Effects of Sex on Growth Performance, Carcass Traits, Blood Biochemical Parameters, and Meat Quality of XueShan Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Growth Performance
2.4. Carcass Traits
2.5. Blood Antioxidant and Anti-Stress Indicators
2.6. Meat Quality
2.7. Statistical Analysis
3. Results and Discussion
3.1. Growth Performance
3.2. Carcass Traits
3.3. Blood Antioxidant and Anti-Stress Indicators
3.4. Meat Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA. Livestock and Poultry: World Markets and Trade; Foreign Agricultural Service: Washington, DC, USA, 2022. [Google Scholar]
- Hossain, K.Z.; Xue, J.; Rabbany, M.G. Consumers’ willingness to pay for GLOBALGAP certified chicken: Empirical evidence from a consumer survey in Bangladesh. Food Control 2021, 130, 108397. [Google Scholar] [CrossRef]
- Kaygisiz, F.; Bolat, B.A.; Bulut, D. Determining Factors Affecting Consumer’s Decision to Purchase Organic Chicken Meat. Braz. J. Poult. Sci. 2019, 21, eRBCA-2019-1060. [Google Scholar] [CrossRef]
- Lusk, J.L. Consumer preferences for and beliefs about slow growth chicken. Poult. Sci. 2018, 97, 4159–4166. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, L.D.L.; Chen, X.; Xu, M.Y.; Ke, J.; Tian, Y. Chicken meat taste preferences, perceived risk of human infection with avian influenza virus, and self-reported chicken meat consumption in China. Prev. Vet. Med. 2022, 203, 105658. [Google Scholar] [CrossRef] [PubMed]
- Bongiorno, V.; Schiavone, A.; Renna, M.; Sartore, S.; Soglia, D.; Sacchi, P.; Gariglio, M.; Castillo, A.; Mugnai, C.; Forte, C.; et al. Carcass Yields and Meat Composition of Male and Female Italian Slow-Growing Chicken Breeds: Bianca di Saluzzo and Bionda Piemontese. Animals 2022, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Meira, M.; Afonso, I.M.; Casal, S.; Lopes, J.C.; Domingues, J.; Ribeiro, V.; Dantas, R.; Leite, J.V.; Brito, N.V. Carcass and Meat Quality Traits of Males and Females of the “Branca” Portuguese Autochthonous Chicken Breed. Animals 2022, 12, 2640. [Google Scholar] [CrossRef] [PubMed]
- Daszkiewicz, T.; Murawska, D.; Kubiak, D.; Han, J. Chemical Composition and Fatty Acid Profile of the Pectoralis major Muscle in Broiler Chickens Fed Diets with Full-Fat Black Soldier Fly (Hermetia illucens) Larvae Meal. Animals 2022, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Ngongolo, K.; Chota, A. Chicken production, flock size, management systems, and challenges in the Dodoma region in Tanzania. Poult. Sci. 2021, 100, 101136. [Google Scholar] [CrossRef] [PubMed]
- Baeza, E.; Chartrin, P.; Meteau, K.; Bordeau, T.; Juin, H.; Le Bihan-Duval, E.; Lessire, M.; Berri, C. Effect of sex and genotype on carcase composition and nutritional characteristics of chicken meat. Br. Poult. Sci. 2021, 51, 344–353. [Google Scholar] [CrossRef]
- Qi, J.; Liu, D.-y.; Zhou, G.-h.; Xu, X.-l. Characteristic Flavor of Traditional Soup Made by Stewing Chinese Yellow-Feather Chickens. J. Food Sci. 2017, 82, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Izuddin, W.I.; Awad, E.A.; Idrus, Z.; Samsudin, A.A.; Mustapha, N.M. Dietary Supplementation of Postbiotics Mitigates Adverse Impacts of Heat Stress on Antioxidant Enzyme Activity, Total Antioxidant, Lipid Peroxidation, Physiological Stress Indicators, Lipid Profile and Meat Quality in Broilers. Animals 2020, 10, 982. [Google Scholar] [CrossRef] [PubMed]
- Song, D.J.; King, A.J. Effects of heat stress on broiler meat quality. World’s Poult. Sci. J. 2015, 71, 701–709. [Google Scholar] [CrossRef]
- Wang, L.; Kong, L.; Hu, X.; Bai, H.; Wang, Z.; Jiang, Y.; Bi, Y.; Chang, G.; Chen, G. Effect of stocking density on performance, meat quality and cecal bacterial communities of yellow feather broilers. Anim. Biotechnol. 2022, 33, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jiang, Y.; Wang, Z.; Chen, G.; Bai, H.; Chang, G. Indigenous, Yellow-Feathered Chickens Body Measurements, Carcass Traits, and Meat Quality Depending on Marketable Age. Animals 2022, 12, 2422. [Google Scholar] [CrossRef] [PubMed]
- NY/T823-2020; Terminology and Measurement Calculation Method for Poultry Performance. China Agriculture Press: Beijing, China, 2020.
