Gastrointestinal Helminths in Wild Felids in the Cerrado and Pantanal: Zoonotic Bioindicators in Important Brazilian Biomes
Abstract
Simple Summary
Abstract
1. Introduction
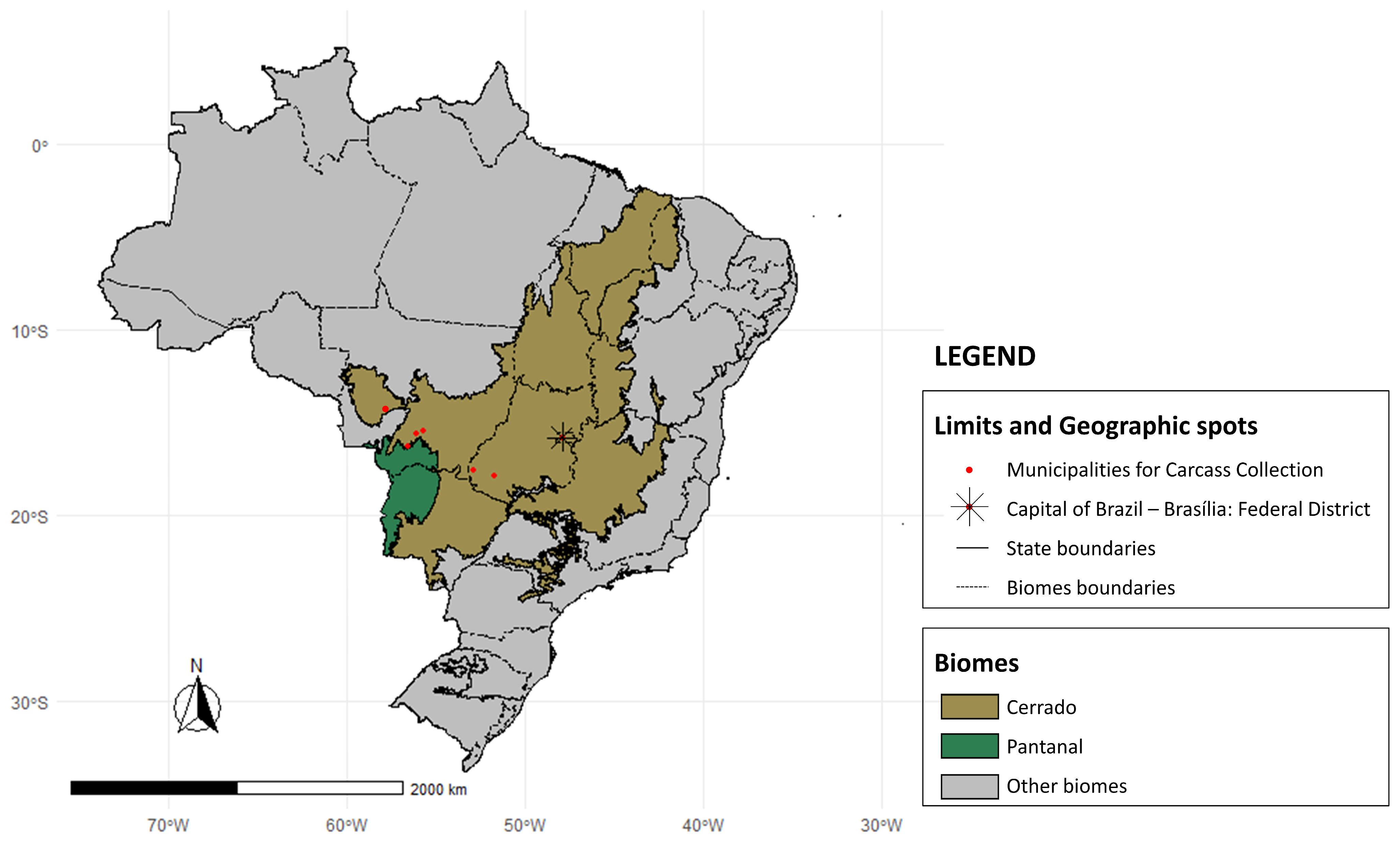
2. Materials and Methods
3. Results
3.1. Hosts and Municipalities
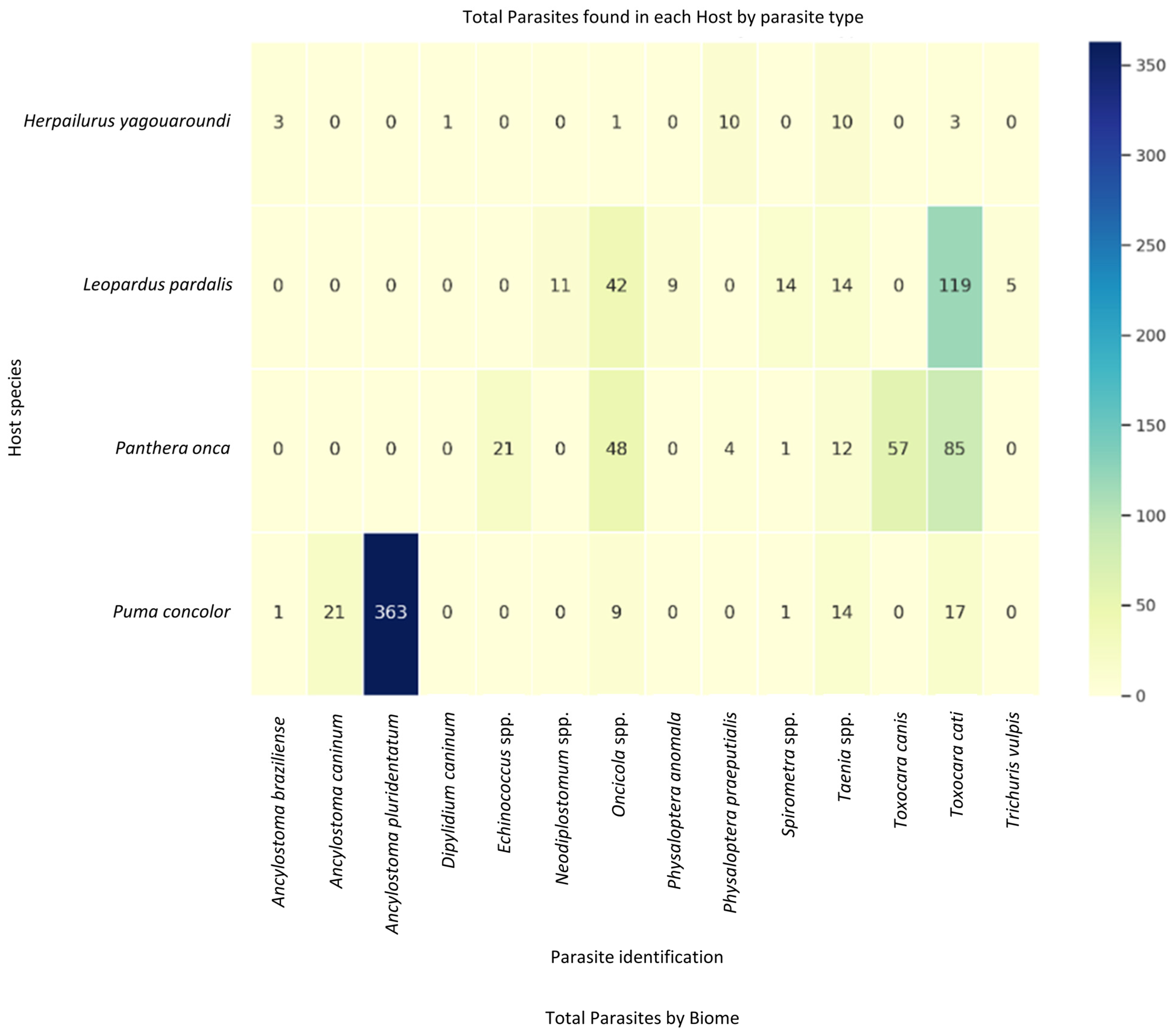
3.2. Parasite Identification and Load
3.3. Taxonomic Distribution
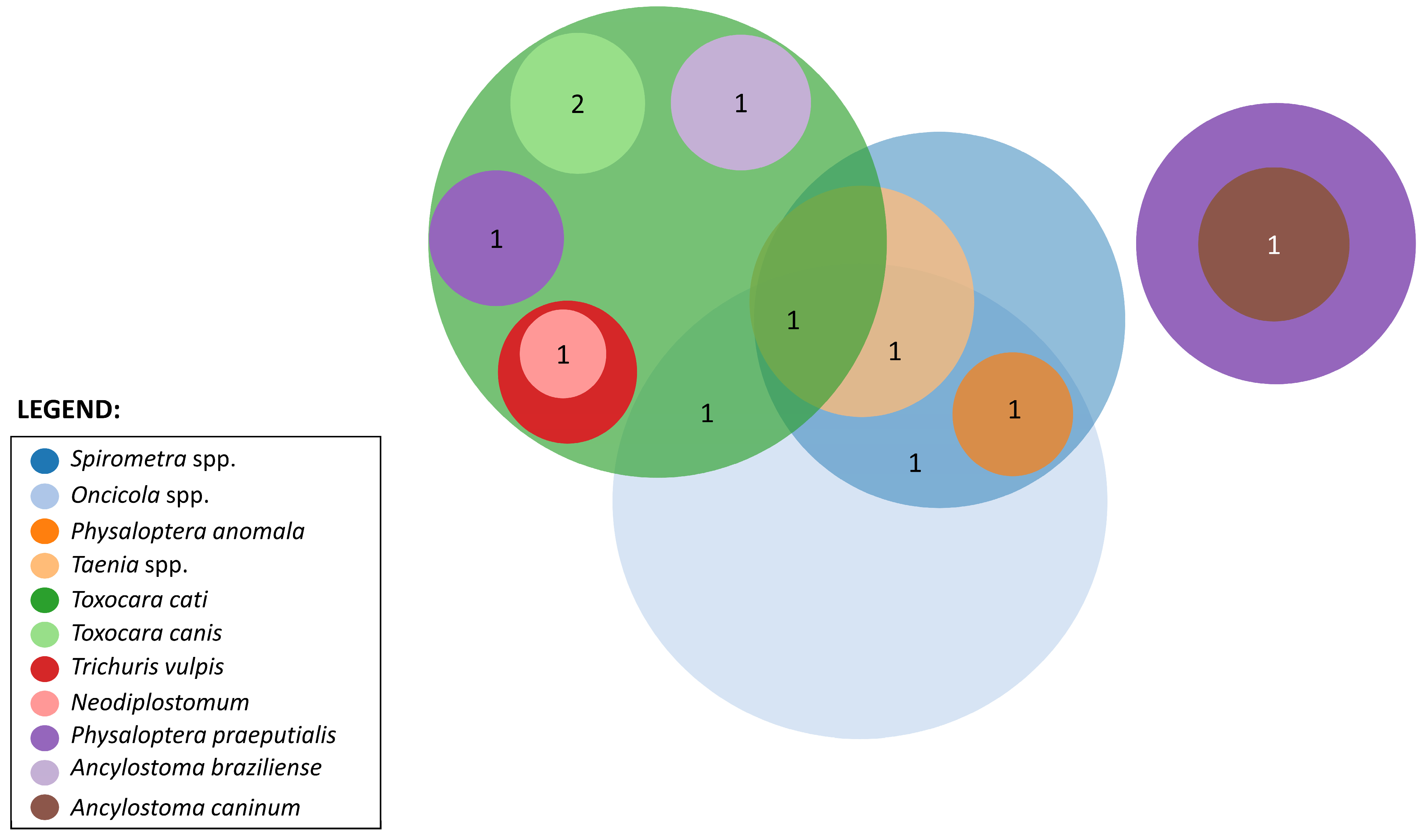
3.4. Co-Infection Patterns
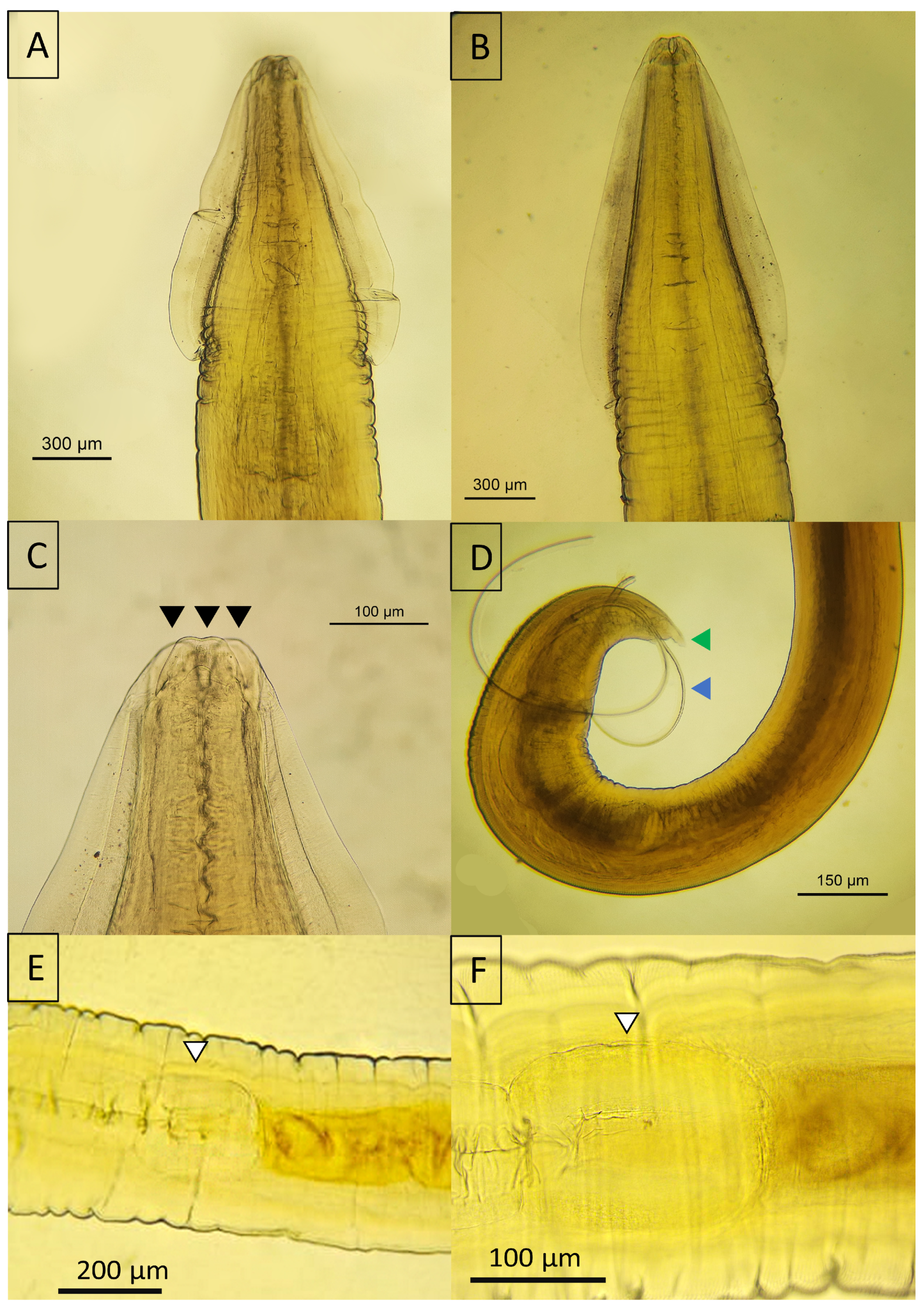
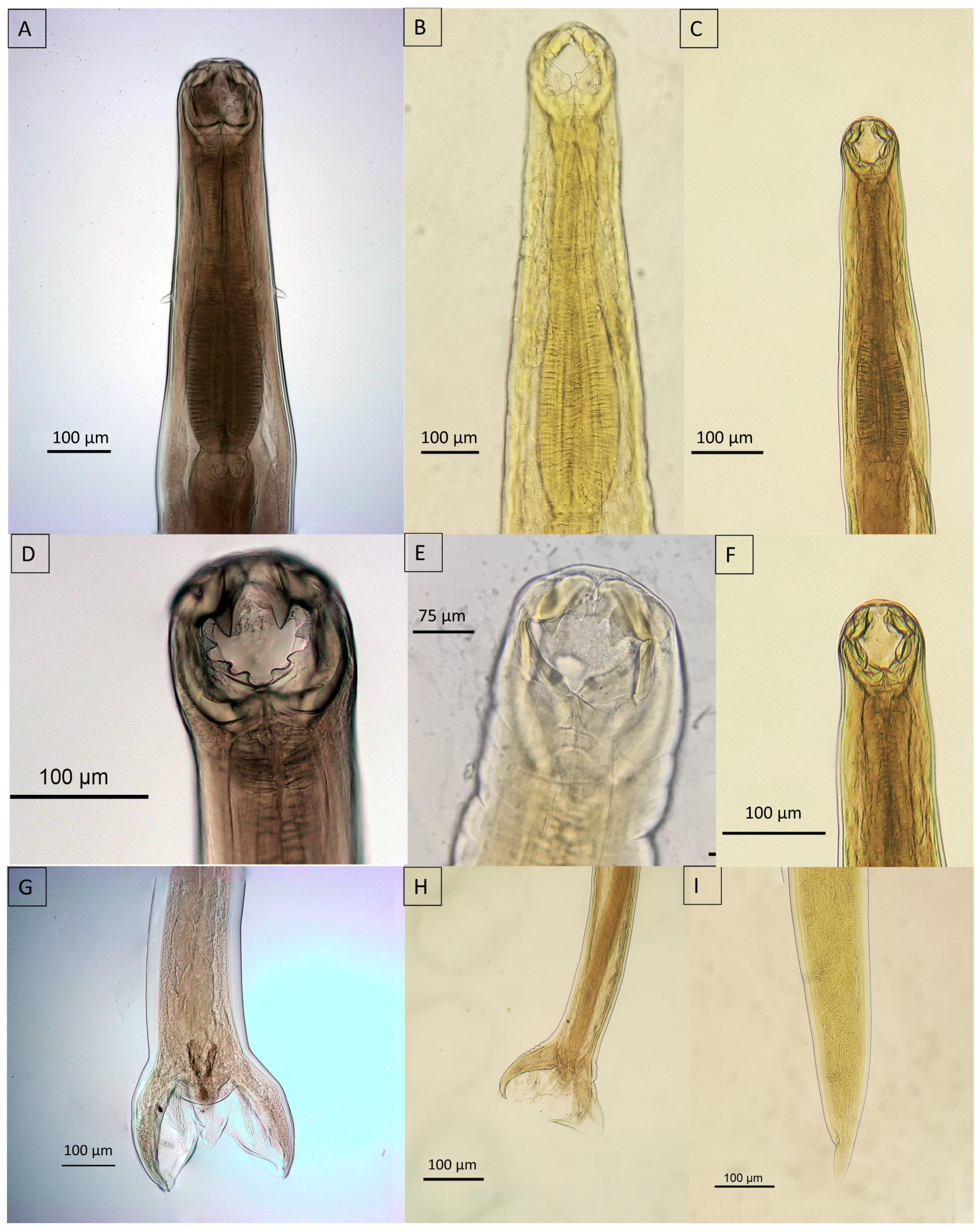
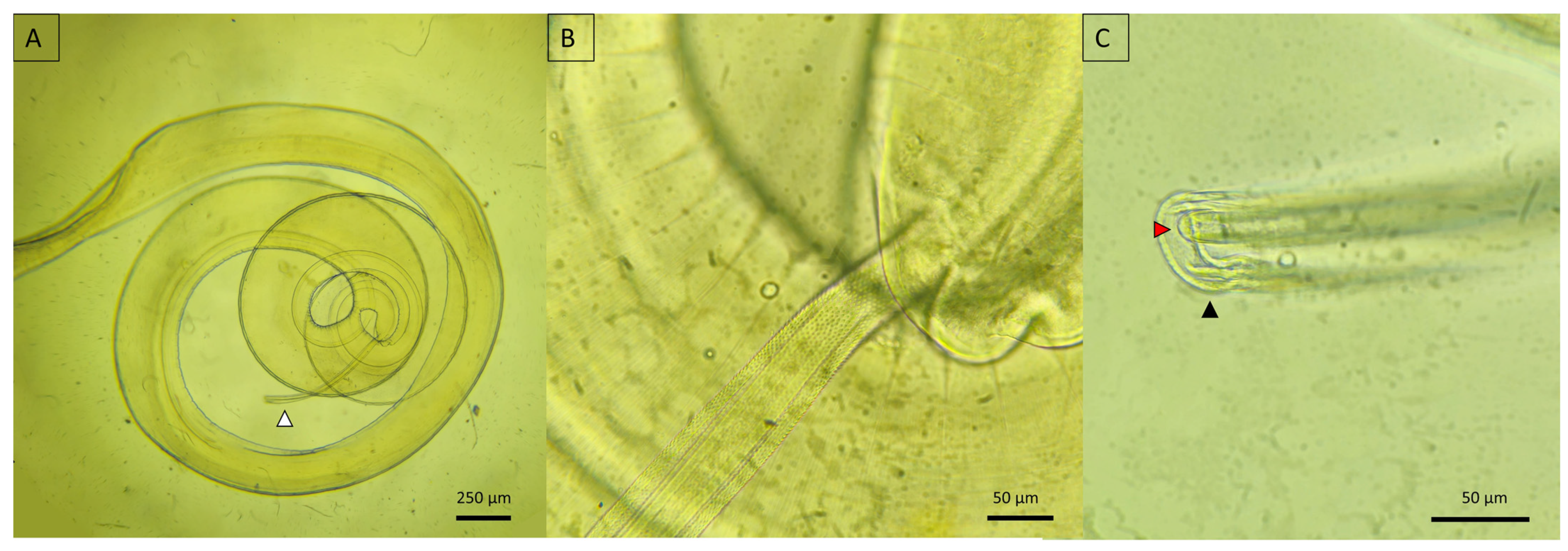
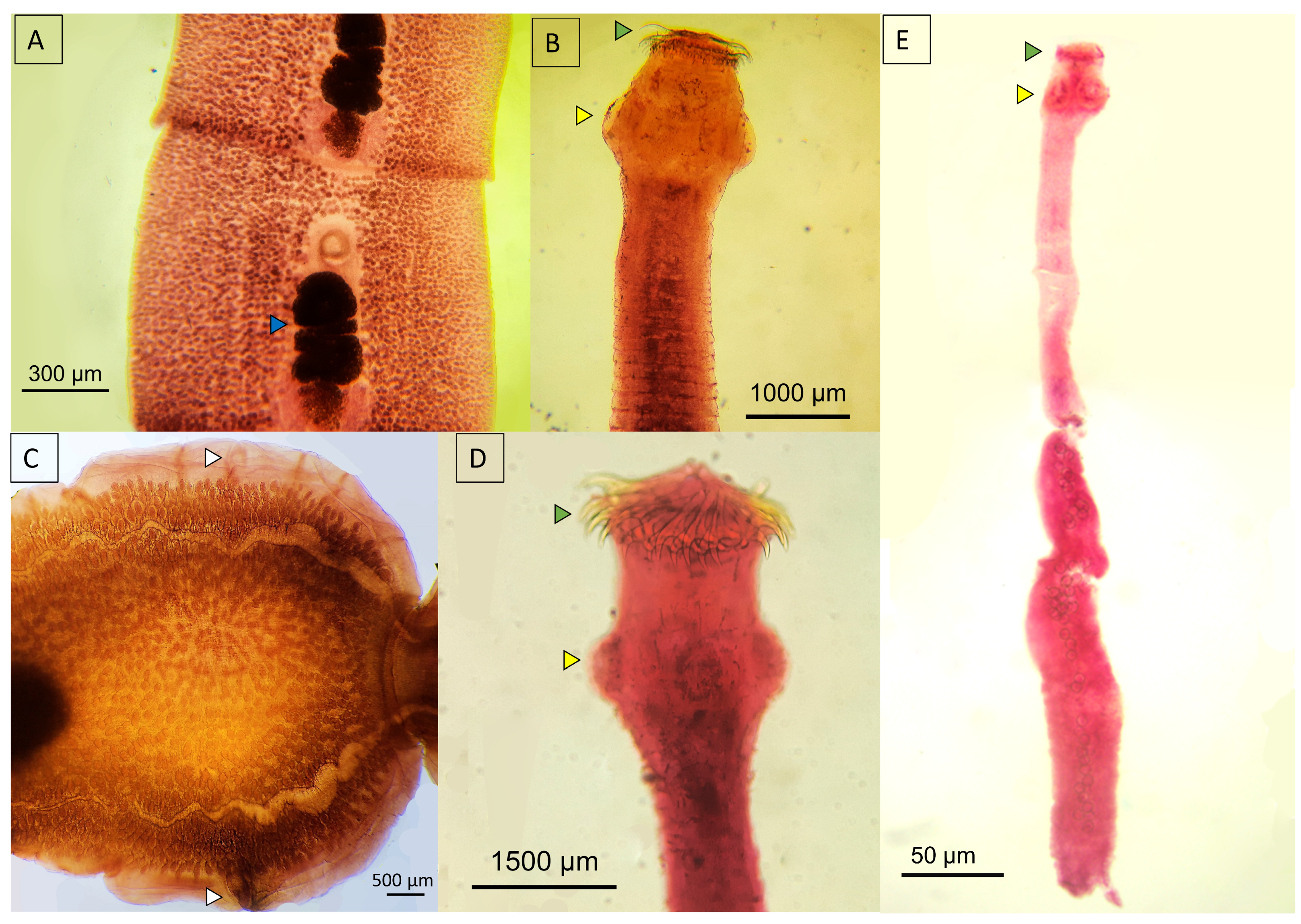
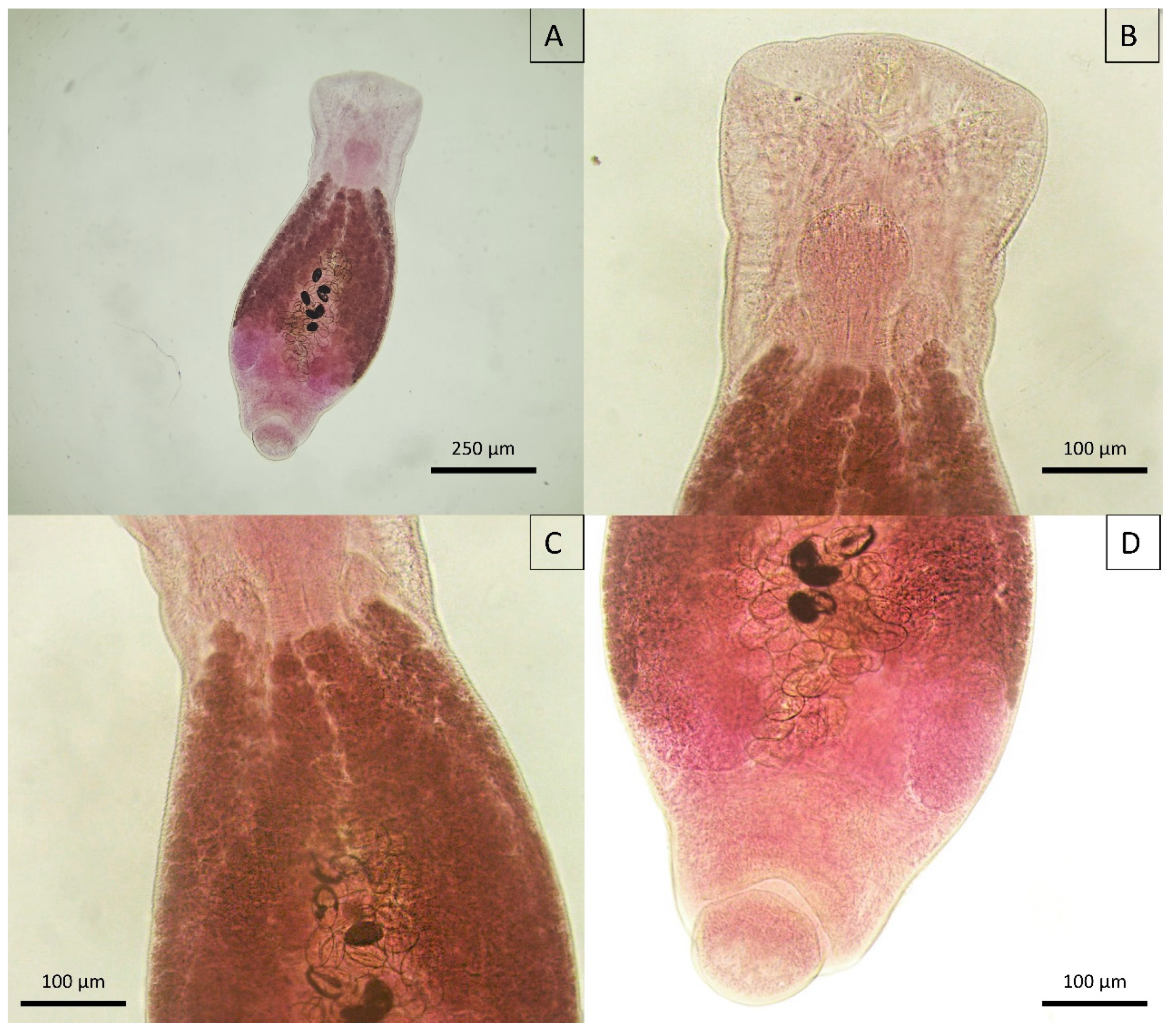
3.5. Morphological Insights
3.5.1. Acanthocephala
3.5.2. Nematoda
3.5.3. Cestoda
3.5.4. Trematoda
4. Discussion
4.1. Parasite Occurrence and Consequences
4.2. Impacts of Environmental Changes and Co-Infections Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Junk, W.; Cunha, C.N.; Wantzen, K.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Harris, M.B.; Tomas, W.; Mourão, G.; Silva, C.J.; Guimarães, E.; Sonoda, F.; Fachim, E. Safeguarding the Pantanal Wetlands: Threats and Conservation Initiatives. Conserv. Biol. 2005, 19, 714–720. [Google Scholar] [CrossRef]
- Ratter, J.; Ribeiro, J.F.; Bridgewater, S. The Brazilian Cerrado Vegetation and Threats to its Biodiversity. Ann. Bot. 1997, 80, 223–230. [Google Scholar] [CrossRef]
- Bortolotto, I.M.; Hiane, P.; Ishii, I.; Souza, P.R.; Campos, R.P.; Gomes, R.B.; Corrêa da Costa, L.B.L. A knowledge network to promote the use and valorization of wild food plants in the Pantanal and Cerrado, Brazil. Reg. Environ. Chang. 2017, 17, 1329–1341. [Google Scholar] [CrossRef]
- Lourival, R.; McCallum, H.; Grigg, G.; Arcângelo, C.; Machado, R.; Possingham, H. A systematic evaluation of the conservation plans for the Pantanal wetland in Brazil. Wetlands 2009, 29, 1189–1201. [Google Scholar] [CrossRef]
- Hernández-Camacho, N.; Zamora-Ledesma, S. The potential of the parasite fauna as an indicator of ecosystem health in the anthropized environments of Mexico. In Mexican Fauna in the Anthropocene; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 569–579. [Google Scholar]
- Fleschutz, M.M.; Gálvez, N.; Pe’er, G.; Davies, Z.G.; Henle, K.; Schüttler, E. Response of a small felid of conservation concern to habitat fragmentation. Biodiv Conserv. 2016, 25, 1447–1463. [Google Scholar] [CrossRef]
- Zanin, M.; Palomares, F.; Brito, D. What we (don’t) know about the effects of habitat loss and fragmentation on felids. Oryx 2015, 49, 96–106. [Google Scholar] [CrossRef]
- Eisenberg, C.; Eisenberg, L. Jaguar (Panthera onca). The Carnivore Way: Coexisting with and Conserving North America’s Predators; Island Press: Washington, DC, USA, 2014; pp. 217–240. [Google Scholar]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Cui, X.; Fan, K.; Liang, X.; Gong, W.; Chen, W.; He, B.; Shen, Y. Virus diversity, wildlife-domestic animal circulation and potential zoonotic viruses of small mammals, pangolins and zoo animals. Nat. Commun. 2023, 14, 2488. [Google Scholar] [CrossRef]
- Esposito, M.M.; Turku, S.; Lehrfield, L.; Shoman, A. The impact of human activities on zoonotic infection transmissions. Animals 2023, 13, 1646. [Google Scholar] [CrossRef]
- Uribe, M.; Payán, E.; Brabec, J.; Vélez, J.; Taubert, A.; Chaparro-Gutiérrez, J.J.; Hermosilla, C. Intestinal parasites of neotropical wild jaguars, pumas, ocelots, and jaguarundis in Colombia: Old friends brought back from oblivion and new insights. Pathogens 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Perrucci, S.; Maestrini, M.; Coppola, F.; Di Marco, M.; Rosso, A.D.; Pacini, M.I.; Zintu, P.; Felicioli, A. Gray wolf (Canis lupus italicus) and red fox (Vulpes vulpes) parasite survey in anthropized and natural areas of Central Italy. Vet. Sci. 2023, 10, 108. [Google Scholar] [CrossRef]
