profile of the whole-body olive flounder were not affected by dietary treatments in the present experiment. Likewise, dietary substitution of fish meal with chicken by-product meal up to 50% level did not alter the carcass composition or AA profile of olive flounder [15], and total substitution of FM with poultry by-product meal did not alter the muscle AA profile of juvenile gilthead seabream (Sparus aurata) [59], except tyrosine and threonine. The incorporation of protein hydrolysates in low-FM diets [46] or different AA patterns in the experimental feeds [36] did not change the proximate composition or whole-body AA profile of olive flounder. Likewise, replacing FM with animal by-product meal [48] and meat meal produced from pig [16] caused no changes in the whole-body chemical composition or AA profile of olive flounder either. Nevertheless, there are also some contradicting studies, where FM replacements with animal and plant proteins affected whole-body proximate composition [14,17] and AA profiles [60,61] of olive flounder.
5. Conclusions
Inclusion of JMM in low-FM diets replacing 50% FM with DBM improved weight gain, SGR, and feed consumption of olive flounder. Comparable weight gain, SGR, and feed intake were obtained in fish fed the DJ40 and DJ50 diets compared to fish fed the Con (60% FM-based) diet. Furthermore, the DJ50 diet led to the highest EPI. Therefore, inclusion of 50% JMM in low-FM feed replacing 50% FM with DBM is the most recommendable treatment based on growth and feed intake of olive flounder and economic return to the farmer.
Author Contributions
M.R.I. contributed to carrying out the investigation, writing—original draft, and data curation. S.H.C. contributed to conceptualization, methodology, writing—review and editing, project administration, supervision, and funding acquisition. T.K. contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2020R1A2C1009903). This research was also supported by the Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (RS-2018-KS181194).
Institutional Review Board Statement
The feeding trial and subsequent handling and sampling of experimental fish were carried out as per the ethical guideline of the Korea Maritime and Ocean University (KMOU IACUC 2022-04).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the conclusion of this article will be made available on request from the authors.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Rome. 2020. Available online: https://doi.org/10.4060/ca9229en (accessed on 20 February 2023).
- KOSIS. Korean Statistical Information Service, Korea. 2023. Available online: https://kosis.kr/eng/ (accessed on 25 February 2022).
- Cha, S.; Lee, J.; Song, C.; Lee, K.; Jeon, Y. Effects of chitosan-coated diet on improving water quality and innate immunity in the olive flounder, Paralichthys olivaceus. Aquaculture 2008, 278, 110–118. [Google Scholar] [CrossRef]
- Kim, J.-D.; Shin, S.-H. Growth, feed utilization and nutrient retention of juvenile olive flounder (Paralichthys olivaceus) fed moist, semi-moist and extruded diets. Asian-Australas. J. Anim. Sci. 2006, 19, 720–726. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Won, S.; Lee, S.; Lee, S.; Farris, N.W.; Bai, S.C. Nutrition and feeding of olive flounder Paralichthys olivaceus: A review. Rev. Fish. Sci. Aquac. 2020, 28, 340–357. [Google Scholar] [CrossRef]
- Han, D.; Shan, X.; Zhang, W.; Chen, Y.; Wang, Q.; Li, Z.; Zhang, G.; Xu, P.; Li, J.; Xie, S.; et al. A revisit to fishmeal usage and associated consequences in Chinese aquaculture. Rev. Aquac. 2016, 10, 493–507. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lee, S.M.; Park, H.G.; Choi, J. Effects of dietary inclusion of chlorella vulgaris on growth, blood biochemical parameters, and antioxidant enzyme activity in olive flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2017, 48, 103–112. [Google Scholar] [CrossRef]
- Zeynali, M.; Bahabadi, M.N.; Morshedi, V.; Ghasemi, A.; Mozanzadeh, M.T. Replacement of dietary fishmeal with Sargassum ilicifolium meal on growth, innate immunity and immune gene mRNA transcript abundance in Lates calcarifer juveniles. Aquac. Nutr. 2020, 26, 1657–1668. [Google Scholar] [CrossRef]
