Association of Dry Period Length with Automatic Milking System, Mastitis, and Reproductive Indicators in Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animals
2.2. Study Data
2.3. Statistical Analysis of Data
3. Results
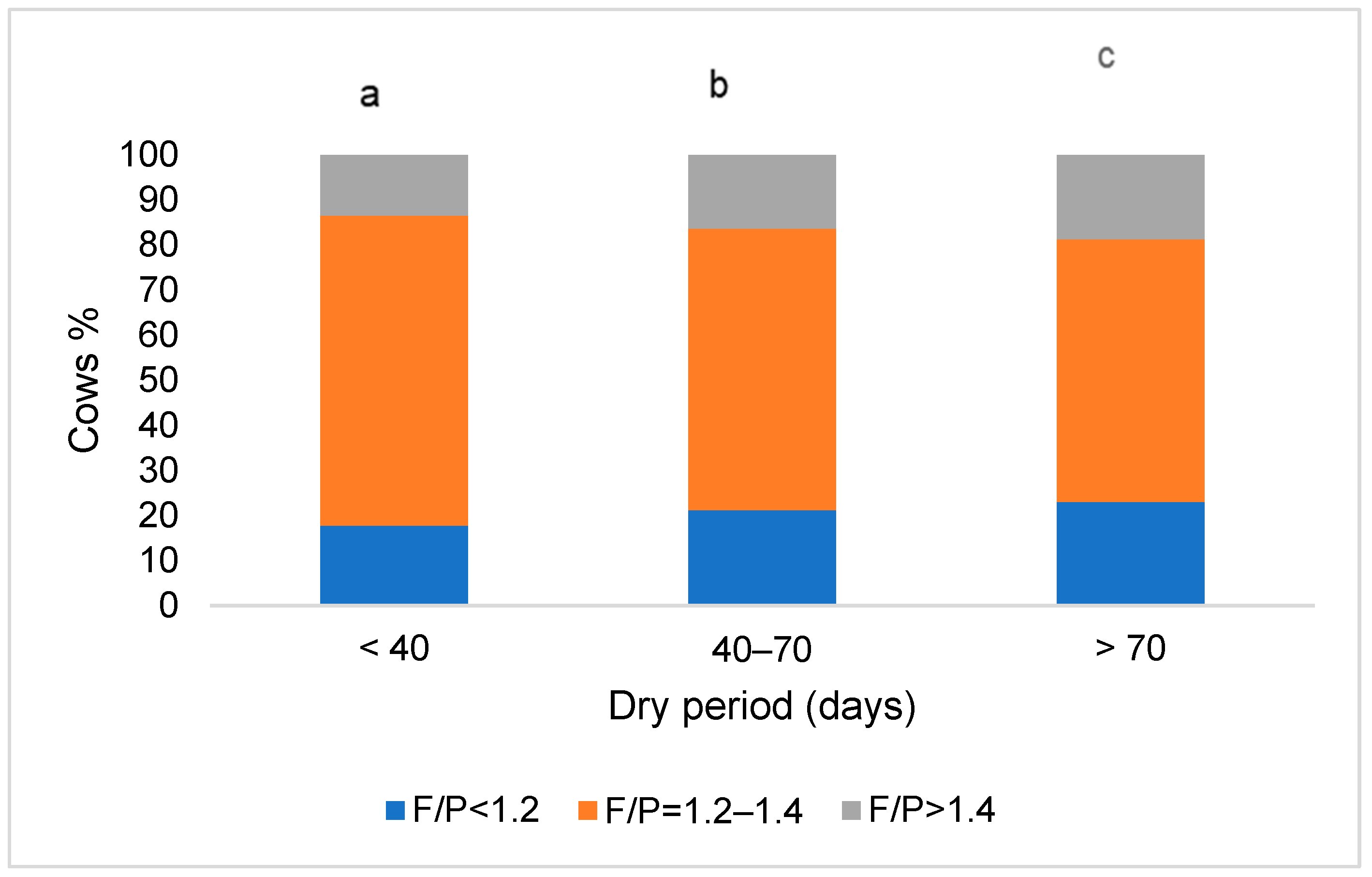
3.1. Milk Characteristics in Cows Based on Their Dry Period Length
3.2. Prevalence of Mastitis Pathogens in Association with Dry Period Length in Cows
3.3. Association of Body Condition Score and Fertility Characteristics with Dry Period Length in Cows
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rastani, R.R.; Grummer, R.R.; Bertics, S.J.; Gümen, A.; Wiltbank, M.C.; Mashek, D.G.; Schwab, M.C. Reducing dry period length to simplify feeding transition cows: Milk production, energy balance, and metabolic profiles. J. Dairy Sci. 2005, 88, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Annen, E.L.; Collier, R.J.; McGuire, M.A.; Vicini, J.L.; Ballam, J.M.; Lormore, M.J. Effect of modified dry period lengths and bovine somatotropin on yield and composition of milk from dairy cows. J. Dairy Sci. 2004, 87, 3746–3761. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.B.; Madsen, T.G.; Larsen, T.; Ingvartsen, K.L.; Nielsen, M.O. The effects of dry period versus continuous lactation on metabolic status and performance in periparturient cows. J. Dairy Sci. 2005, 88, 3530–3541. [Google Scholar] [CrossRef] [PubMed]
- Watters, R.D.; Guenther, J.N.; Brickner, A.E.; Rastani, R.R.; Crump, P.M.; Clark, P.W.; Grummer, R.R. Effects of dry period length on milk production and health of dairy cattle. J. Dairy Sci. 2008, 91, 2595–2603. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, J.T.; Enevoldsen, C. Effect of dry period length on milk production in subsequent lactation. J. Dairy Sci. 1991, 74, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Bachman, K.C.; Schairer, M.L.J. Invited review: Bovine studies on optimal lengths of dry periods. J. Dairy Sci. 2003, 86, 3027–3037. [Google Scholar] [CrossRef] [PubMed]
- Church, G.T.; Fox, L.K.; Gaskins, C.T.; Hancock, D.D.; Gay, J.M. The effect of a shortened dry period on intramammary infections during the subsequent lactation. J. Dairy Sci. 2008, 91, 4219–4225. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Capuco, A.V.; De Spiegeleer, B.; Peelman, L.; Stevens, M.; Collier, R.J.; Burvenich, C. An integrated view on how the management of the dry period length of lactating cows could affect mammary biology and defence. J. Anim. Physiol. Anim. Nutr. 2010, 94, e7–e30. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Hammon, D.S.; Evjen, I.M.; Dhiman, T.R.; Goff, J.P.; Walters, J.L. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 2006, 113, 21–29. [Google Scholar] [CrossRef]