- Fagre, A.C.; Cohen, L.E.; Eskew, E.A.; Farrell, M.; Glennon, E.; Joseph, M.B.; Albery, G.F. Assessing the risk of human-to-wildlife pathogen transmission for conservation and public health. Ecol. Lett. 2022, 25, 1534–1549. [Google Scholar] [CrossRef]
- Lempp, C.; Jungwirth, N.; Grilo, M.; Reckendorf, A.; Ulrich, A.; van Neer, A.; Bodewes, R.; Pfankuche, V.; Bauer, C.; Osterhaus, A.; et al. Pathological findings in the red fox (Vulpes vulpes), stone marten (Martes foina), and raccoon dog (Nyctereutes procyonoides), with special emphasis on infectious and zoonotic agents in Northern Germany. PLoS ONE 2017, 12, e0175469. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Rabozzi, G.; Bonizzi, L.; Crespi, E.; Somaruga, C.; Sokooti, M.; Tabibi, R.; Colosio, C. Emerging Zoonoses: The “One Health Approach”. Saf. Health Work. 2012, 3, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mackenstedt, U.; Jenkins, D.; Romig, T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasit. Wildl. 2015, 4, 71–79. [Google Scholar] [CrossRef]
- Devaux, C.A.; Mediannikov, O.; Medkour, H.; Raoult, D. Infectious disease risk across the growing human population. Microb. Pathog. 2019, 126, 139–149. [Google Scholar]
- Rainova, I.; Harizanov, R.; Kaftandjiev, I.; Tsvetkova, N.; Mikov, O.; Jordanova, D. Zoonotic helminths in Bulgaria. Acta Zool. Bulg. 2019, 71, 279–283. [Google Scholar]
- Hoffmann, R.P. Diagnóstico de Parasitismo Veterinário; Porto Alegre: Sulina, Romania, 1987. [Google Scholar]
- Amato, J.F.R. 8. Platelmintos (temnocefálidos, trematódeos, cestóides, cestodários) e acantocéfalos. In Manual de Técnicas para a Preparacão de Coleções Zoológicas; Sociedade Brasileira de Zoologia: São Paulo, Brazil, 1985. [Google Scholar]
- Vicente, J.J.; Rodrigues, H.O.; Gomes, D.C.; Pinto, R.M. Nematodes of Brazil. Part V: Nematodes of mammals. Rev. Bras. Zool. 1997, 14 (Suppl. 1), 1–452. [Google Scholar] [CrossRef]
- Khalil, L.F.; Jones, A.; Bray, R.A. Keys to the Cestode Parasites of Vertebrates; CABI International: London, UK, 1994. [Google Scholar]
- Schmidt, G.D. Revision of the Class Archiacanthocephala Meyer, 1931 (Phylum Acanthocephala), with Emphasis on Oligacanthorhynchidae Southwell et Macfie, 1925. J. Parasitol. 1972, 58, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.I.; Jones, A.; Bray, R.A. Keys to the Trematoda; CABI International: London, UK, 2002; Volume 2. [Google Scholar]
- Anderson, R.C.; Chabaud, A.G.; Willmott, S. Keys to the Nematode Parasites of Vertebrates; Archival Volume; CABI International: London, UK, 2009. [Google Scholar]
- Gibbons, L.M. Keys to the Nematode Parasites of Vertebrates; Supplementary Volume; CABI International: London, UK, 2010. [Google Scholar]
- Thatcher, V.E. Some hookworms of the genus Ancylostoma from Colombia and Panama. Proc. Helminthol. Soc. Wash. 1971, 38, 109–116. [Google Scholar]
- Ortlepp, M.A. The nematode genus Physaloptera. Rud. Proc. Zool. Soe. 1922, 2, 999–1107. [Google Scholar]
- Ortlepp, M.A. Some undescribed species ofthe nematode genus Physaloptera Rud., together with a key to the sufficiently known forms. Ondepstepoort J. Vet. Sei Anim. Indust. 1937, 9, 71–84. [Google Scholar]
- Yevstafieva, V.A.; Kravchenko, S.O.; Gutyj, B.V.; Melnychuk, V.V.; Kovalenko, P.N.; Volovyk, L.B. Morphobiological analysis of Trichuris vulpis (Nematoda, Trichuridae), obtained from domestic dogs. Regul. Mech. Biosyst. 2019, 10, 165–176. [Google Scholar] [CrossRef]
- Yasuda, F.; Nishikawa, H.; Ujihashi, M.; Watanabe, S. Studies on the life-history of Dipylidium caninum (Linnaeus, 1758). II. The ecology of the mature proglottids and the cellophane-tape method of diagnosis based on the biological characteristics of the proglottids. Bull. Nippon. Vet. Zootech. Coll. 1970, 18, 122–128. [Google Scholar]
- Dubois, G. Étude de quelques strigéidés d’Australie et notes sur le genre Fibricola Dubois 1932. Ann. Parasitol. Hum. Comp. 1937, 15, 231–247. [Google Scholar] [CrossRef]
