Effect of Guanidinoacetic Acid on Production Performance, Serum Biochemistry, Meat Quality and Rumen Fermentation in Hu Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Moral Statement
2.2. Experimental Design and Feeding Management
2.3. Growth Performance
2.4. Sample Collection
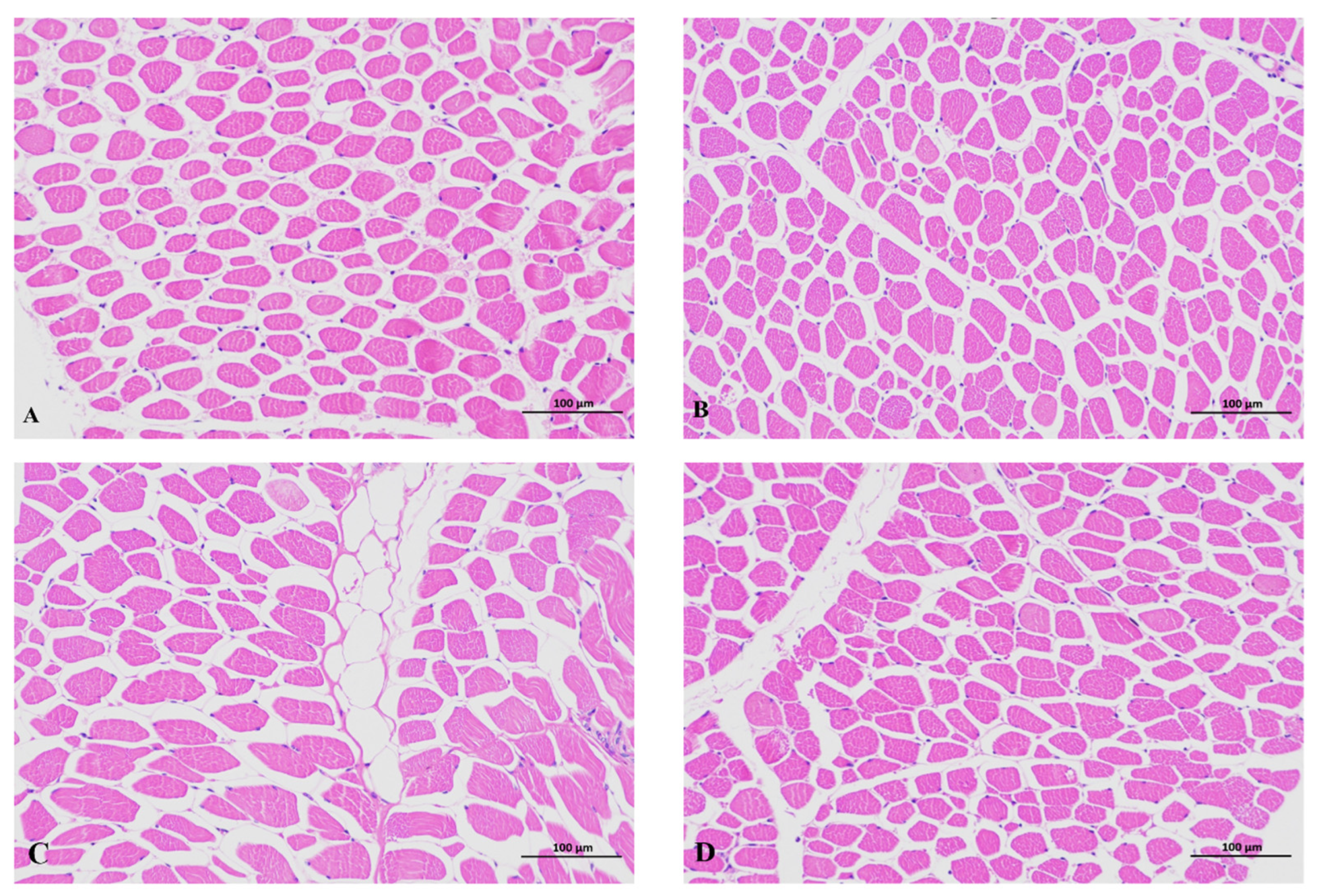
2.5. Meat Quality and Histological Analyses
2.6. Serum Biochemical Indexes and Antioxidant Capacity
2.7. Rumen Fermentation
2.8. Quantitative Real-Time PCR for Gene Expression
2.9. Statistics Analysis
3. Results
3.1. Effects of GAA Supplementation on Growth Performance, Carcass Traits and Meat Quality
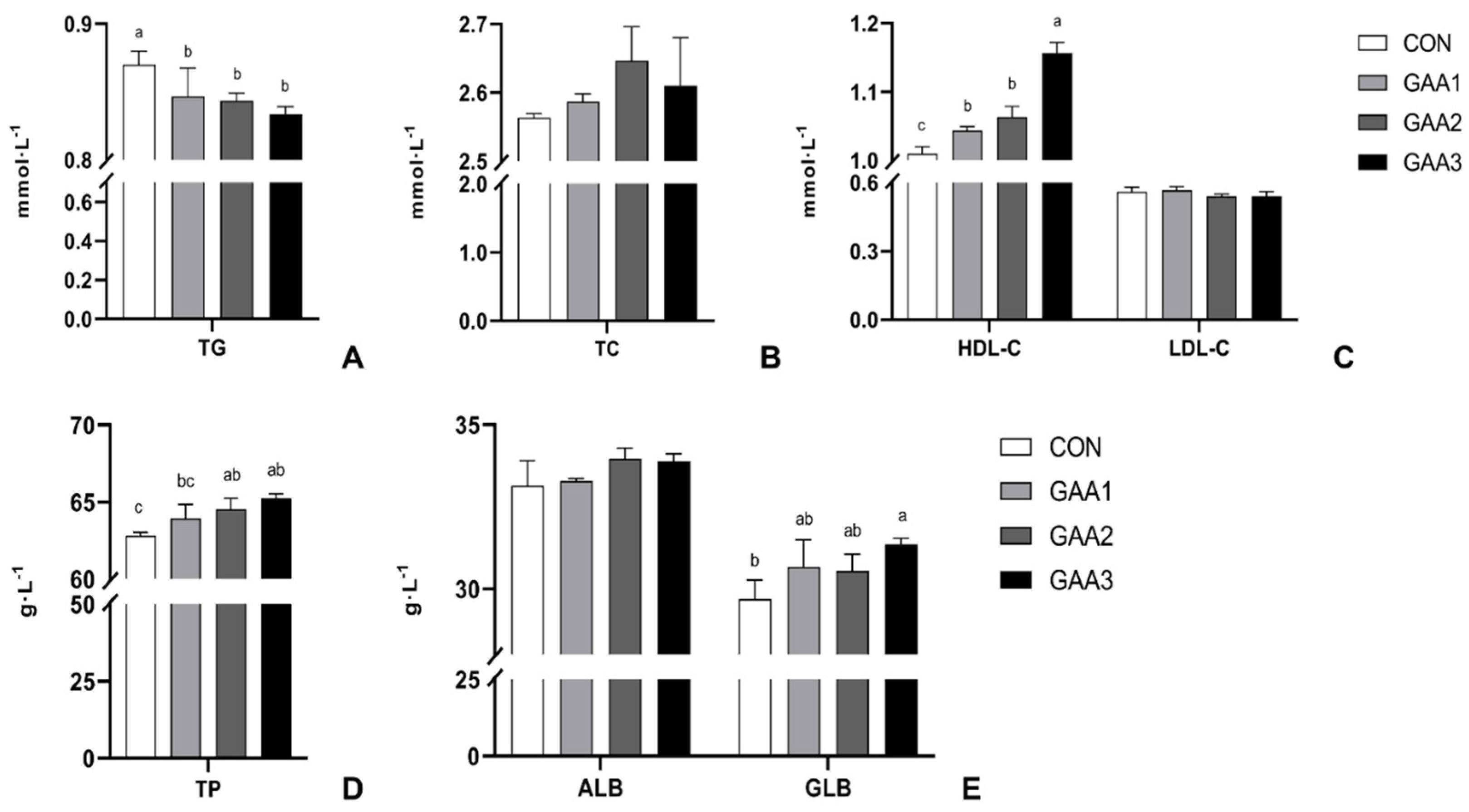
3.2. GAA Supplementation Alters Serum Lipid Profile and Protein Levels
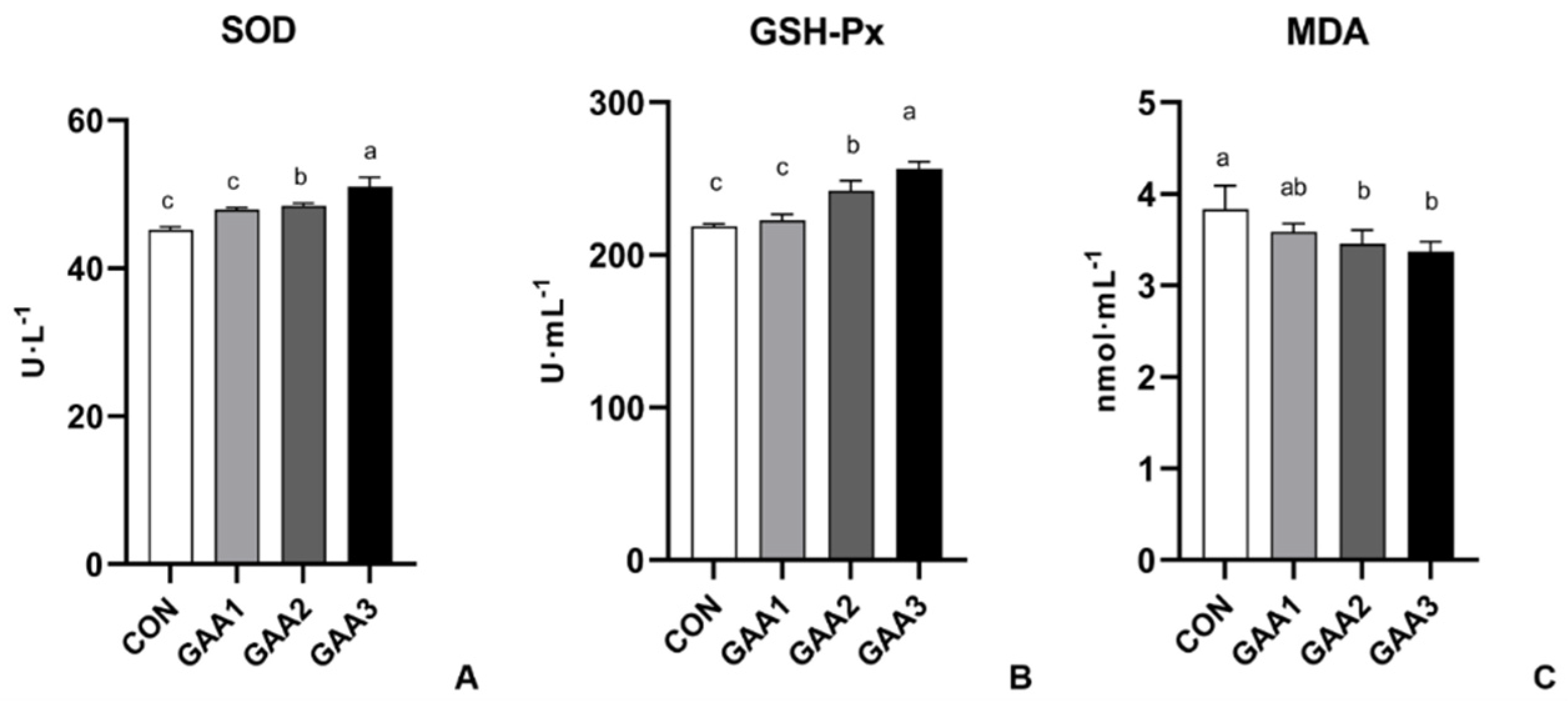
3.3. GAA Supplementation Enhanced the Antioxidant Capacity on Serum
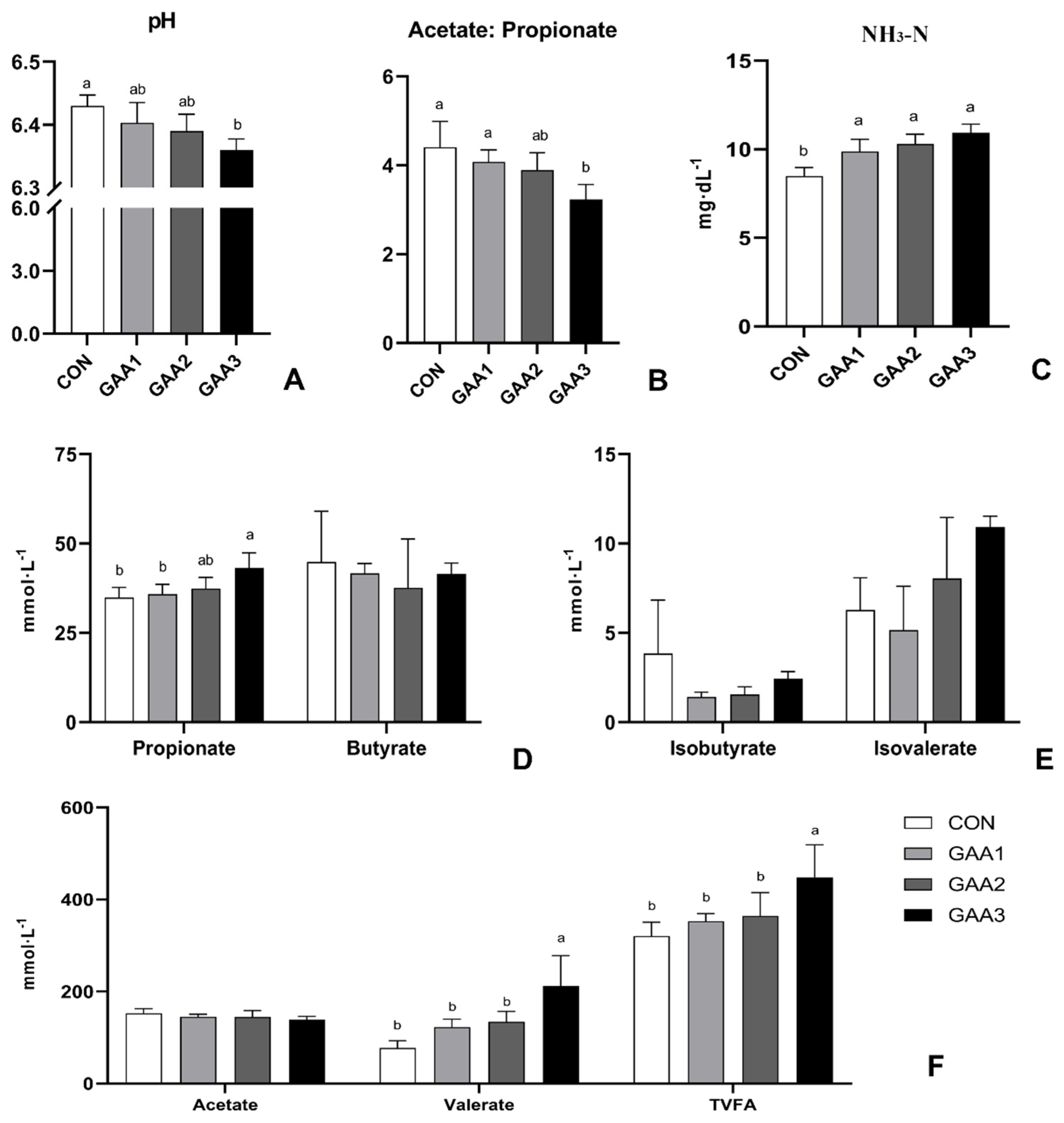
3.4. Modulation of Rumen pH, VFA Profile, and NH3−N Content by GAA Supplementation
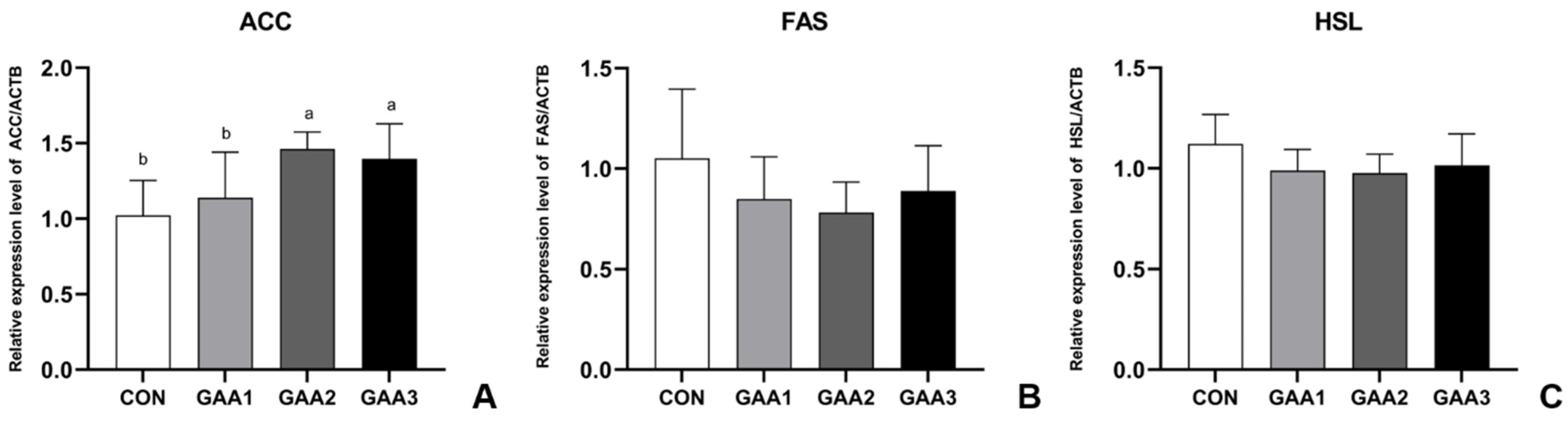
3.5. GAA Supplementation Affects Fatty Acid Metabolism in Longissimus Dorsi Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, D.T.; Gai, X.R.; Yang, L.B.; Li, J.T.; Lai, W.Q.; Sun, X.L.; Zhang, L.Y. Effects of guanidinoacetic acid on growth performance, creatine and energy metabolism, and carcass characteristics in growing-finishing pigs. J. Anim. Sci. 2018, 96, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Stojanovic, M.D.; Olcina, G. Oxidant-Antioxidant Capacity of Dietary Guanidinoacetic Acid. Ann. Nutr. Metab. 2015, 67, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Xu, D.; He, L.; Zhu, X.; Yin, J. Dietary guanidinoacetic acid supplementation improves water holding capacity and lowers free amino acid concentration of fresh meat in finishing pigs fed with various dietary protein levels. Anim. Nutr. 2022, 11, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, B.; La, K.V.; La, H.; Doan, V.; Carpena, E.M.; Rademacher, M.; Channarayapatna, G. Supplementation of guanidinoacetic acid to pig diets: Effects on performance, carcass characteristics, and meat quality. J. Anim. Sci. 2018, 96, 2332–2341. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; McBreairty, L.E.; Ryan, R.A.; Randunu, R.; Walsh, C.J.; Martin, G.M.; Brunton, J.A.; Bertolo, R.F. Effects of supplemental creatine and guanidinoacetic acid on spatial memory and the brain of weaned Yucatan miniature pigs. PLoS ONE 2020, 15, e0226806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Li, H.H.; Zhang, G.J.; Zhang, Y.H.; Liu, B.; Huang, S.; Guyader, J.; Zhong, R.Z. Supplementation of guanidinoacetic acid and rumen-protected methionine increased growth performance and meat quality of Tan lambs. Anim. Biosci. 2022, 35, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Hu, S.; Wang, J.; Abudukelimu, A.; Wang, Y.; Li, X.; Wu, H.; Meng, Q.; Zhou, Z. Effect of Guanidinoacetic Acid Supplementation on Growth Performance, Rumen Fermentation, Blood Indices, Nutrient Digestion, and Nitrogen Metabolism in Angus Steers. Animals 2024, 14, 401. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. Creatine metabolism and the urea cycle. Mol. Genet. Metab. 2010, 100, S49–S52. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Wijekoon, E.P.; Warford-Woolgar, L.; Trottier, N.L.; Brosnan, M.E.; Brunton, J.A.; Bertolo, R.F.P. Creatine Synthesis Is a Major Metabolic Process in Neonatal Piglets and Has Important Implications for Amino Acid Metabolism and Methyl Balance. J. Nutr. 2009, 139, 1292–1297. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Song, P.; Zhao, J.; Zhang, J.; Zhao, J. Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 2022, 192, 108906. [Google Scholar] [CrossRef]
- Ardalan, M.; Batista, E.D.; Titgemeyer, E.C. Effect of post-ruminal guanidinoacetic acid supplementation on creatine synthesis and plasma homocysteine concentrations in cattle. J. Anim. Sci. 2020, 98, skaa072. [Google Scholar] [CrossRef]
- Duan, B.B.; Xu, J.W.; Xing, T.; Li, J.L.; Zhang, L.; Gao, F. Creatine nitrate supplementation strengthens energy status and delays glycolysis of broiler muscle via inhibition of LKB1/AMPK pathway. Poult. Sci. 2022, 101, 101653. [Google Scholar] [CrossRef]
- Córdova-Noboa, H.A.; Oviedo-Rondón, E.O.; Sarsour, A.H.; Barnes, J.; Sapcota, D.; López, D.; Gross, L.; Rademacher-Heilshorn, M.; Braun, U. Effect of guanidinoacetic acid supplementation on live performance, meat quality, pectoral myopathies and blood parameters of male broilers fed corn-based diets with or without poultry by-products. Poult. Sci. 2018, 97, 2494–2505. [Google Scholar] [CrossRef]