- McArt, J.A.; Nydam, D.V.; Oetzel, G.R. A field trial on the effect of propylene glycol on displaced abomasum, removal from herd, and reproduction in fresh cows diagnosed with subclinical ketosis. J. Dairy Sci. 2012, 95, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Leal Yepes, F.A.; Overton, T.R.; Wakshlag, J.J.; Lock, A.L.; Ryan, C.M.; Nydam, D.V. Dry period plane of energy: Effects on feed intake, energy balance, milk production, and composition in transition dairy cows. J. Dairy Sci. 2015, 98, 3366–3382. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.P.; Tervit, R.M.; Laven, A.L. Effects of dry period length on milk production and somatic cell count in subsequent lactations in dairy cows. J. Anim. Sci. Biotechnol. 2013, 2, 1–10. [Google Scholar]
- Van Knegsel, H.A.J.; Metz, J.H.M.; van den Borne, M.A.M.; Schukken, Y.H.; van der Zwaag, H.G.J.M. Effect of dry period length on lactation performance of dairy cows: A meta-analysis. J. Dairy Sci. 2007, 90, 2720–2729. [Google Scholar]
- O’Brien, H.R.; O’Neill, E.J.; McCarthy, M.A.; Griffin, M. Effects of extended dry periods on lactation performance and milk quality in dairy cows. J. Dairy Res. 2012, 79, 337–346. [Google Scholar]
- Soyeurt, H.; Bruwier, D.; Romnee, J.M.; Gengler, N.; Bertozzi, C.; Veselko, D.; Dardenne, P. Genetic parameters of dairy traits measured in Walloon herds and their relationships with somatic cell counts. J. Dairy Sci. 2005, 88, 4297–4305. [Google Scholar]
- Shingfield, K.J.; Reynolds, C.K.; Hervás, G.; Griinari, J.M.; Wallace, R.J. Duodenal infusion of butterfat increases feed intake and decreases plasma concentrations of nonesterified fatty acids in lactating dairy cows. J. Nutr. 2006, 136, 1820–1827. [Google Scholar]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited Review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 91, 407–426. [Google Scholar] [CrossRef]
- Riekerink, R.G.; Barkema, H.W.; Stryhn, H.; Kelton, D.F. The effect of season on somatic cell count and the incidence of clinical mastitis. J. Dairy Sci. 2007, 90, 1704–1715. [Google Scholar] [CrossRef]
- Gröhn, Y.T.; Wilson, D.J.; González, R.N.; Hertl, J.A.; Schulte, H.; Bennett, G.; Schukken, Y.H. Effect of pathogen-specific clinical mastitis on milk yield in dairy cows. J. Dairy Sci. 2004, 87, 3358–3374. [Google Scholar] [CrossRef]
- Bradley, A.J.; Green, M.J. An investigation of the impact of intramammary antibiotic dry cow therapy on clinical coliform mastitis. J. Dairy Sci. 2001, 84, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. Investigation of mastitis problems on farms. Veterinary Clinics of North America. Food Anim. Pract. 2003, 19, 47–73. [Google Scholar] [CrossRef]
- López-Gatius, F. Is fertility declining in dairy cattle? A retrospective study in northeastern Spain. Theriogenology 2003, 60, 89–99. [Google Scholar] [CrossRef]
- Butler, W.R.; Smith, R.D. Interrelationships between energy balance and postpartum reproductive function in dairy cattle. J. Dairy Sci. 1989, 72, 767–783. [Google Scholar] [CrossRef] [PubMed]
- Guadagnini, M.; Amodeo, P.; Biscarini, F.; Bolli, A.; Moroni, P. Observational study on dry period length and its associations with milk production, culling risk, and fertility in Italian dairy farms. J. Dairy Sci. 2023, 106, 2630–2641. [Google Scholar] [CrossRef]