- Carrera-Játiva, P.D.; Acosta-Jamett, G. Influence of habitat alteration on the structure of helminth communities in small mammals: A systematic review and critical appraisal of theory and current evidence. Parasitol. Res. 2023, 122, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.W.; Cunningham, M.W.; Kinsella, J.M.; McLaughlin, G.; Forrester, D.J. Gastrointestinal helminths of free-ranging Florida panthers (Puma concolor coryi) and the efficacy of the current anthelmintic treatment protocol. J. Wildl. Dis. 2006, 42, 402–406. [Google Scholar] [CrossRef]
- Quintana, T.; Johnson, W.L.; Ritchie, D.; Smith, V.; Martin, K.A.; McMahan, K.; Brewer, M.; Jesudoss Chelladurai, J.J. Genetic characterization of the zoonotic parasite Ancylostoma caninum in the central and eastern United States. J. Helminthol. 2023, 97, e37. [Google Scholar] [CrossRef]
- Stocker, T.; Scott, I.; Šlapeta, J. Unambiguous identification of Ancylostoma caninum and Uncinaria stenocephala in Australian and New Zealand dogs from faecal samples. Austral. Vet. J. 2023, 101, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Evason, M.D.; Weese, J.S.; Polansky, B.J.; Leutenegger, C.M.; Evason, M.D. Emergence of canine hookworm treatment resistance: Novel detection of Ancylostoma caninum anthelmintic resistance markers by fecal PCR in 11 dogs from Canada. Am. J. Vet. Res. 2023, 84, ajvr.23.05.0116. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.M.P.; Aires, C.G.; Alves-Sobrinho, A.V.; Moraes, I.S.; Moreira, C.N.; Amaral, A.V.C.; Saturnino, K.C.; Braga, Í.A.; de Campos Pacheco, R.; de Souza Ramos, D.G. Gastrointestinal parasites of wild carnivores from conservation institutions in the Cerrado of Goiás, Brazil. Braz. J. Vet. Parasitol. 2023, 32, e004823. [Google Scholar] [CrossRef] [PubMed]
- Noronha, D.; Vicente, J.J.; Pinto, R.M. A survey of new records for nematodes from mammals deposited in the Helminthological collection of the Institute Oswaldo Cruz (CHIOC). Rev. Bras. Zool. 2002, 19, 945–949. [Google Scholar] [CrossRef]
- Vieira, F.M.; Luque, J.L.; Muniz-Pereira, L.C. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008, 1721, 1–23. [Google Scholar] [CrossRef]
- Santos, M.J.; Pinto, B.M.; Santos-Reis, M. Trophic niche partitioning between two native and two exotic carnivores in SW Portugal. Web. Ecol. 2007, 7, 53–62. [Google Scholar] [CrossRef]
- Coati, N.; Schnieder, T.; Epe, C. Vertical transmission of Toxocara cati Schrank 1788 (Anisakidae) in the cat. Parasitol. Res. 2004, 92, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Maciag, L.; Morgan, E.R.; Holland, C. Toxocara: Time to let cati ‘out of the bag’. Trends Parasitol. 2022, 38, 280–289. [Google Scholar] [CrossRef]
- Bowman, D.D.; Montgomery, S.P.; Zajac, A.M.; Eberhard, M.L.; Kazacos, K.R. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010, 26, 162–167. [Google Scholar] [CrossRef]
- Gavignet, B.; Piarroux, R.; Aubin, F.; Millon, L.; Humbert, P. Manifestações cutâneas da toxocaríase humana. J. Am. Acad. Dermatol. 2008, 59, 1031–1042. [Google Scholar] [CrossRef]
- Ma, G.; Holland, C.V.; Wang, T.; Hofmann, A.; Fan, C.K.; Maizels, R.M.; Hotez, P.J.; Gasser, R.B. Human toxocariasis. Lancet Infect. Dis. 2018, 18, e14–e24. [Google Scholar] [CrossRef] [PubMed]
- Axelerad, A.D.; Stroe, A.Z.; Gogu, A.E.; Pusztai, A.; Jianu, D.C.; Daniel, D.; Axelerad, D.D. Espectro clínico de sintomas em toxocaríase cerebral. Exper. Ther. Med. 2020, 21, 1–11. [Google Scholar]
- Chihai, O.; Rusu, Ş.; Tălămbuţă, N.; Nistreanu, V.; Larion, A.; Savin, A.; Naforniţa, N. Parasite fauna diversity in Red Fox (Vulpes vulpes) from natural and anthropized ecosystems of the Republic of Moldova. Sustainable Use and Protection of Animal World in the Context of Climate Change. In Proceedings of the Xth International Conference of Zoologists, Chisinau, Moldova, 16–17 September 2021; pp. 180–186. [Google Scholar]
- Hayasaki, M.; Ohishi, I.; Munakata, A. Incidence of stomach worm, Physaloptera praeputialis von Linstow, 1889, in two cats and a dog in Tokyo, Japan. JAPN J. Parasitol. 1982, 31, 499–506. [Google Scholar]