- Amiri, M.; Ghasemi, H.A.; Hajkhodadadi, I.; Khaltabadi Farahani, A.H. Efficacy of guanidinoacetic acid at different dietary crude protein levels on growth performance, stress indicators, antioxidant status, and intestinal morphology in broiler chickens subjected to cyclic heat stress. Anim. Feed Sci. Technol. 2019, 254, 114208. [Google Scholar] [CrossRef]
- Khalil, S.; Saenbungkhor, N.; Kesnava, K.; Sivapirunthep, P.; Sitthigripong, R.; Jumanee, S.; Chaosap, C. Effects of Guanidinoacetic Acid Supplementation on Productive Performance, Pectoral Myopathies, and Meat Quality of Broiler Chickens. Animals 2021, 11, 3180. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Fu, Y.; Li, Y.; Jiang, Y.; Zhou, G.; Gao, F. Creatine Monohydrate and Guanidinoacetic Acid Supplementation Affects the Growth Performance, Meat Quality, and Creatine Metabolism of Finishing Pigs. J. Agric. Food Chem. 2018, 66, 9952–9959. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, S.M.S.; Bauer, L.; Htoo, J.K.; Asmussen, S.; Richert, S. 282 Effect of dietary supplementation with guanidinoacetic acid, a creatine precursor, in gestating and lactating sows on sow and litter performance. J. Anim. Sci. 2024, 102, 176–177. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, C.; Wu, Z.Z.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Zhang, Y.L.; Pei, C.X.; et al. Effects of guanidinoacetic acid supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Animal 2020, 14, 2535–2542. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, J.Z.; Wang, D.H.; Wu, M.J.; Zheng, C.; Wu, Z.Z.; Wang, C.; Liu, Q.; Zhang, J.; Guo, G.; et al. Effects of guanidinoacetic acid and coated folic acid supplementation on growth performance, nutrient digestion and hepatic gene expression in Angus bulls. Br. J. Nutr. 2020, 126, 510–517. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, C.; Pan, J.; Ma, C.; Wang, C.; Li, X.; Cai, W.; Yang, K. Effects of dietary guanidinoacetic acid on growth performance, guanidinoacetic acid absorption and creatine metabolism of lambs. PLoS ONE 2022, 17, e0264864. [Google Scholar] [CrossRef]
- Safety and efficacy of guanidinoacetic acid for chickens for fattening, breeder hens and roosters, and pigs. EFSA J. 2016, 14, 4394. [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of guanidinoacetic acid for all animal species (Alzchem Trostberg GmbH). EFSA J. 2022, 20, 7269. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Deng, K.; Liu, Z.; Su, Y.; Fan, Y.; Zhang, Y.; Wang, F. Comparison of muscle fiber characteristics and meat quality between newborn and adult Haimen goats. Meat Sci. 2024, 207, 109361. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.C.; Zeng, X.Z.; Zhu, J.L.; Liu, Y.; Ye, Q.H.; Qiao, S.Y.; Zeng, X.F. Dietary N-Carbamylglutamate Supplementation in a Reduced Protein Diet Affects Carcass Traits and the Profile of Muscle Amino Acids and Fatty Acids in Finishing Pigs. J. Agric. Food Chem. 2017, 65, 5751–5758. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, J.C.; Lategan, P.M. Gas chromatographic determination of C2-C5 fatty acids in aqueous media with a Porapak N column. J. Chromatogr. 1976, 124, 63–65. [Google Scholar] [CrossRef] [PubMed]
- GA, B.; JH, K. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci 1980, 63, 64–75. [Google Scholar]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. 2021, 49, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, N.; Koca, S.; Golzar Adabi, S.; Kahraman, N.; Bhaya, M.N.; Bozkurt, M.F. Effects of dietary energy level and guanidino acetic acid supplementation on growth performance, carcass quality and intestinal architecture of broilers. Czech J. Anim. Sci. 2021, 66, 281–291. [Google Scholar] [CrossRef]
- Majdeddin, M.; Golian, A.; Kermanshahi, H.; De Smet, S.; Michiels, J. Guanidinoacetic acid supplementation in broiler chickens fed on corn-soybean diets affects performance in the finisher period and energy metabolites in breast muscle independent of diet nutrient density. Br. Poult. Sci. 2018, 59, 443–451. [Google Scholar] [CrossRef]
- Yan, Z.; Yan, Z.; Liu, S.; Yin, Y.; Yang, T.; Chen, Q. Regulative Mechanism of Guanidinoacetic Acid on Skeletal Muscle Development and Its Application Prospects in Animal Husbandry: A Review. Front. Nutr. 2021, 8, 714567. [Google Scholar] [CrossRef]
- Du, M.; Zhao, J.X.; Yan, X.; Huang, Y.; Nicodemus, L.V.; Yue, W.; McCormick, R.J.; Zhu, M.J. Fetal muscle development, mesenchymal multipotent cell differentiation, and associated signaling pathways1,2. J. Anim. Sci. 2011, 89, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lealiifano, A.K.; Cadogan, D.J.; White, S.; Grigg, H.C.; Brewster, C.J. 68. In-feed guanidinoacetic acid improves meat quality in finisher pigs. Anim. Sci. Proc. 2021, 12, 230. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Xin, J.; Xu, L.; Li, M.; Yu, H.; Zhang, W.; Ge, Y.; Li, Y.; Qu, M. Effects of Dietary Guanidinoacetic Acid on the Feed Efficiency, Blood Measures, and Meat Quality of Jinjiang Bulls. Front. Vet. Sci. 2021, 8, e684295. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Warner, R.D.; Rosenvold, K. Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: A review. Anim. Prod. Sci. 2014, 54, 375–395. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.Y.; Zhang, K.Y.; Tian, G.; Bai, S.P.; Ding, X.M.; Wang, J.P.; Peng, H.W.; Lv, L.; Xuan, Y.; Zeng, Q.F. Effects of dietary corn germ meal levels on growth performance, serum biochemical parameters, meat quality, and standardized ileal digestibility of amino acids in Pekin ducks. Poult. Sci. 2022, 101, 101779. [Google Scholar] [CrossRef] [PubMed]
- Kokoszyński, D.; Piwczyński, D.; Arpášová, H.; Hrnčar, C.; Saleh, M.; Wasilewski, R. A comparative study of carcass characteristics and meat quality in genetic resources Pekin ducks and commercial crossbreds. Asian-Australas. J. Anim. Sci. 2019, 32, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wan, X.L.; Zhang, L.L.; Wang, C.; He, J.T.; Bai, K.W.; Zhang, X.H.; Zhao, L.G.; Wang, T. Effect of different doses of fermented Ginkgo biloba leaves on serum biochemistry, antioxidant capacity hepatic gene expression in broilers. Anim. Feed Sci. Technol. 2019, 248, 132–140. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Huang, C.; Wang, D.; Chang, D.; Shi, X.; Chen, Y.; Chen, H. Positive effects of Mulberry leaf extract on egg quality, lipid metabolism, serum biochemistry, and antioxidant indices of laying hens. Front. Vet. Sci. 2022, 9, e1005643. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, S.; Fan, S.; Jin, Z.; Bao, Q.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Comparison of growth performance, meat quality and blood biochemical indexes of Yangzhou goose under different feeding patterns. Poult. Sci. 2023, 103, 103349. [Google Scholar] [CrossRef]
- Liu, H.N.; Liu, Y.; Hu, L.L.; Suo, Y.L.; Zhang, L.; Jin, F.; Feng, X.A.; Teng, N.; Li, Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014, 93, 347–353. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Xing, T.; Zhang, L.; Gao, F. Effects of guanidinoacetic acid and complex antioxidant supplementation on growth performance, meat quality, and antioxidant function of broiler chickens. J. Sci. Food Agric. 