- Bae, K.M.; Kim, K.W.; Lee, S.M. Evaluation of rice distillers dried grain as a partial replacement for fish meal in the practical diet of the juvenile olive flounder Paralichthys olivaceus. Fish. Aquat. Sci. 2015, 18, 151–158. [Google Scholar] [CrossRef]
- Lim, S.-J.; Lee, K.-J. Supplemental iron and phosphorus increase dietary inclusion of cottonseed and soybean meal in olive flounder (Paralichthys olivaceus). Aquac. Nutr. 2008, 14, 423–430. [Google Scholar] [CrossRef]
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Li, X.; Fang, T.; Wang, J.; Wang, Z.; Guan, D.; Sun, H.; Yun, X.; Zhou, J. The efficiency of adding amino acid mixtures to a diet free of fish meal and soybean meal as an attractant in yellow river carp (Cyprinus carpio var.). Aquac. Rep. 2022, 24, 101189. [Google Scholar] [CrossRef]
- Gunathilaka, B.E.; Jeong, S.; Cho, M.; Kim, K.; Hur, S.; Lee, S.; You, S.; Lee, S. Effects of Dietary Fish Meal Replacement with Alternative Protein Ingredients and Their Combinations on Growth Performance, Feed Utilization, Fillet Composition, and Biochemical Parameters of Red Seabream (Pagrus major). Aquac. Nutr. 2023, 83739, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, I.C.; Kim, K.T.; Cho, S.H.; Yoo, J.Y. Response of dietary substitution of fishmeal with various protein sources on growth, body composition and blood chemistry of olive flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846). Fish Physiol. Biochem. 2012, 38, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.S.; Lee, K.W.; Kim, J.; Yun, A.; Jeong, H.S.; Lee, M.J.; Baek, S.I.; Cho, S.H.; Kim, K.W.; Lim, S.G.; et al. Dietary substitution effect of fish meal with chicken by-product meal on growth, feed utilization, body composition, haematology and non-specific immune responses of olive flounder (Paralichthys olivaceus). Aquac. Nutr. 2020, 27, 315–326. [Google Scholar] [CrossRef]
- Ha, M.S.; Cho, S.H.; Kim, T. Dietary substitution of fish meal by meat meal: Effects on juvenile olive flounder (Paralichthys olivaceus) growth performance, feed utilization, haematology, biochemical profile, and disease resistance against Streptococcus iniae. Aquac. Nutr. 2021, 27, 1888–1902. [Google Scholar] [CrossRef]
- Kim, H.S.; Jung, W.G.; Myung, S.H.; Cho, S.H.; Kim, D.S. Substitution effects of fishmeal with tuna by product meal in the diet on growth, body composition, plasma chemistry and amino acid profiles of juvenile olive flounder (Paralichthys olivaceus). Aquaculture 2014, 431, 92–98. [Google Scholar] [CrossRef]
- JGP. JGP STD65 Duck Meal. 2023. Available online: https://jgpears.com/std65-duck-meat-meal/ (accessed on 25 February 2023).
- FAOSTAT. Production of Livestock Primary (Duck, Meat). 2023. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 8 November 2023).
- Hancz, C. Feed efficiency, nutrient sensing and feeding stimulation in aquaculture: A review. Acta Agric. Kapos 2020, 24, 35–54. [Google Scholar] [CrossRef]
- Biswas, P.R.P.; Patel, A.B.; Saha, H. Effect of dietary incorporation of chemo-attractants on growth and survival during seed rearing of Ompok bimaculatus (Bloch). Turk. J. Fish. Aquat. Sci. 2018, 18, 491–499. [Google Scholar] [CrossRef]
- Kader, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Bulbul, M. Supplemental effects of some crude ingredients in improving nutritive values of low fishmeal diets for red sea bream, Pagrus major. Aquaculture 2010, 308, 136–144. [Google Scholar] [CrossRef]
- Carr, W.E.S.; Netherton, J.C., III; Gleeson, R.A.; Derby, C.D. Stimulants of feeding behavior in fish: Analyses of tissues of diverse marine organisms. Biol. Bull. 1996, 190, 149–160. [Google Scholar] [CrossRef]
- Jeong, H.S.; Cho, S.H. Inclusion effect of jack mackerel meal as feed stimulants in diets replacing different levels of fish meal with various animal protein sources on growth performance of olive flounder (Paralichthys olivaceus). Aquac. Rep. 2023, 28, 101450. [Google Scholar] [CrossRef]