- Groher, T.; Heitkämper, K.; Umstätter, C. Digital technology adoption in livestock production with a special focus on ruminant farming. Animal 2020, 11, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Da Borso, F.; Chiumenti, A.; Sigura, M.; Pezzuolo, A. Influence of automatic feeding systems on design and management of dairy farms. J. Agric. Eng. 2017, 48, 48–52. [Google Scholar] [CrossRef]
- Von Keyserlingk, M.A.G.; Weary, D.M. The Impact of Automatic Milking Systems on Milk Quality, Animal Welfare, and Productivity. Animal 2018, 12, 661–674. [Google Scholar]
- Cole, J.B.; VanRaden, P.M. Long-Term Effects of Automatic Milking Systems on Farm Efficiency and Herd Management. J. Dairy Sci. 2020, 103, 5084–5093. [Google Scholar]
- Jacobs, J.A.; Siegford, J.M. Invited review: The impact of automatic milking systems on dairy cow management, behavior, health, and welfare. J. Dairy Sci. 2012, 95, 2227–2247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, W.; Li, C.; Wang, W. Innovation, practical benefits and prospects for the future development of automatic milking systems. Front. Agric. Sci. Eng. 2017, 4, 37–47. [Google Scholar] [CrossRef]
- Van Knegsel, H.A.J.; van den Borne, M.A.M.; Dijkstra, J.; van der Veen, M. Real-time monitoring of milk quality with automatic milking systems: A review. J. Dairy Sci. 2012, 95, 1852–1862. [Google Scholar]
- Weersink, A.; Bartus, D.J.R.A.; Hoekstra, M.C.C. Comparison of automatic milking systems and traditional milking systems: Milk production, milk quality, and herd health. J. Dairy Sci. 2014, 97, 6934–6943. [Google Scholar]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Janzekovic, M.; Brus, M.; Mursec, B.; Vinis, P.; Stajnko, D.; Cus, F. Mastitis Detection Based on Electric Conductivity of Milk. J. Achiev. Mater. Manuf. Eng. 2009, 34, 39–46. [Google Scholar]
- Tyrrell, H.F.; Reid, J.T. Prediction of the energy value of cow’s milk. J. Dairy Sci. 1965, 48, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Antanaitis, R.; Džermeikaitė, K.; Januškevičius, V.; Šimonytė, I.; Baumgartner, W. In-Line Registered Milk Fat-to-Protein Ratio for the Assessment of Metabolic Status in Dairy Cows. Animals 2023, 13, 3293. [Google Scholar] [CrossRef] [PubMed]
- Atalay, H. Milk fat/protein ratio in ketosis and acidosis. Balikesir Sağlik Bilim. Derg./Balikesir Health Sci. J. 2019, 8, 143–146. [Google Scholar]
- Silva, S.J.; Schmitt, D.A.; Zieminski, J.K.; Hegg, K.A.P.; Anderson, J.E.R. Impact of Dry Period Length on Dairy Cow Performance and Health: A Review of Recent Advances. J. Dairy Sci. 2023, 106, 2523–2540. [Google Scholar]
- Van Knegsel, A.T.M.; Remmelink, G.J.; Jorjong, S.; Fievez, V.; Kemp, B. Effect of dry period length and dietary energy source on energy balance, milk yield, and milk composition of dairy cows. J. Dairy Sci. 2014, 97, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.L.; Boettcher, P.J.; Dekkers, J.C.M.; Petitclerc, D.; Schaeffer, L.R. Relationships between energy balance and health traits of dairy cattle in early lactation. J. Dairy Sci. 2000, 83, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- O’Callahan, D.; Lozano, J.M.; Fahey, J.; Gath, V.; Snijders, S.; Boland, M.P. Relationships between Nutrition and Fertility in Cattle; British Society of Animal Science Occasional Publication No. 26; Nottingham University Press: Edinburgh, UK, 2001; pp. 147–160. [Google Scholar]
