Effect of the TGF-β/BMP Signaling Pathway on the Proliferation of Yak Pulmonary Artery Smooth Muscle Cells under Hypoxic Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Culture of Yak PASMCs
2.3. Purification of Primary Yak PASMCs
2.4. Determination of Growth Curves of PASMCs
2.5. Immunofluorescence Staining
2.6. Establishment of a Hypoxia Model of Yak PASMCs
2.7. Cell Proliferation and Scratch Test
2.8. Extraction of Total Cellular Proteins
2.9. Western Blotting
2.10. Data Analysis
3. Results
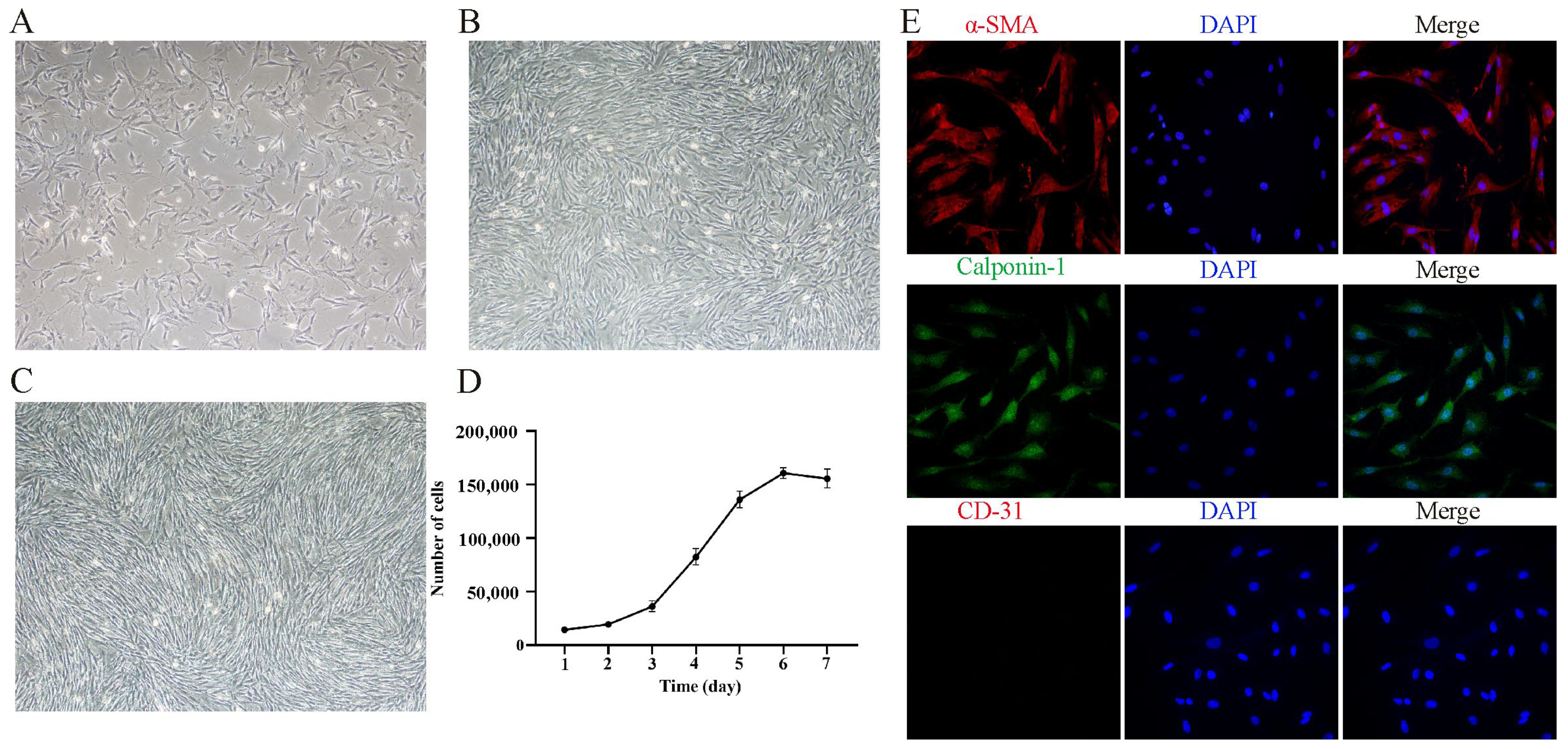
3.1. Isolation, Culture, and Characterization of Yak PASMCs In Vitro
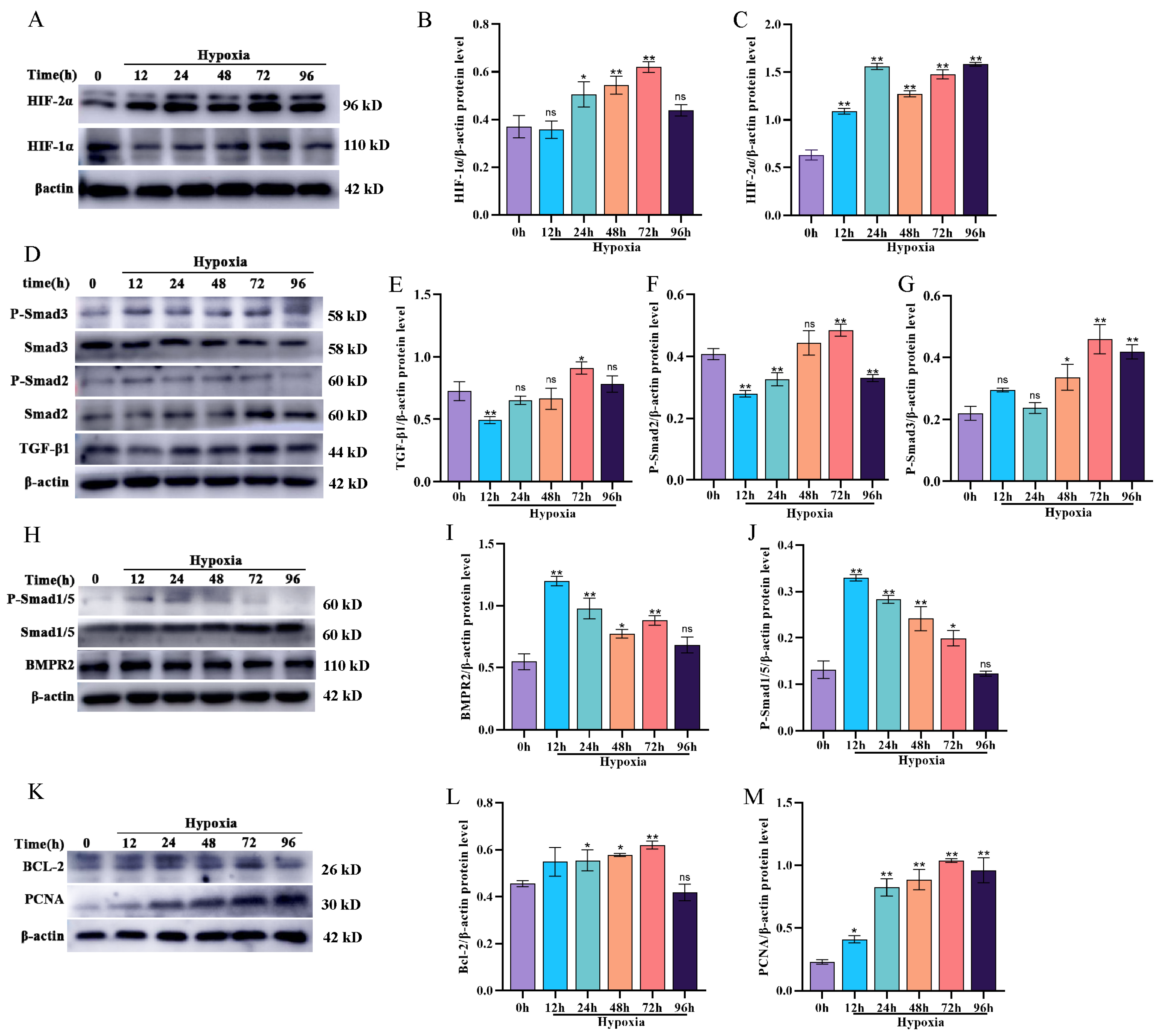
3.2. Effect of Hypoxia on BMP/TGF-β Signaling Pathway-Related Protein Expression and Proliferation in Yak PASMCs
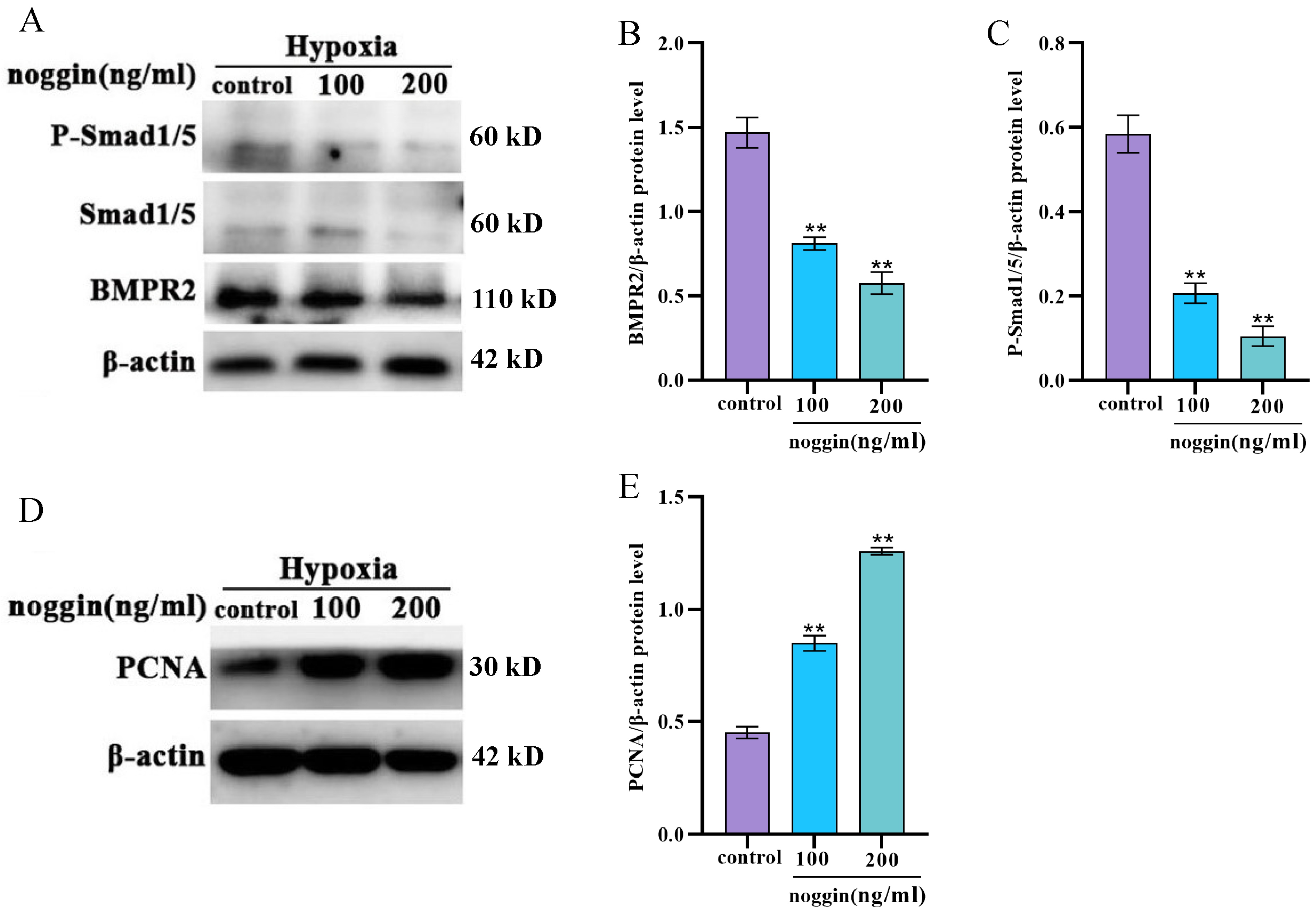
3.3. Selection of Drug Concentrations for TGF-β1 Recombinant Protein and Noggin
3.4. TGF-β1 Activates the TGF-β Signaling Pathway and Promotes the Proliferation of Yak PASMCs
3.5. Inhibition of the BMP Signaling Pathway Increases the Proliferation of Yak PASMCs
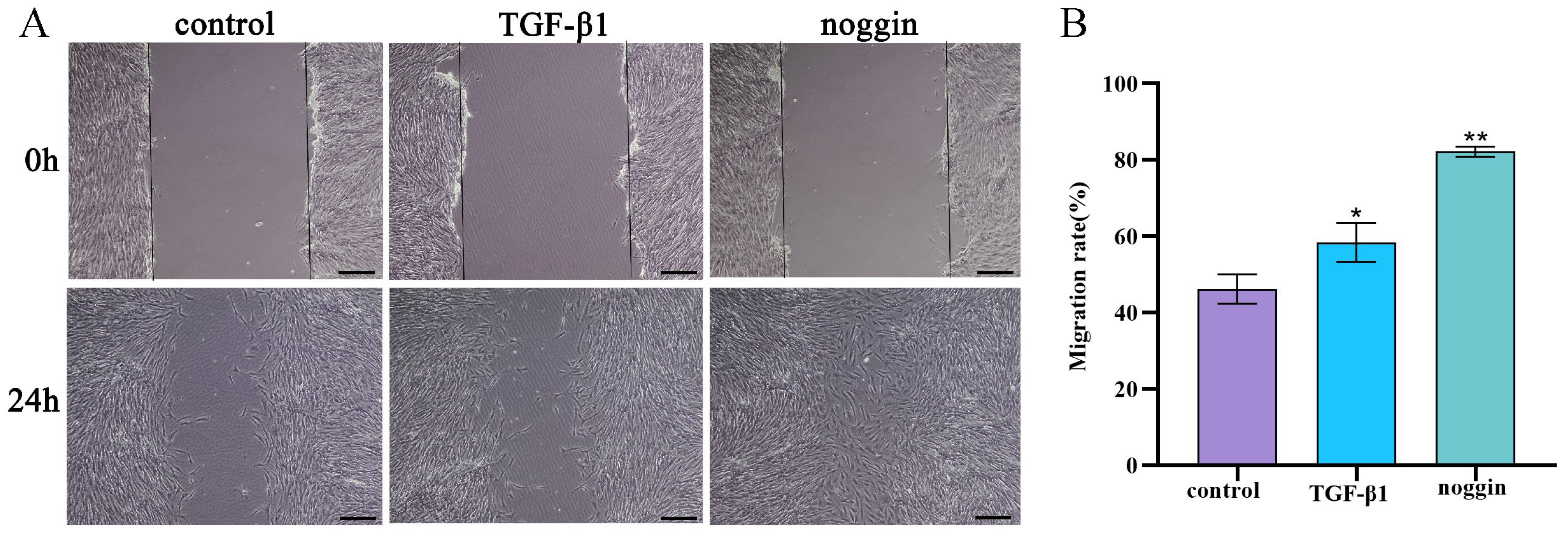
3.6. TGF-β1 and Noggin Promote the Migration of Yak PASMCs
4. Discussion
5. Conclusions and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lan, D.; Xiong, X.; Ji, W.; Li, J.; Mipam, T.D.; Ai, Y.; Chai, Z. Transcriptome profile and unique genetic evolution of positively selected genes in yak lungs. Genetica 2018, 146, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, Q.; He, Y.; Yang, L.; Zhang, X.; Shi, P.; Yang, L.; Liu, Z.; Zhang, F.; Liu, F.; et al. The Transcriptomic Landscape of Yaks Reveals Molecular Pathways for High Altitude Adaptation. Genome Biol. Evol. 2019, 11, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Durmowicz, A.G.; Hofmeister, S.; Kadyraliev, T.K.; Aldashev, A.A.; Stenmark, K.R. Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J. Appl. Physiol. 1993, 74, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Mizuno, S.; Sakai, A.; Matsukawa, S.; Kojonazarov, B.; Zamirbek, B.; Umeda, Y.; Morikawa, M.; Anzai, M.; Ishizuka, T.; et al. Blunted activation of Rho-kinase in yak pulmonary circulation. BioMed Res. Int. 2015, 2015, 720250. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Tucker, A.; Reeves, J.T.; Will, D.H.; Grover, R.F. The genetic factor influencing pulmonary hypertension in cattle at high altitude. Cardiovasc. Res. 1974, 8, 745–749. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Fagan, K.A.; Frid, M.G. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ. Res. 2006, 99, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.A.R.; Lawrie, A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol. Med. 2017, 23, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Ruopp, N.F.; Cockrill, B.A. Diagnosis and Treatment of Pulmonary Arterial Hypertension: A Review. JAMA 2022, 327, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Koizumi, T.; Ruan, Z.; Wang, Z.; Chen, Q.; Sakai, A. Nitric oxide inhibitor altitude-dependently elevates pulmonary arterial pressure in high-altitude adapted yaks. Respir. Physiol. Neurobiol. 2005, 146, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, H.; Cui, Y.; Yu, S.; Li, S.; Afedo, S.Y.; Wang, Y.; Bai, X.; He, J. Regulatory effects of HIF-1α and HO-1 in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells in yak. Cell. Signal. 2021, 87, 110140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Zhou, J.; Yao, Y.; Li, R.; Zhou, M.; Chen, S.; Qiao, Z.; Yang, K. Combined transcriptome and proteome analysis of yak PASMCs under hypoxic and normoxic conditions. PeerJ 2022, 10, e14369. [Google Scholar] [CrossRef] [PubMed]