- Kim, H.S.; Cho, S.H. Dietary inclusion effect of feed ingredients showing high feeding attractiveness to rockfish (Sebastes schlegeli Hilgendorf 1880) on the growth performance, feed utilization, condition factor, and whole-body composition of fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 231, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.I.; Cho, S.H.; Kim, H.S. Dietary inclusion effect of various levels of jack mackerel meal on the growth performance, feed efficiency and whole body composition of rockfish (Sebastes schlegeli). Fish. Aquat. Sci. 2021, 24, 311–317. [Google Scholar] [CrossRef]
- Hosokawa, H.; Takii, K.; Shimeno, S.; Takeda, M. Identification of feeding stimulants for yellowtail in muscle extract of jack mackerel. Aquac. Sci. 2001, 49, 225–229. [Google Scholar] [CrossRef]
- Ikeda, I.; Okamoto, Y.; Oda, K. Identification of feeding stimulants for Japanese flounder in muscle extract of jack mackerel. Aquac. Sci. 2012, 60, 195–198. [Google Scholar] [CrossRef]
- Takakuwa, F.; Masumoto, T.; Fukada, H. Identification of feeding stimulants for greater amberjack Seriola dumerili in muscle tissue of jack mackerel Trachurus japonicus. Fish. Sci. 2019, 85, 387–395. [Google Scholar] [CrossRef]
- Kim, H.S.; Baek, S.I.; Lee, K.W.; Jeong, H.S.; Cho, S.H. Attractiveness of various protein sources to juvenile rockfish (Sebastes schlegeli, Hilgendorf 1880). J. Appl. Aquac. 2019, 32, 205–220. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, J.; Olowe, O.S.; Cho, S.H. Dietary optimum inclusion level of jack mackerel meal for olive flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846). Aquaculture 2022, 559, 738432. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemists. The Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lange, S.; Guđmundsdottir, B.K.; Magnadottir, B. Humoral immune parameters of cultured Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol. 2001, 11, 523–535. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Moñino, A.V.; Tomás Vidal, A.; Salvador, V.J.M.; Pla Torres, M.; Jover Cerdá, M. Soybean meal as a protein source in gilthead sea bream (Sparus aurata L.) diets: Effects on growth and nutrient utilization. Aquac. Res. 2007, 38, 82–90. [Google Scholar] [CrossRef]
- Alam, S.; Teshima, S.; Yaniharto, D.; Koshio, S.; Ishikawa, M. Influence of different dietary amino acid patterns on growth and body composition of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2002, 210, 359–369. [Google Scholar] [CrossRef]
- Forster, I.; Ogata, H.Y. Lysine requirement of juvenile Japanese flounder Paralichthys olivaceus and juvenile red sea bream Pagrus major. Aquaculture 1998, 161, 131–142. [Google Scholar] [CrossRef]
- Alam, M.S.; Teshima, S.; Ishikawa, M.; Koshio, S. Methionine requirement of juvenile Japanese flounder Paralichthys olivaceus. J. World Aquac. Soc. 2000, 31, 618–626. [Google Scholar] [CrossRef]
- Hasanthi, M.; Kim, M.G.; Lim, H.; Lim, J.; Hur, S.W.; Lee, S.; Lee, B.J.; Kim, K.W.; Lee, K.J. The dietary requirement for threonine in juvenile olive flounder (Paralichthys olivaceus). Fish. Aquat. Sci. 2023, 26, 58–68. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S. Requirement of dietary n-3 highly unsaturated fatty acids for juvenile flounder (Paralichthys olivaceus). Aquaculture 2004, 229, 315–323. [Google Scholar] [CrossRef]
- Senzui, A.; Masumoto, T.; Fukada, H. Neuropeptide Y expression in response to sensory organ-detected fish meal soluble components and orally fed fish meal-based diet in yellowtail Seriola quinqueradiata. Aquaculture 2020, 514, 734512. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Kong, F.; Song, X.; Tan, Q. The attractive effects of amino acids and some classical substances on grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2021, 47, 1489–1505. [Google Scholar] [CrossRef] [PubMed]
- Derby, C.D.; Sorensen, P.W. Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. J. Chem. Ecol. 2008, 34, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K. Use of defatted soybean meal as a substitute for fish meal in diets of Japanese flounder (Paralichthys olivaceus). Aquaculture 1999, 179, 3–11. [Google Scholar] [CrossRef]
- Tharaka, K.; Benitez-Santana, T.; Gunathilaka, B.E.; Kim, M.G.; Lee, C.; Shin, J.; Lee, K.J. Evaluation of Antarctic krill (Euphausia superba) meal supplementation in diets for olive flounder (Paralichthys olivaceus). Aquac. Res. 2020, 51, 2291–2302. [Google Scholar] [CrossRef]