- NY/T1180-2006; Determination of Meat Tenderness Shear Force Method. China Agriculture Press: Beijing, China, 2006.
- Katemala, S.; Molee, A.; Thumanu, K.; Yongsawatdigul, J. Meat quality and Raman spectroscopic characterization of Korat hybrid chicken obtained from various rearing periods. Poult. Sci. 2021, 100, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Song, S.; Park, T.S.; Kim, G.D. Proteolysis and changes in meat quality of chicken pectoralis major and iliotibialis muscles in relation to muscle fiber type distribution. Poult. Sci. 2022, 101, 102185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, C.; Peng, H.; Yin, H.; Wang, Y.; Hu, Y.; Yu, C.; Jiang, X.; Du, H.; Li, Q.; et al. Effects of Slaughter Age on Muscle Characteristics and Meat Quality Traits of Da-Heng Meat Type Birds. Animals 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Abbood, A.A.; Jawad, H.S. Borreria latifolia effects as natural supplementation on growth performance of village chicken. Biochem. Cell. Arch. 2021, 21, 5421–5426. [Google Scholar]
- Mosca, F.; Zaniboni, L.; Iaffaldano, N.; Sayed, A.A.; Mangiagalli, M.G.; Pastorelli, G.; Cerolini, S. Free-Range Rearing Density for Male and Female Milanino Chickens: Growth Performance and Stress Markers. J. Appl. Poult. Res. 2019, 28, 1342–1348. [Google Scholar] [CrossRef]
- Sohn, S.H.; Cho, E.J.; Kim, K.G.; Shin, K.B.; Lee, S.G. Comparison of Growth Performance and Stress Response between Male and Female Korean Native Commercial Chickens. Korean J. Poult. Sci. 2022, 49, 89–98. [Google Scholar] [CrossRef]
- Charuta, A.; Dzierzęcka, M.; Komosa, M.; Kalinowski, Ł.; Pierzchała, M. Age- and Sex-related Differences of Morphometric, Densitometric and Geometric Parameters of Tibiotarsal Bone in Ross Broiler Chickens. Folia Biol.-Krakow 2013, 61, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Malomane, D.K.; Norris, D.; Banga, C.B.; Ngambi, J.W. Use of factor scores for predicting body weight from linear body measurements in three South African indigenous chicken breeds. Trop. Anim. Health Prod. 2014, 46, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Tyasi, T.L.; Qin, N.; Jing, Y.; Mu, F.; Zhu, H.Y.; Liu, D.; Yuan, S.; Xu, R. Assessment of relationship between body weight and body measurement traits of indigenous Chinese Dagu chickens using path analysis. Indian J. Anim. Res. 2017, 51, 588–593. [Google Scholar]