- Rausch, R.L.; Maser, C.; Hoberg, E.P. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J. Wildl. Dis. 1983, 19, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Scholz, T.; Uhlirova, M.; Ditrich, O. Helminth parasites of cats from the Vientiane province, Laos, as indicators of the occurrence of causative agents of human parasitosis. Parasite 2003, 10, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Naem, S.; Farshid, A.A.; Marand, V.T. Pathological findings on natural infection with Physaloptera praeputialis in cats. Vet. Arch. 2006, 76, 315–321. [Google Scholar]
- Guerrero, J.H.M.; Solis, M.E.P.; Ramos, J.J.Z.; Alferez, F.R.; Casio, H.H. Report of Physaloptera praeputialis (Von Linstow) in mountain lion (Puma concolor, Linneaus, 1771). J. Animal. Vet. Adv. 2010, 9, 601–603. [Google Scholar]
- Borji, H.; Razmi, G.; Ahmadi, A.; Karami, H.; Yaghfoori, S.; Abedi, V. A survey on endoparasites and ectoparasites of stray cats from Mashhad (Iran) and association with risk factors. J. Parasit. Dis. 2011, 35, 202–206. [Google Scholar] [CrossRef]
- Cantó, G.J.; García, M.P.; García, A.; Guerrero, M.J. The prevalence and abundance of helminth parasites in stray dogs from the city of Queretaro in central Mexico. J. Helminthol. 2011, 85, 263–269. [Google Scholar] [CrossRef]
- Torres, F.D.; Otranto, D. Dogs, cats, parasites, and humans in Brazil: Opening the black box. Parasite Vectors 2014, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Quadros, R.M.; Marques, S.M.T.; Moura, A.B.D.; Antonelli, M. First arasitosthe nematode Physaloptera praeputialis parasitizing a Jaguarandi. Neotrop. Biol. Conserv. 2014, 9, 186–189. [Google Scholar]
- Hajipour, N.; Imani Baran, A.; Yakhchali, M.; Banan Khojasteh, S.M.; Sheikhzade Hesari, F.; Esmaeilnejad, B.; Arjmand, J. A survey study on gastrointestinal parasites of stray cats in Azarshahr (East Azerbaijan province, Iran). J. Parasit. Dis. 2016, 40, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.G.S.; Santos, A.R.G.L.O.; Freitas, L.C.; Correa, S.H.R.; Kempe, G.V.; Morgado, T.O.; Aguiar, D.M.; Wolf, R.W.; Rossi, R.V.; Sinkoc, A.L.; et al. Endoparasites of wild animals from three biomes in the state of Mato Grosso, Brazil. Braz. J. Vet. Animal Sci. 2016, 68, 571–578. [Google Scholar] [CrossRef]
- Hobmaier, M. Extramammalian phase of Physaloptera maxillaris Molin, 1860 (Nematoda). J. Parasitol. 1941, 27, 233. [Google Scholar] [CrossRef]
- Petri, L.H.; Ameel, D.J. Studies on the life cycle of Physaloptera rara Hall and Wigdor, 1918 and Physaloptera praeputialis Linstow, 1889. J. Parasitol. 1950, 36, 40. [Google Scholar]
- Goldberg, S.R.; Bursey, C.R. Gastrointestinal helminths of seven gekkonid lizard species (Sauria: Gekkonidae) from Oceania. J. Nat. Hist. 2002, 36, 2249–2264. [Google Scholar] [CrossRef]
- Widmer, E.A. Development of third-stage Physaloptera larvae from Crotalus viridis Rafinesque, 1818 in cats with notes on pathology of the larvae in the reptile. J. Wildl. Dis. 1970, 6, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, R.J.; Anderson, R.C. Development of Physaloptera maxillaris (Nematoda: Physalopteroidea) in skunk (Mephitis mephitis) and the role of paratenic and other hosts in its life cycle. Can. J. Zool. 1976, 54, 313–323. [Google Scholar] [CrossRef]
- Velikanov, V.P.; Sharpillo, V.P. Experimental identification of Physaloptera praeputialis and Pterygodermatites cahirensis larvae (Nematoda, Spirurata) from paratenic hosts. Vestn. Zool. 2002, 6, 25–29. (In Russian) [Google Scholar]