2021, 101, 3961–3968. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Advanced physiological roles of guanidinoacetic acid. Eur. J. Nutr. 2015, 54, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Barnes, W.S.; Wu, G.; Song, W.; Demaree, S. Direct Antioxidant Properties of Creatine. Biochem. Biophys. Res. Commun. 2002, 290, 47–52. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Wang, C.; Guo, G.; Huo, W.; Xia, C.; Chen, L.; Zhang, Y.; Pei, C.; Liu, Q. Effects of guanidinoacetic acid supplementation on lactation performance, nutrient digestion and rumen fermentation in Holstein dairy cows. J. Sci. Food Agric. 2022, 103, 1522–1529. [Google Scholar] [CrossRef]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal pH regulation and nutritional consequences of low pH. Anim. Feed Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kim, I.H.; Cho, J.H.; Yoo, J.S.; Kim, H.J.; Shin, S.O. Utilization of δ-aminolevulinic acid for livestock: Blood characteristics and immune organ weight in broilers. J. Anim. Feed. Sci. 2008, 17, 215. [Google Scholar] [CrossRef]
- Brunetto, A.L.R.; Giacomelli, C.M.; Favero, J.F.; Bissacotti, B.F.; Copeti, P.M.; Morsch, V.M.; de Oliveira, F.d.C.; Wagner, R.; Alves, R.; Pereira, W.A.B.; et al. Phytogenic blend in the diet of growing Holstein steers: Effects on performance, digestibility, rumen volatile fatty acid profile, and immune and antioxidant responses. Anim. Feed Sci. Technol. 2023, 297, 115595. [Google Scholar] [CrossRef]
- Stepanchenko, N.; Stefenoni, H.; Hennessy, M.; Nagaraju, I.; Wasson, D.E.; Cueva, S.F.; Räisänen, S.E.; Dechow, C.D.; Pitta, D.W.; Hristov, A.N. Microbial composition, rumen fermentation parameters, enteric methane emissions, and lactational performance of phenotypically high and low methane-emitting dairy cows. J. Dairy Sci. 2023, 106, 6146–6170. [Google Scholar] [CrossRef]
- Thao, N.T.; Wanapat, M.; Cherdthong, A.; Kang, S. Effects of Eucalyptus Crude Oils Supplementation on Rumen Fermentation, Microorganism and Nutrient Digestibility in Swamp Buffaloes. Asian-Australas. J. Anim. Sci. 2014, 27, 46–54. [Google Scholar] [CrossRef]