- Khosravi, S.; Bui, H.T.D.; Herault, M.; Fournier, V.; Kim, K.; Lee, B.; Kim, K.; Lee, K. Supplementation of protein hydrolysates to a low-fishmeal diet improves growth and health status of juvenile olive flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2018, 49, 897–911. [Google Scholar] [CrossRef]
- Kroon, F.; Streten, C.; Harries, S. A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PLoS ONE 2017, 12, e0174762. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, S.H.; Kim, T.; Hur, S.W. Substitution effect of fish meal with various sources of animal by-product meals in feed on growth, feed utilization, body composition, haematology and non-specific immune response of olive flounder (Paralichthys olivaceus, Temminck & Schlegel, 1846). Aquac. Res. 2021, 52, 2802–2817. [Google Scholar] [CrossRef]
- Kader, M.A.; Bulbul, M.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Nguyen, B.T.; Komilus, C.F. Effect of complete replacement of fishmeal by dehulled soybean meal with crude attractants supplementation in diets for red sea bream, Pagrus major. Aquaculture 2012, 350–353, 109–116. [Google Scholar] [CrossRef]
- Khosravi, S.; Rahimnejad, S.; Herault, M.; Fournier, V.; Lee, C.R.; Dio Bui, H.T.; Jeong, J.B.; Lee, K.J. Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immunol. 2015, 45, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.B.; Gatlin, D.M. Evaluation of different sulfur amino acid compounds in the diet of red drum, Sciaenops ocellatus, and sparing value of cystine for methionine. Aquaculture 2004, 24, 465–477. [Google Scholar] [CrossRef]
- Twibell, R.G.; Wilson, K.A.; Brown, P.B. Dietary sulfur amino acid requirement of juvenile yellow perch fed the maximum cystine replacement value for methionine. J. Nutr. 2000, 130, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Aminikhoei, Z.; Kim, K.D.; Lee, S.M. Growth and fatty acid composition of juvenile olive flounder Paralichthys olivaceus fed diets containing different levels and ratios of eicosapentaenoic acid and docosahexaenoic acid. Fish. Aquat. Sci. 2014, 17, 95–103. [Google Scholar] [CrossRef][Green Version]
- Lee, S.; Aya, F.A.; Won, S.; Hamidoghli, A.; Bai, S.C. Effects of replacing dietary fish oil with beef tallow on growth performance, serological parameters, and fatty acid composition in juvenile olive flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2020, 51, 393–406. [Google Scholar] [CrossRef]
- Peng, D.; Peng, B.; Li, J.; Zhang, Y.; Luo, H.; Xiao, Q.; Tang, S.; Liang, X.F. Effects of three feed attractants on the growth, biochemical indicators, lipid metabolism and appetite of Chinese perch (Siniperca chuatsi). Aquac. Rep. 2022, 23, 101075. [Google Scholar] [CrossRef]
- Bui, H.T.D.; Khosravi, S.; Fournier, V.; Herault, M.; Lee, K.J. Growth performance, feed utilization, innate immunity, digestibility and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture 2014, 418–419, 11–16. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Wang, H.; Xie, F.; Wang, T. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immunol. 2012, 32, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Oncul, F.O.; Aya, F.A.; Hamidoghli, A.; Won, S.; Lee, G.; Han, K.R.; Bai, S.C. Effects of the dietary fermented tuna by-product meal on growth, blood parameters, nonspecific immune response, and disease resistance in juvenile olive flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2019, 50, 65–77. [Google Scholar] [CrossRef]
- Sabbagh, M.; Schiavone, R.; Brizzi, G.; Sicuro, B.; Zilli, L.; Vilella, S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture 2019, 511, 734220. [Google Scholar] [CrossRef]
- Deng, J.; Mai, K.; Ai, Q.; Zhang, W.; Wang, X.; Xu, W.; Liufu, Z. Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2006, 258, 503–513. [Google Scholar] [CrossRef]
- Alam, M.S.; Watanabe, W.O.; Carroll, P.M.; Gabel, J.E.; Corum, M.A.; Seaton, P.; Wedegaertner, T.C.; Rathore, K.S.; Dowd, M.K. Evaluation of genetically improved (glandless) and genetically modified low-gossypol cottonseed meal as alternative protein sources in the diet of juvenile southern flounder Paralichthys lethostigma reared in a recirculating aquaculture system. Aquaculture 2018, 489, 36–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).