- Kalyanasundaram, A.; Henry, C.; Brym, M.Z.; Kendall, R.J. Molecular identification of Physaloptera sp. from wild northern bobwhite (Colinus virginianus) in the Rolling Plains ecoregion of Texas. Parasitol. Res. 2018, 117, 2963–2969. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarli, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Mendoza Roldan, J.; Otranto, D. Zoonotic parasites associated with predation by dogs and cats. Parasite Vectors 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Sonnenberg, B. An apparent case of human infection with the whipworm of dogs, Trichuris vulpis (Froelich 1789). J. Parasitol. 1956, 42, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.J.; Columbus, S.T.; Aldeen, W.E.; Davis, M.; Carroll, K.C. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J. Clin. Microbiol. 2002, 40, 2703–2704. [Google Scholar] [CrossRef]
- Kirkova, Z.; Georgieva, D.; Raychev, E. Study on the prevalence of trichurosis in different categories of dogs and wild carnivores. Bulg. J. Vet. Vet. Med. 2006, 9, 141–147. [Google Scholar]
- Traversa, D. Are we paying too much attention to cardio-pulmonary nematodes and neglecting old-fashioned worms like Trichuris vulpis? Parasit. Vectors 2011, 4, 32. [Google Scholar] [CrossRef]
- Bartsocas, C.S.; Von Graevenitz, A.; Blodgett, F. Dipylidium infection in a 6-month-old infant. J. Pediatr. 1966, 69, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Neira, O.P.; Jofré, M.L.; Muñoz, S.N. Infection by Dipylidium caninum: Case presentation and literature review. Chil. J. Infectol. 2008, 25, 465–471. [Google Scholar]
- Benitez-Bolivar, P.; Rondón, S.; Ortiz, M.; Díaz-Díaz, J.; León, C.; Riveros, J.; Molina, H.; González, C. Morphological and molecular characterization of the parasite Dipylidium caninum infecting an infant in Colombia: A case report. Parasit. Vectors 2022, 15, 463. [Google Scholar] [CrossRef]
- East, M.L.; Kurze, C.; Wilhelm, K.; Benhaiem, S.; Hofer, H. Factors influencing Dipylidium sp. infection in a free-ranging social carnivore, the spotted hyena (Crocuta crocuta). Int. J. Parasitol. Parasites Wildl. 2013, 2, 257–265. [Google Scholar] [CrossRef]
- Nichol, S.; Ball, S.J.; Snow, K.R. Prevalence of intestinal parasites in feral cats in some urban areas of England. Vet. Parasitol. 1981, 9, 107–110. [Google Scholar] [CrossRef]
- Coman, B.J.; Jones, E.H.; Driesen, M.A. Helminth parasites and arthropods of feral cats. Austral. Vet. J. 1981, 57, 324–327. [Google Scholar] [CrossRef]
- Waap, H.; Gomes, J.; Nunes, T. Parasite communities in stray cat populations from Lisbon, Portugal. J. Helminthol. 2014, 88, 389–395. [Google Scholar] [CrossRef]
- Gomez-Puerta, L.A.L.; Mayor, P. The red brocket deer (Mazama americana) as a new intermediate host of Taenia omissa (Taeniidae). Parasitol. Int. 2021, 85, 102439. [Google Scholar] [CrossRef]
- Chinchilla-Carmona, M.; Valerio-Campos, I.; Gutiérrez-Espeleta, G.A.; Soto-Fournier, S.; Vanegas-Pissa, J.C.; Salom-Pérez, R.; Arroyo-Arce, S. Intestinal parasites found in fecal samples of wild cats of Costa Rica. Int. J. Vet. Sci. 2020, 9, 153–156. [Google Scholar]
- Bally, A.; Francis-Charles, S.; Ackbar, T.; Beharrylal, Y.; Charles, R.; Basu, A.; Suepaul, R. The prevalence of gastrointestinal parasites and seroprevalence of Toxoplasma gondii in captive ocelots (Leopardus pardalis) in Trinidad, West Indies. Vet. Med. Int. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Arrabal, J.P.; Arce, L.F.; Macchiaroli, N.; Kamenetzky, L. Ecological and molecular associations between Neotropical wild felids and Taenia (Cestoda: Taeniidae) in the Atlantic Forest: A new report for Taenia omissa. Parasitol. Res. 2023, 122, 2999–3012. [Google Scholar] [CrossRef]
- Carmena, D.; Cardona, G.A. Equinococose em espécies carnívoras selvagens: Epidemiologia, diversidade genotípica e implicações para a saúde pública veterinária. Vet. Parasitol. 2014, 202, 69–94. [Google Scholar] [CrossRef]
- Schwantes, J.B.; de Souza Quevedo, P.; D’Ávila, M.F.; De Paula, A.A.; Fortes, V.B.; Sganzerla Graichen, D.A. Another piece of the puzzle: Echinococcus oligarthrus recorded in jaguarundis (Herpailurus yagouaroundi) in southern Brazil. J. Wildl. Dis. 2021, 57, 936–941. [Google Scholar] [CrossRef]
- Kakundi, E.M. Molecular Epidemiology of Echinococcus and Taenia Species in Dogs from Cystic Echinococcosis Endemic Areas in Kenya. Doctoral Dissertation, University of Nairobi, Nairobi, Kenya, 2020. [Google Scholar]