- Abbas Raza, S.H.; Zhong, R.; Wei, X.; Zhao, G.; Zan, L.; Pant, S.D.; Schreurs, N.M.; Lei, H. Investigating the Role of KLF6 in the Growth of Bovine Preadipocytes: Using Transcriptomic Analyses to Understand Beef Quality. J. Agric. Food Chem. 2024, 72, 9656–9668. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Wang, W.; Zhu, X.C.; Ye, W.J.; Cai, H.; Wu, P.L.; Huang, X.Y.; Wang, L.X. The potential of asiaticoside for TGF-β1/Smad signaling inhibition in prevention and progression of hypoxia-induced pulmonary hypertension. Life Sci. 2015, 137, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhou, Q.; Tang, H.; Chen, T.; Li, J.; Raychaudhuri, P.; Yuan, J.X.; Zhou, G. Smooth muscle cell-specific FoxM1 controls hypoxia-induced pulmonary hypertension. Cell. Signal. 2018, 51, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Siques, P.; Pena, E.; Brito, J.; El Alam, S. Oxidative Stress, Kinase Activation, and Inflammatory Pathways Involved in Effects on Smooth Muscle Cells During Pulmonary Artery Hypertension Under Hypobaric Hypoxia Exposure. Front. Physiol. 2021, 12, 690341. [Google Scholar] [CrossRef] [PubMed]
- Pak, O.; Aldashev, A.; Welsh, D.; Peacock, A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur. Respir. J. 2007, 30, 364–372. [Google Scholar] [CrossRef]
- Udjus, C.; Cero, F.T.; Halvorsen, B.; Behmen, D.; Carlson, C.R.; Bendiksen, B.A.; Espe, E.K.S.; Sjaastad, I.; Løberg, E.M.; Yndestad, A.; et al. Caspase-1 induces smooth muscle cell growth in hypoxia-induced pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L999–L1012. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhu, M.M.; Peng, Y.; Machireddy, N.; Evans, C.E.; Machado, R.; Zhang, X.; Zhao, Y.Y. Therapeutic Targeting of Vascular Remodeling and Right Heart Failure in Pulmonary Arterial Hypertension with a HIF-2α Inhibitor. Am. J. Respir. Crit. Care Med. 2018, 198, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Pullamsetti, S.S.; Mamazhakypov, A.; Weissmann, N.; Seeger, W.; Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Investig. 2020, 130, 5638–5651. [Google Scholar] [CrossRef]
- Doerr, M.; Morrison, J.; Bergeron, L.; Coomber, B.L.; Viloria-Petit, A. Differential effect of hypoxia on early endothelial-mesenchymal transition response to transforming growth beta isoforms 1 and 2. Microvasc. Res. 2016, 108, 48–63. [Google Scholar] [CrossRef]
- Mingyuan, X.; Qianqian, P.; Shengquan, X.; Chenyi, Y.; Rui, L.; Yichen, S.; Jinghong, X. Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget 2018, 9, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mickael, C.; Kassa, B.; Gebreab, L.; Robinson, J.C.; Koyanagi, D.E.; Sanders, L.; Barthel, L.; Meadows, C.; Fox, D.; et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat. Commun. 2017, 8, 15494. [Google Scholar] [CrossRef] [PubMed]
- Sanada, T.J.; Sun, X.Q.; Happé, C.; Guignabert, C.; Tu, L.; Schalij, I.; Bogaard, H.J.; Goumans, M.J.; Kurakula, K. Altered TGFβ/SMAD Signaling in Human and Rat Models of Pulmonary Hypertension: An Old Target Needs Attention. Cells 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Dong, M.; Luo, Y.; Zhao, F.; Li, Y. Danshensu prevents hypoxic pulmonary hypertension in rats by inhibiting the proliferation of pulmonary artery smooth muscle cells via TGF-β-smad3-associated pathway. Eur. J. Pharmacol. 2018, 820, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, J.; Chen, Y.; Lv, T.; Wang, X.; Liu, C.; Xue, H.; He, K.; Sun, L. The miR-182/Myadm axis regulates hypoxia-induced pulmonary hypertension by balancing the BMP- and TGF-β-signalling pathways in an SMC/EC-crosstalk-associated manner. Basic Res. Cardiol. 