- Tyasi, T.L.; Qin, N.; Niu, X.; Sun, X.; Chen, X.; Zhu, H.; Zhang, F.; Xu, R. Prediction of carcass weight from body measurement traits of Chinese indigenous Dagu male chickens using path coefficient analysis. Indian J. Anim. Sci. 2018, 88, 744–748. [Google Scholar] [CrossRef]
- Nualhnuplong, P.; Wattanachant, C. Effects of Age at Slaughter and Sex on Carcass Characteristics and Meat Quality of Betong Chicken. Pertanika J. Trop. Agric. Sci. 2020, 43, 343–357. [Google Scholar]
- De Freitas Gottardi, C.P.F.; Oliveira, A.F.G.; de Souza, A.R.Q.; Ferreira, B.R.; Abaker, O.E.P.; Abaker, P. Effect of sex on productive performance and characteristics of carcass cut chicken. Rev. Agric. Neotrop. 2019, 6, 52–58. [Google Scholar]
- Ikusika, O.O.; Falowo, A.B.; Mpendulo, C.T.; Zindove, T.J.; Okoh, A.I. Effect of strain, sex and slaughter weight on growth performance, carcass yield and quality of broiler meat. Open Agric. 2020, 5, 607–616. [Google Scholar] [CrossRef]
- Chen, M.J.; Xie, W.Y.; Pan, N.X.; Wang, X.Q.; Yan, H.C.; Gao, C.Q. Methionine improves feather follicle development in chick embryos by activating Wnt/beta-catenin signaling. Poult. Sci. 2020, 99, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.G.; Zhang, M.; Liu, Y.F.; Shan, Y.J.; Tu, Y.J.; Ju, X.J.; Zou, J.M.; Shu, J.T.; Wu, J.F.; Xie, J.F. A gene co-expression network analysis of the candidate genes and molecular pathways associated with feather follicle traits of chicken skin. J. Anim. Breed. Genet. 2021, 138, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Darnell, D.K.; Zhang, L.S.; Hannenhalli, S.; Yaklichkin, S.Y. Developmental expression of chicken FOXN1 and putative target genes during feather development. Int. J. Dev. Biol. 2014, 58, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z.; Chen, G.; Luo, Q.; Nie, Q.; Zhang, X.; Luo, W. Characterization of Chicken Skin Yellowness and Exploration of Genes Involved in Skin Yellowness Deposition in Chicken. Front. Physiol. 2021, 12, 585089. [Google Scholar] [CrossRef]
- Ferrante, V.; Mugnai, C.; Ferrari, L.; Marelli, S.P.; Spagnoli, E.; Lolli, S. Stress and reactivity in three Italian chicken breeds. Ital. J. Anim. Sci. 2016, 15, 303–309. [Google Scholar] [CrossRef]
- Ambwani, S.; Dolma, R.; Sharma, R.; Kaur, A.; Singh, H.; Ruj, A.; Ambwani, T.K. Modulation of inflammatory and oxidative stress biomarkers due to dexamethasone exposure in chicken splenocytes. Vet. Immunol. Immunopathol. 2023, 262, 110632. [Google Scholar] [CrossRef] [PubMed]