- Frank, D.; Joo, S.-T.; Warner, R. Consumer Acceptability of Intramuscular Fat. Korean J. Food Sci. Anim. Resour. 2016, 36, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-M.; Li, F.-Q.-Y.; Li, X.-Y.; Jiao, D.-R.; Liu, X.-D.; Lv, X.-Y.; Zhao, J.-X. Guanidinoacetic Acid Attenuates Adipogenesis through Regulation of miR−133a in Sheep. Animals 2023, 13, 3108. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Huang, Z.; Li, Q.; Liu, Z.; Hao, C.; Shi, G.; Dai, R.; Xie, Z. Developmental Changes of the FAS and HSL mRNA Expression and Their Effects on the Content of Intramuscular Fat in Kazak and Xinjiang Sheep. J. Genet. Genom. 2007, 34, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, X.; Yang, Y.; Niu, W.; Kou, Q.; Wang, X.; Chen, Y. PPARγ, FAS, HSL mRNA and protein expression during Tan sheep fat-tail development. Electron. J. Biotechnol. 2015, 18, 122–127. [Google Scholar] [CrossRef]
- Recazens, E.; Mouisel, E.; Langin, D. Hormone-sensitive lipase: Sixty years later. Prog. Lipid Res. 2021, 82, 101084. [Google Scholar] [CrossRef]
| Dietary Ingredient | Contents |
|---|---|
| Bamboo shoot shells silage | 60 |
| Corn | 17 |
| Bran | 12 |
| Soybean meal | 6 |
| NaCl | 1 |
| CaHCO3 | 1.4 |
| NaHCO3 | 1.2 |
| Premix (1) | 1.4 |
| Total: | 100 |
| Nutrient levels (2) | |
| Metabolizable energy (MJ/Kg) | 9.68 |
| Crude protein | 11.55 |
| Neutral detergent fiber | 58.46 |
| Acid detergent fiber | 36.32 |
| Calcium | 0.75 |
| Phosphorus | 0.50 |
| Items | Primer Sequences (5′−3′) | Genebank Number | Product Length (bp) |
|---|---|---|---|
| ACC | F: CCTGTCCGCCATTGACAT | NM_001009256.1 | 171 |
| R: TAGGCGATATAAGCCCTTCG | |||
| FAS | F: CAGTCGGTTGGATCGAGCAT | XM_015098375.1 | 151 |
| R: AGAAGGAGGGTGGCTTTTGG | |||
| HSL | F: TACAAACGCAACGAGACTGG | NM_001128154.1 | 172 |
| R: ACGATAGCACCTGGATCTCG | |||
| ACTB | F: CCCTGGAGAAGAGCTACGAG | NM_001009784.3 | 131 |
| R: GGTAGTTTCGTGAATGCCGC |
| Items | Groups | p-Value | |||
|---|---|---|---|---|---|
| CON | GAA−1 | GAA−2 | GAA−3 | ||
| Growth performance | |||||
| Initial BW (kg) | 17.45 ± 2.53 | 17.66 ± 3.28 | 17.99 ± 3.04 | 17.71 ± 2.79 | 0.960 |
| Final BW (kg) | 28.05 ± 2.60 | 28.75 ± 3.01 | 29.45 ± 3.65 | 29.42 ± 3.20 | 0.872 |
| ADFI (kg) | 1.17 ± 0.17 | 1.17 ± 0.22 | 1.26 ± 0.21 | 1.24 ± 0.23 | 0.934 |
| ADG (g) | 117.85 ± 1.57 b | 123.31 ± 3.76 ab | 127.43 ± 3.24 a | 130.12 ± 2.39 a | 0.021 |
| F/G | 9.71 ± 1.41 | 9.51 ± 1.81 | 10.15 ± 1.71 | 9.95 ± 1.85 | 0.969 |
| Carcass traits | |||||
| Live weight (kg) | 28.50 ± 0.50 | 28.50 ± 0.50 | 28.83 ± 0.29 | 29.00 ± 0.50 | 0.482 |