2021, 116, 53. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Aikeremu, N.; Shi, W.Y.; Tang, X.C.; Gao, R.J.; Kong, L.J.; Zhang, J.R.; Qin, W.J.; Zhang, A.M.; Ma, K.T.; et al. Inhibition of KIR2.1 decreases pulmonary artery smooth muscle cell proliferation and migration. Int. J. Mol. Med. 2022, 50, 119. [Google Scholar] [CrossRef] [PubMed]
- Andre, P.; Joshi, S.R.; Briscoe, S.D.; Alexander, M.J.; Li, G.; Kumar, R. Therapeutic Approaches for Treating Pulmonary Arterial Hypertension by Correcting Imbalanced TGF-β Superfamily Signaling. Front. Med. 2021, 8, 814222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, L.; Zhong, Y.; Flanders, K.C.; Roberts, J.D., Jr. Transforming growth factor-β stimulates Smad1/5 signaling in pulmonary artery smooth muscle cells and fibroblasts of the newborn mouse through ALK1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L615–L627. [Google Scholar] [CrossRef] [PubMed]
- Happé, C.; Kurakula, K.; Sun, X.Q.; da Silva Goncalves Bos, D.; Rol, N.; Guignabert, C.; Tu, L.; Schalij, I.; Wiesmeijer, K.C.; Tura-Ceide, O.; et al. The BMP Receptor 2 in Pulmonary Arterial Hypertension: When and Where the Animal Model Matches the Patient. Cells 2020, 9, 1422. [Google Scholar] [CrossRef]
- Orriols, M.; Gomez-Puerto, M.C.; Ten Dijke, P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell. Mol. Life Sci. CMLS 2017, 74, 2979–2995. [Google Scholar] [CrossRef] [PubMed]
- Hashimi, S.M. Exogenous noggin binds the BMP-2 receptor and induces alkaline phosphatase activity in osteoblasts. J. Cell. Biochem. 2019, 120, 13237–13242. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.H.; Brazil, D.P. Bone morphogenetic proteins and their antagonists: Current and emerging clinical uses. Br. J. Pharmacol. 2014, 171, 3620–3632. [Google Scholar] [CrossRef]