- Rama Rao, S.V.; Prakash, B.; Rajkumar, U.; Raju, M.V.L.N.; Srilatha, T.; Reddy, E.P.K. Effect of supplementing germinated sprouts of pulses on performance, carcass variables, immune and oxidative stress indicators in broiler chickens reared during tropical summer season. Trop. Anim. Health Prod. 2018, 50, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lei, X.; Luo, J.; Everaert, N.; Zhao, G.; Wen, J.; Yang, Y. The effect of Epigallocatechin-3-gallate on small intestinal morphology, antioxidant capacity and anti-inflammatory effect in heat-stressed broilers. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Cell culture, oxidative stress, and antioxidants: Avoiding pitfalls. Biomed. J. 2014, 37, 99–105. [Google Scholar] [CrossRef]
- Shayan-Nasr, M.; Ghaniei, A.; Eslami, M.; Zadeh-Hashem, E. Ameliorative role of trans- ferulic acid on induced oxidative toxicity of rooster semen by β-cyfluthrin during low temperature liquid storage. Poult. Sci. 2021, 100, 101308. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, K.Y.; Bai, S.P.; Wang, J.P.; Zeng, Q.F.; Peng, H.W.; Xuan, Y.; Su, Z.W.; Ding, X.M. The impacts of egg storage time and maternal dietary vitamin E on the growth performance and antioxidant capacity of progeny chicks. Poult. Sci. 2021, 100, 101142. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Zaytsoff, S.J.; Montina, T.; Inglis, G.D. Corticosterone-Mediated Physiological Stress Alters Liver, Kidney, and Breast Muscle Metabolomic Profiles in Chickens. Animals 2021, 11, 3056. [Google Scholar] [CrossRef] [PubMed]
- Zaytsoff, S.J.; Brown, C.L.; Montina, T.; Metz, G.A.; Abbott, D.W.; Uwiera, R.R.; Inglis, G.D. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 2019, 9, 19225. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Matarneh, S.K.; Gerrard, D.; Tan, J. Modelling of energy metabolism and analysis of pH variations in postmortem muscle. Meat Sci. 2021, 182, 108634. [Google Scholar] [CrossRef] [PubMed]
- Rosenvold, K.; Andersen, H.J. Factors of significance, for pork quality—A review. Meat Sci. 2003, 64, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Weng, K.; Li, Y.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Comparison of muscle fiber characteristics and glycolytic potential between slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101649. [Google Scholar] [CrossRef]