- Santos, E.G.N.; Chame, M.; Chagas-Moutinho, V.A.; Santos, C.P. Morphology and molecular analysis of Oncicola venezuelensis (Acanthocephala: Oligacanthorhynchidae) from the ocelot Leopardus pardalis in Brazil. J. Helminthol. 2017, 91, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Sianto, L.; de Souza, M.V.; Chame, M.; da Luz, M.D.F.; Guidon, N.; Pessis, A.M.; Araújo, A. Helminths in feline coprolites up to 9000 years in the Brazilian Northeast. Parasitol. Int. 2014, 63, 851–857. [Google Scholar] [CrossRef]
- Palmer, J.P.S.; Dib, L.V.; Lobão, L.F.; Pinheiro, J.L.; Ramos, R.C.F.; Uchoa, C.M.A.; Bastos, O.M.P.; Silva, M.E.M.; Nascimento, J.L.; Pissinatti, A.; et al. Oncicola venezuelensis (Marteau, 1977) (Acanthocephala: Oligacanthorhynchidae) in Puma concolor in Rio de Janeiro, Brazil. Braz. J. Vet. Parasitol. 2020, 29, e009620. [Google Scholar] [CrossRef]
- Richardson, D.J. Acanthocephala. In Encyclopedia of Life Sciences; Wiley InterScience, Ed.; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Núñez, V.; Drago, F. Phylum Acanthocephala. In Macroparasites: Parasitology and Biology; Drago, F.B., Ed.; National University of La Plata: La Plata, Argentina, 2017; pp. 112–127. [Google Scholar]
- Becker, A.A.M.J.; Rajeev, S.; Freeman, M.A.; Beierschmitt, A.; Savinon, V.; Wulcan, J.M.; Bolfa, P. Acanthocephalan extraintestinal Oncicola venezuelensis (Oligacanthorhynchidae) em mangustos indianos pequenos (Herpestes auropunctatus) e macacos-verdes africanos (Chlorocebus aethiops sabaeus). Vet. Pathol. 2019, 56, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Achatz, T.J.; Pulis, E.E.; Woodyard, E.T.; Rosser, T.G.; Martens, J.R.; Weinstein, S.; Fecchio, A.; McAllister, C.T.; Carrion Bonilla, C.; Tkach, V. Molecular phylogenetic analysis of Neodiplostomum and Fibricola (Digenea, Diplostomidae) does not support host-based systematics. Parasitology 2022, 149, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Bogan, J.; Garner, M.; Childress, A.; Wellehan, J. Larval Neodiplostomum americanum in the lung of a sugar glider (Petaurus breviceps). J. Exot. Pet. Med. 2019, 30, 72–75. [Google Scholar] [CrossRef]
- Izrailskaia, A.V.; Besprozvannykh, V. Neodiplostomum cf. seoulense (Seo, Rim, Lee, 1964) sensu Pyo et al., 2014 (Trematoda: Diplostomidae Poirier, 1886): Morphology, life cycle, and phylogenetic relationships. J. Helminthol. 2023, 97, e61. [Google Scholar] [CrossRef]
- Alho, C.J.R. Concluding remarks: Overall impacts on biodiversity and future perspectives for conservation in the Pantanal biome. Braz. J. Biol. 2011, 71, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.P.G.; Rosa, C.A.; Bager, A. Seasonal variation of wildlife roadkill on highway MG 354, South of Minas Gerais—Brazil. Biotemas 2012, 25, 73–79. [Google Scholar]
- Clevenger, A.P.; Chruszcz, B.; Gun, S.K. Spatial patterns and factors influencing small vertebrate fauna road-kill aggregations. Biol. Conserv. 2003, 109, 15–26. [Google Scholar] [CrossRef]
- Pitman, M.R.P.L.; Oliveira, T.G.; Paula, R.C.; Indrusiak, C. Manual de Identificação, Prevenção e Controle de Predação por Carnívoros; IBAMA: Brasília, Brazil, 2002; 83p. [Google Scholar]
- Holmes, J.C. Parasites as threats to biodiversity in shrinking ecosystems. Biodiv. Conserv. 1996, 5, 975–983. [Google Scholar] [CrossRef]
- Cleaveland, S.; Haydon, D.T.; Taylor, L. Overviews of pathogen emergence: Which pathogens emerge, when and why? Wildlife and emerging zoonotic diseases: The biology, circumstances and consequences of cross-species transmission. Curr. Top. Microbiol. Immunol. 2007, 315, 85–111. [Google Scholar]
- Mukarati, N.L.; Vassilev, G.D.; Tagwireyi, W.M.; Tavengwa, M. Occurrence, prevalence and intensity of internal parasite infections in African lions (Panthera leo) in enclosures at a recreation park in Zimbabwe. J. Zoo Wildl. Med. 2013, 44, 686. [Google Scholar] [CrossRef]