- Tielemans, B.; Delcroix, M.; Belge, C.; Quarck, R. TGFβ and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov. Today 2019, 24, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Bloch, D.B.; ten Dijke, P.; Goumans, M.J.; Hata, A.; Smith, J.; Yu, P.B.; Bloch, K.D. Targeting BMP signalling in cardiovascular disease and anaemia. Nat. Rev. Cardiol. 2016, 13, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Chouvarine, P.; Legchenko, E.; Hoffmann, N.; Geldner, J.; Borchert, P.; Jonigk, D.; Mozes, M.M.; Hansmann, G. PPARγ Links BMP2 and TGFβ1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab. 2017, 25, 1118–1134.e7. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Wang, K.; Wang, B.; Cui, Y.; Zhang, Q. Effect of the TGF-β/BMP Signaling Pathway on the Proliferation of Yak Pulmonary Artery Smooth Muscle Cells under Hypoxic Conditions. Animals 2024, 14, 2072. https://doi.org/10.3390/ani14142072
He J, Wang K, Wang B, Cui Y, Zhang Q. Effect of the TGF-β/BMP Signaling Pathway on the Proliferation of Yak Pulmonary Artery Smooth Muscle Cells under Hypoxic Conditions. Animals. 2024; 14(14):2072. https://doi.org/10.3390/ani14142072
Chicago/Turabian StyleHe, Junfeng, Kejin Wang, Biao Wang, Yan Cui, and Qian Zhang. 2024. "Effect of the TGF-β/BMP Signaling Pathway on the Proliferation of Yak Pulmonary Artery Smooth Muscle Cells under Hypoxic Conditions" Animals 14, no. 14: 2072. https://doi.org/10.3390/ani14142072
APA StyleHe, J., Wang, K., Wang, B., Cui, Y., & Zhang, Q. (2024). Effect of the TGF-β/BMP Signaling Pathway on the Proliferation of Yak Pulmonary Artery Smooth Muscle Cells under Hypoxic Conditions. Animals, 14(14), 2072. https://doi.org/10.3390/ani14142072