- Liu, L.; Cui, H.; Xing, S.; Zhao, G.; Wen, J. Effect of Divergent Selection for Intramuscular Fat Content on Muscle Lipid Metabolism in Chickens. Animals 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Ma, X.F.; Li, W.T.; Jiang, R.R.; Han, R.L.; Li, G.X.; Wang, Y.B.; Li, Z.Y.; Tian, Y.D.; et al. Identification of differentially expressed genes and pathways between intramuscular and abdominal fat-derived preadipocyte differentiation of chickens in vitro. BMC Genom. 2019, 20, 734. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Liu, J.; Zhang, J.; Xv, M.; He, D. A Novel Chicken Meat Quality Evaluation Method Based on Color Card Localization and Color Correction. IEEE Access 2020, 8, 170093–170100. [Google Scholar] [CrossRef]
| Items | 1–28 d | 29–63 d | 64–110 d |
|---|---|---|---|
| Ingredient (%) | |||
| Corn | 55.82 | 62.49 | 69.30 |
| Flour | 2.00 | 2.00 | 2.00 |
| Soybean meal | 33.10 | 25.20 | 17.20 |
| Corn protein flour | 1.00 | 2.00 | 3.00 |
| Soybean oil | 1.31 | 1.96 | 2.27 |
| Stone powder | 1.41 | 1.24 | 1.25 |
| Calcium hydrogen phosphate | 1.36 | 1.11 | 0.98 |
| Choline chloride | 1.00 | 1.00 | 1.00 |
| Premix | 3.00 | 3.00 | 3.00 |
| Nutritional level (%) | |||
| Crude protein | 21.00 | 18.50 | 16.00 |
| Metabolizable energy (MJ/kg) | 12.13 | 12.55 | 12.97 |
| Ca | 0.94 | 0.80 | 0.75 |
| Available phosphorus | 0.38 | 0.33 | 0.30 |
| Digestible lysine | 1.05 | 0.90 | 0.80 |
| Sex | ||||
|---|---|---|---|---|
| Items | Male | Female | SEM | p-Value |
| Initial BW (g) | 467.40 a | 418.40 b | 11.525 | 0.029 |
| Initial Body slope length (cm) | 12.43 | 12.18 | 0.108 | 0.257 |
| Initial Keel length (cm) | 6.12 | 5.96 | 0.060 | 0.193 |
| Initial Chest width (cm) | 4.88 | 4.74 | 0.036 | 0.063 |
| Initial Chest depth (cm) | 7.17 a | 6.85 b | 0.077 | 0.032 |
| Initial Shank length (cm) | 6.93 a | 6.62 b | 0.059 | 0.006 |
| Initial Shank girth (cm) | 3.15 | 3.01 | 0.039 | 0.075 |
| Finial BW (g) | 2526.0 a | 2204.9 b | 35.412 | <0.001 |
| Final Body slope length (cm) | 25.31 a | 20.97 b | 0.215 | <0.001 |
| Final Keel length (cm) | 18.70 a | 14.31 b | 0.249 | <0.001 |
| Final Chest width (cm) | 8.58 | 8.21 | 0.138 | 0.198 |
| Final Chest depth (cm) | 9.48 | 9.22 | 0.115 | 0.267 |
| Final Shank length (cm) | 9.02 a | 7.69 b | 0.100 | <0.001 |
| Final Shank girth (cm) | 5.05 a | 4.29 b | 0.049 | <0.001 |
| Daily BW gain (g) | 25.10 a | 21.79 b | 0.432 | 0.001 |
| Daily Body slope length gain (mm) | 1.57 a | 1.07 b | 0.023 | <0.001 |