- Clark, N.J.; Seddon, J.M.; Šlapeta, J.; Wells, K. Parasite spread at the domestic animal—Wildlife interface: Anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephalides spp.) infestation in wild mammals. Parasit. Vectors 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; He, Y.-D.; Theodorou, P.; Yang, C.-F. The effects of urbanization on pollinators and pollination: A meta-analysis. Ecol. Lett. 2023, 26, 1629–1642. [Google Scholar] [CrossRef]
- Zhu, S.; VanWormer, E.; Shapiro, K. More people, more cats, more parasites: Human population density and temperature variation predict prevalence of Toxoplasma gondii oocyst shedding in free-ranging domestic and wild felids. PLoS ONE 2023, 18, e0286808. [Google Scholar] [CrossRef]
- Almeida, G.G.; Coscarelli, D.; Melo, M.N.; Melo, A.L.; Pinto, H.A. Identificação molecular de Spirometra spp. em alguns animais selvagens do Brasil. Parasitol. Int. 2016, 65, 428–431. [Google Scholar] [CrossRef]
- Oda, F.H.; Borteiro, C.; da Graca, R.J.; Tavares, L.E.R.; Crampet, A.; Guerra, V.; Lima, F.S.; Bellay, S.; Karling, L.C.; Castro, L.; et al. Parasitism by larval cestodes of the genus Spirometra in South American amphibians and reptiles: New records from Brazil and Uruguay. Acta Tropica 2016, 164, 150–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gong, T.; Chen, S.; Liu, Q.; Zhou, H.; He, J.; Wu, Y.; Li, F.; Liu, Y. Epidemiology, diagnosis, and prevention of sparganosis in Asia. Animals 2022, 12, 1578. [Google Scholar] [CrossRef]
- Mamede, S.B.; Alho, C.J.R. Response of wild mammals to the seasonal shrinking and expansion of habitats due to the flooding regime of the Pantanal, Brazil. Braz. J. Biol. 2006, 66, 991–998. [Google Scholar] [CrossRef]
- Maracahipes-Santos, L.; Lenza, E.; Santos, J.O.; Mews, H.A.; Oliveira, B. Effects of soil and space on the woody species composition and vegetation structure of three Cerrado phytophysiognomies in the Cerrado-Amazon transition. Braz. J. Biol. 2017, 77, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.S.; Pontes, M.R.; Neto, M.C.; Campião, K.M.; Anjos, L.A. Helminths of eight anuran species from a remnant riparian forest in the Cerrado biome, Brazil. Herpetol. Notes 2020, 13, 463–478. [Google Scholar]
- Oliveira, S.S.; Sousa Machado, H.T.; Castro Araújo, K.; Sousa Silva, C.; Ávila, R.W. Nematodes associated with Leptodactylus cf. mystaceus (Anura: Leptodactylidae) in agricultural landscapes of Ibiapaba plateau, Ceará state, Brazil. Caldasia 2024, 46. in press. [Google Scholar] [CrossRef]
- Maizels, R.M. Parasite immunomodulation and polymorphisms of the immune system. J. Biol. 2009, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Petney, T.N.; Andrews, R.H. Multiparasite communities in animals and humans: Frequency, structure and pathogenic significance. Int. J. Parasitol. 1998, 28, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Nkuo-Akenji, T.K.; Chi, P.C.; Cho, J.F.; Ndamukong, K.K.; Sumbele, I. Malaria and helminth co-infection in children living in a malaria endemic setting of Mount Cameroon and predictors of anemia. J. Parasitol. 2006, 96, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Boel, M.; Carrara, V.I.; Rijken, M.; Proux, S.; Nacher, M.; Pimanpanarak, M.; McGready, R. Complex interactions between soil-transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PloS Negl. Trop. Dis. 2010, 4, e887. [Google Scholar] [CrossRef] [PubMed]
- Ezenwa, V.; Jolles, A. From host immunity to pathogen invasion: The effects of helminth coinfection on the dynamics of microparasites. Integr. Comp. Biol. 2011, 51, 540–551. [Google Scholar] [CrossRef]
- Griffiths, E.C.; Pedersen, A.B.; Fenton, A.; Petchey, O.L. The nature and consequences of coinfection in humans. J. Infect. 2013, 63, 200–206. [Google Scholar] [CrossRef]




| Host | Host ID | Biome | Location | Helminth Species | n(+) |
|---|---|---|---|---|---|
| Herpailurus yagouaroundi | UFJ-LPPV-156 | Pantanal | Poconé, MT | Physaloptera praeputialis | 10 |