| Carcass weight (kg) | 12.35 ± 0.10 b | 12.35 ± 0.30 b | 12.45 ± 0.26 b | 13.17 ± 0.40 a | 0.023 |
| Dressing percentage (%) | 43.34 ± 1.11 | 43.34 ± 1.26 | 43.19 ± 1.25 | 45.40 ± 1.14 | 0.147 |
| Eye muscle area (cm2) | 11.67 ± 1.38 | 11.47 ± 1.06 | 13.25 ± 0.90 | 12.92 ± 1.76 | 0.318 |
| Items | Groups | p-Value | |||
|---|---|---|---|---|---|
| CON | GAA−1 | GAA−2 | GAA−3 | ||
| pH24h | 5.22 ± 0.21 | 5.27 ± 0.19 | 5.36 ± 0.17 | 5.38 ± 0.40 | 0.890 |
| Drip loss (%) | 7.15 ± 2.85 a | 6.48 ± 1.52 ab | 5.01 ± 0.93 ab | 3.41 ± 0.55 b | 0.027 |
| Shear force (N) | 17.21 ± 0.9 b | 21.97 ± 1.55 ab | 23.49 ± 2.21 a | 25.71 ± 1.66 a | 0.034 |
| Intramuscular fat (%) | 5.42 ± 0.66 | 6.00 ± 0.75 | 6.33 ± 0.75 | 6.58 ± 0.82 | 0.328 |
| Muscle fiber diameter (um) | 27.77 ± 3.81 b | 28.08 ± 2.73 ab | 30.46 ± 3.32 a | 30.42 ± 3.50 a | 0.048 |
| Total muscle fibers (N) | 225.44 ± 46.75 | 202.56 ± 81.15 | 171.11 ± 25.77 | 213 ± 25.75 | 0.145 |
| Total muscle fiber area (mm2) | 0.15 ± 0.01 | 0.14 ± 0.04 | 0.16 ± 0.03 | 0.15 ± 0.01 | 0.362 |
| Muscle fiber density (N/mm2) | 1550.10 ± 298.57 a | 1375.38 ± 298.47 a | 1468.34 ± 161.05 a | 1089.78 ± 2 69.89 b | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Du, Z.; Fan, X.; Qin, L.; Liu, W.; Zhang, Y.; Ren, J.; Ye, C.; Liu, Q. Effect of Guanidinoacetic Acid on Production Performance, Serum Biochemistry, Meat Quality and Rumen Fermentation in Hu Sheep. Animals 2024, 14, 2052. https://doi.org/10.3390/ani14142052
Jin H, Du Z, Fan X, Qin L, Liu W, Zhang Y, Ren J, Ye C, Liu Q. Effect of Guanidinoacetic Acid on Production Performance, Serum Biochemistry, Meat Quality and Rumen Fermentation in Hu Sheep. Animals. 2024; 14(14):2052. https://doi.org/10.3390/ani14142052
Chicago/Turabian StyleJin, Huayun, Zhijian Du, Xiaoyu Fan, Liwen Qin, Weiwei Liu, Yan Zhang, Jingnan Ren, Changchuan Ye, and Qinghua Liu. 2024. "Effect of Guanidinoacetic Acid on Production Performance, Serum Biochemistry, Meat Quality and Rumen Fermentation in Hu Sheep" Animals 14, no. 14: 2052. https://doi.org/10.3390/ani14142052
APA StyleJin, H., Du, Z., Fan, X., Qin, L., Liu, W., Zhang, Y., Ren, J., Ye, C., & Liu, Q. (2024). Effect of Guanidinoacetic Acid on Production Performance, Serum Biochemistry, Meat Quality and Rumen Fermentation in Hu Sheep. Animals, 14(14), 2052. https://doi.org/10.3390/ani14142052