| Daily Keel length gain (mm) | 1.53 a | 1.01 b | 0.028 | <0.001 |
| Daily Chest width (mm) | 0.45 | 0.42 | 0.017 | 0.408 |
| Daily Chest depth (mm) | 0.28 | 0.29 | 0.019 | 0.847 |
| Daily Shank length (mm) | 0.26 a | 0.13 b | 0.012 | <0.001 |
| Daily Shank girth (mm) | 0.23 a | 0.15 b | 0.007 | <0.001 |
| Sex | ||||
|---|---|---|---|---|
| Items | Male | Female | SEM | p-Value |
| Carcass yield (%) | 85.61 b | 87.74 a | 0.499 | 0.044 |
| Semi-eviscerated yield (%) | 79.37 | 80.06 | 0.660 | 0.608 |
| Eviscerated yield (%) | 67.51 | 66.50 | 0.591 | 0.400 |
| Breast muscle yield (%) | 18.18 b | 21.01 a | 0.355 | 0.001 |
| Leg muscle yield (%) | 25.89 a | 21.41 b | 0.257 | <0.001 |
| Lean meat yield (%) | 44.07 a | 42.43 b | 0.372 | 0.037 |
| Dressed weight (g) | 2197.58 a | 1859.46 b | 42.410 | 0.001 |
| Sex | |||||
|---|---|---|---|---|---|
| Items | Male | Female | SEM | p-Value | |
| Follicle density (piece/cm2) | Back | 4.21 a | 3.56 b | 0.075 | <0.001 |
| Abdomen | 3.59 a | 2.86 b | 0.052 | <0.001 | |
| Skin color | L* | 69.14 | 67.61 | 0.323 | 0.021 |
| a* | 7.90 a | 5.18 b | 0.220 | <0.001 | |
| b* | 17.19 | 15.68 | 0.461 | 0.108 | |
| Spotted skin level | Points | 3.33 a | 2.93 b | 0.887 | p < 0.001 Z = −3.535 |
| Sex | ||||
|---|---|---|---|---|
| Items | Male | Female | SEM | p-Value |
| SOD (U/mL) | 84.54 b | 102.49 a | 2.906 | 0.021 |
| MDA (nmol/mL) | 2.41 | 2.03 | 0.083 | 0.059 |
| GSH-PX (U/mL) | 357.61 b | 386.03 a | 5.569 | 0.043 |
| T-AOC (U/mL) | 12.30 b | 13.80 a | 0.298 | 0.045 |
| CAT (U/mL) | 65.13 b | 84.85 a | 1.076 | <0.001 |
| CORT (ng/mL) | 4.84 a | 3.70 b | 0.178 | 0.019 |
| CK (U/L) | 1555.05 | 1531.03 | 134.923 | 0.923 |
| Items | Sex | pH | Shear Force (N) | Water Loss Rate (%) | Meat Color | Proximate Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH1 | pH24 | L* | a* | b* | Moisture (%) | Protein (%) | Intramuscular Fat (%) | Collagen (%) | ||||
| Breast muscle | Male | 6.32 a | 6.31 a | 15.42 a | 10.13 | 47.44 | 12.72 | 12.81 | 72.19 a | 25.97 | 0.64 b | 0.25 |
| Female | 6.17 b | 6.13 b | 12.49 b | 9.50 | 48.01 | 15.27 | 14.36 | 71.53 b | 26.02 | 1.00 a | 0.24 | |
| SEM | 0.019 | 0.013 | 0.512 | 0.714 | 0.956 | 0.764 | 0.863 | 0.097 | 0.107 | 0.057 | 0.030 | |
| p-value | <0.001 | 0.002 | 0.008 | 0.664 | 0.769 | 0.107 | 0.378 | 0.002 | 0.789 | 0.004 | 0.931 | |
| Leg muscle | Male | 5.96 b | 5.93 b | 19.59 | 13.14 b | 54.51 b | 3.06 a | 14.68 | 74.74 a | 21.87 | 2.89 b | 0.52 |