| UFJ-LPPV-78 | Cerrado | Cuiabá, MT | Taenia spp. | 10 | |
| UFJ-LPPV-80 | Cerrado | Cuiabá, MT | Dipylidium caninum | 1 | |
| UFJ -LPPV-85 | Cerrado | Cuiabá, MT | Ancylostoma braziliense | 3 | |
| UFJ-LPPV-86 | Cerrado | Cuiabá, MT | Oncicola spp. | 1 | |
| UFJ-LPPV-304 | Cerrado | Jataí, GO | Toxocara cati | 3 | |
| Leopardus pardalis | UFJ-LPPV-133 | Pantanal | Poconé, MT | Spirometra spp. | 5 |
| Oncicola spp. | 4 | ||||
| UFJ-LPPV-154 | Pantanal | Poconé, MT | Toxocara cati | 5 | |
| UFJ-LPPV-155 | Pantanal | Poconé, MT | Physaloptera anomala | 9 | |
| Spirometra spp. | 9 | ||||
| Oncicola spp. | 32 | ||||
| UFJ-LPPV-216 | Cerrado | Tangará da Serra, MT | Oncicola spp. | 3 | |
| Taenia spp. | 14 | ||||
| UFJ-LPPV-303 | Cerrado | Cuiabá, MT | Toxocara cati | 6 | |
| Trichuris vulpis | 5 | ||||
| Neodiplostomum spp. | 11 | ||||
| UFJ-LPPV-302 | Cerrado | Cuiabá, MT | Toxocara cati | 75 | |
| UFJ-LPPV-301 | Cerrado | Cuiabá, MT | Toxocara cati | 24 | |
| Oncicola spp. | 3 | ||||
| UFJ-LPPV-75 | Cerrado | Mineiros, GO | Toxocara cati | 9 | |
| Panthera onca | UFJ-LPPV-140 | Pantanal | Poconé, MT | Toxocara cati | 62 |
| Toxocara canis | 51 | ||||
| UFJ -LPPV-77 | Cerrado | Chapada dos Guimarães, MT | Spirometra spp. | 1 | |
| UFJ-LPPV-79 | Cerrado | Cuiabá, MT | Taenia spp. | 12 | |
| UFJ-LPPV-82 | Cerrado | Jataí, GO | Toxocara cati | 10 | |
| UFJ-LPPV-81 | Cerrado | Jataí, GO | Toxocara cati | 5 | |
| Physaloptera praeputialis | 4 | ||||
| UFJ-LPPV-83 | Cerrado | Cuiabá, MT | Oncicola spp. | 48 | |
| UFJ-LPPV-84 | Cerrado | Chapada dos Guimarães, MT | Echinococcus spp. | 21 | |
| UFJ-LPPV-298 | Cerrado | Cuiabá, MT | Toxocara cati | 8 | |
| Toxocara canis | 6 | ||||
| Puma concolor | UFJ-LPPV-153 | Pantanal | Poconé, MT | Oncicola spp. | 9 |
| UFJ-LPPV-74 | Cerrado | Mineiros, GO | Toxocara cati | 1 | |
| Ancylostoma braziliense | 1 | ||||
| UFJ-LPPV-215 | Cerrado | Jataí, GO | Toxocara cati | 14 | |
| UFJ-LPPV-76 | Cerrado | Chapada dos Guimarães, MT | Ancylostoma pluridentatum | 363 | |
| Ancylostoma caninum | 21 | ||||
| UFJ-LPPV-299 | Cerrado | Jataí, GO | Taenia spp. | 14 | |
| Spirometra spp. | 1 | ||||
| Toxocara cati | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, I.d.S.; Silva, V.L.d.B.; Andrade-Silva, B.E.d.; Gomes, A.P.N.; Urzedo, N.F.d.; Abolis, V.B.; Gonçalves, R.d.S.; Arpon, K.V.; Assis-Silva, Z.M.d.; Silva, L.F.d.; et al. Gastrointestinal Helminths in Wild Felids in the Cerrado and Pantanal: Zoonotic Bioindicators in Important Brazilian Biomes. Animals 2024, 14, 1622. https://doi.org/10.3390/ani14111622
Moraes IdS, Silva VLdB, Andrade-Silva BEd, Gomes APN, Urzedo NFd, Abolis VB, Gonçalves RdS, Arpon KV, Assis-Silva ZMd, Silva LFd, et al. Gastrointestinal Helminths in Wild Felids in the Cerrado and Pantanal: Zoonotic Bioindicators in Important Brazilian Biomes. Animals. 2024; 14(11):1622. https://doi.org/10.3390/ani14111622
Chicago/Turabian StyleMoraes, Iago de Sá, Victória Luiza de Barros Silva, Beatriz Elise de Andrade-Silva, Ana Paula Nascimento Gomes, Nicoly Ferreira de Urzedo, Vitória Breda Abolis, Renata de Souza Gonçalves, Karina Varella Arpon, Zara Mariana de Assis-Silva, Lizandra Fernandes da Silva, and et al. 2024. "Gastrointestinal Helminths in Wild Felids in the Cerrado and Pantanal: Zoonotic Bioindicators in Important Brazilian Biomes" Animals 14, no. 11: 1622. https://doi.org/10.3390/ani14111622
APA StyleMoraes, I. d. S., Silva, V. L. d. B., Andrade-Silva, B. E. d., Gomes, A. P. N., Urzedo, N. F. d., Abolis, V. B., Gonçalves, R. d. S., Arpon, K. V., Assis-Silva, Z. M. d., Silva, L. F. d., Zago, E. A., Gonçalves, M. B., Braga, Í. A., Saturnino, K. C., Colodel, E. M., Júnior, A. M., Pacheco, R. d. C., & Ramos, D. G. d. S. (2024). Gastrointestinal Helminths in Wild Felids in the Cerrado and Pantanal: Zoonotic Bioindicators in Important Brazilian Biomes. Animals, 14(11), 1622. https://doi.org/10.3390/ani14111622