- Egyedy, A.F.; Ametaj, B.N. Mastitis: Impact of Dry Period, Pathogens, and Immune Responses on Etiopathogenesis of Disease and its Association with Periparturient Diseases. Dairy 2022, 3, 881–906. [Google Scholar] [CrossRef]
- Hogan, J.; Smith, K.L. Managing environmental mastitis. Vet. Clin. Food Anim. Pract. 2012, 28, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Santschi, D.E.; Lefebvre, D.M.; Cue, R.I.; Girard, C.L.; Pellerin, D. Incidence of metabolic disorders and reproductive performance following a short (35-d) or conventional (60-d) dry period management in commercial Holstein herds. J. Dairy Sci. 2011, 94, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, P.J.; De Vries, A. Effect of days to conception in the previous lactation on the risk of death and live culling around calving. J. Dairy Sci. 2010, 93, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.M.; Schutz, M.M. An Interdisciplinary Review of Body Condition Scoring for Dairy Cattle. Prof. Anim. Sci. 2008, 24, 507–529. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 769–5801. [Google Scholar] [CrossRef]






| Component | Amount (kg/cow/day) |
|---|---|
| Rapeseed 36% | 1.2 |
| Grass silage (27% DM) | 8 |
| Maize silage (27% DM) | 1.2 |
| Wheat straws | 7.5 |
| Water | 4.3 |
| Dry cow mineral and vitamin | 0.250 |
| Group of Cows | n | Dry Period Length (Days) | Milk Yield, kg | Milk Fat % | Milk Protein % | Milk Lactose % |
|---|---|---|---|---|---|---|
| 1 | 977 | <40 | 38.21 a ± 0.27 | 4.01 a ± 0.02 | 3.36 a ± 0.01 | 4.60 a ± 0.01 |
| 2 | 2015 | 40–70 | 44.81 b ± 0.21 | 4.14 b ± 0.01 | 3.42 b ± 0.01 | 4.64 b ± 0.01 |
| 3 | 870 | >70 | 40.77 c ± 0.16 | 4.03 a ± 0.02 | 3.45 b ± 0.01 | 4.62 ab ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jukna, V.; Meškinytė, E.; Antanaitis, R.; Juozaitienė, V. Association of Dry Period Length with Automatic Milking System, Mastitis, and Reproductive Indicators in Cows. Animals 2024, 14, 2065. https://doi.org/10.3390/ani14142065
Jukna V, Meškinytė E, Antanaitis R, Juozaitienė V. Association of Dry Period Length with Automatic Milking System, Mastitis, and Reproductive Indicators in Cows. Animals. 2024; 14(14):2065. https://doi.org/10.3390/ani14142065
Chicago/Turabian StyleJukna, Vigilijus, Edita Meškinytė, Ramūnas Antanaitis, and Vida Juozaitienė. 2024. "Association of Dry Period Length with Automatic Milking System, Mastitis, and Reproductive Indicators in Cows" Animals 14, no. 14: 2065. https://doi.org/10.3390/ani14142065
APA StyleJukna, V., Meškinytė, E., Antanaitis, R., & Juozaitienė, V. (2024). Association of Dry Period Length with Automatic Milking System, Mastitis, and Reproductive Indicators in Cows. Animals, 14(14), 2065. https://doi.org/10.3390/ani14142065