| Female | 6.39 a | 6.17 a | 19.60 | 17.19 a | 58.26 a | 1.00 b | 16.59 | 73.10 b | 21.88 | 4.01 a | 0.47 | |
| SEM | 0.056 | 0.030 | 1.250 | 0.529 | 0.484 | 0.269 | 0.576 | 0.160 | 0.096 | 0.158 | 0.029 | |
| p-value | 0.001 | <0.001 | 0.997 | 0.001 | 0.001 | 0.001 | 0.110 | <0.001 | 0.934 | 0.001 | 0.437 | |
| Sex | |||||
|---|---|---|---|---|---|
| Items | Male | Female | SEM | p-Value | |
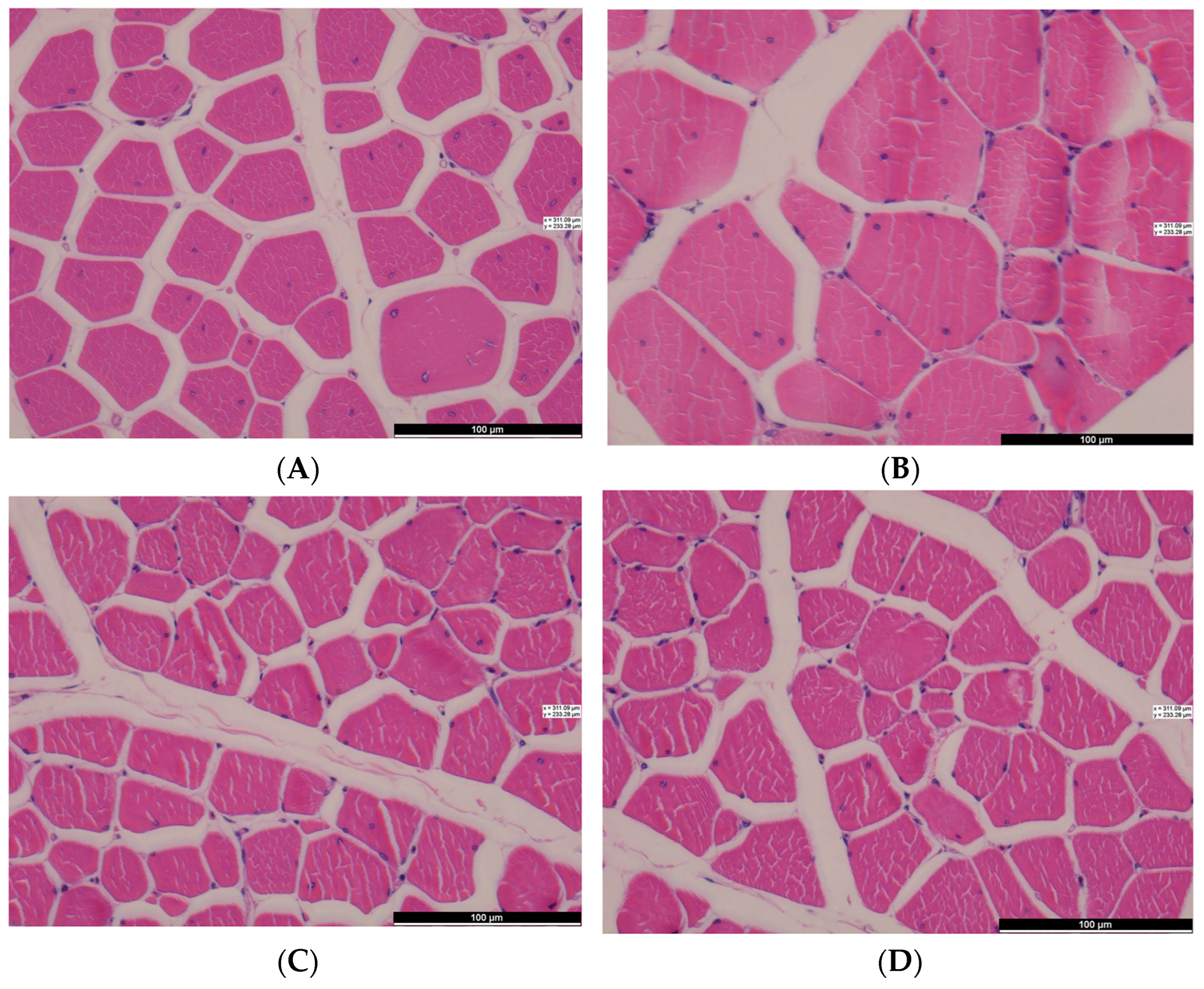
| Breast muscle | Diameter (μm) | 52.32 a | 46.66 b | 0.667 | <0.001 |
| Cross-sectional area (μm2) | 2318.03 a | 1656.17 b | 37.781 | <0.001 | |
| Density (piece/mm2) | 319.21 b | 494.09 a | 27.554 | 0.013 | |
| Leg muscle | Diameter (μm) | 56.59 a | 43.21 b | 0.987 | <0.001 |
| Cross-sectional area (μm2) | 2759.09 a | 1306.46 b | 51.140 | <0.001 | |
| Density(piece/mm2) | 236.52 b | 398.09 a | 21.031 | 0.005 | |
| Sex | ||||||
|---|---|---|---|---|---|---|
| Items | Mean Rank | Male | Female | SEM | Z-Value | p-Value |
| Breast meat | Mouthfeel | 7.82 a | 7.37 b | 0.90 | −2.980 | 0.003 |
| Tenderness | 7.16 b | 7.76 a | 0.99 | −3.052 | 0.002 | |
| Flavor | 7.69 | 7.43 | 0.91 | −1.432 | 0.152 | |
| Soup | Aroma | 8.51 | 8.39 | 0.77 | −1.276 | 0.202 |
| Oiliness | 6.37 | 6.27 | 1.51 | −0.557 | 0.578 | |
| Flavor | 8.33 a | 7.94 b | 0.50 | −3.585 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Jiang, Y.; Wang, Z.; Chen, G.; Chang, G.; Bai, H. Effects of Sex on Growth Performance, Carcass Traits, Blood Biochemical Parameters, and Meat Quality of XueShan Chickens. Animals 2024, 14, 1556. https://doi.org/10.3390/ani14111556
Yuan C, Jiang Y, Wang Z, Chen G, Chang G, Bai H. Effects of Sex on Growth Performance, Carcass Traits, Blood Biochemical Parameters, and Meat Quality of XueShan Chickens. Animals. 2024; 14(11):1556. https://doi.org/10.3390/ani14111556
Chicago/Turabian StyleYuan, Chunyou, Yong Jiang, Zhixiu Wang, Guohong Chen, Guobin Chang, and Hao Bai. 2024. "Effects of Sex on Growth Performance, Carcass Traits, Blood Biochemical Parameters, and Meat Quality of XueShan Chickens" Animals 14, no. 11: 1556. https://doi.org/10.3390/ani14111556
APA StyleYuan, C., Jiang, Y., Wang, Z., Chen, G., Chang, G., & Bai, H. (2024). Effects of Sex on Growth Performance, Carcass Traits, Blood Biochemical Parameters, and Meat Quality of XueShan Chickens. Animals, 14(11), 1556. https://doi.org/10.3390/ani14111556







