The Effects of Bacillus amyloliquefaciens SC06 on Behavior and Brain Function in Broilers Infected by Clostridium perfringens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Behavioral Traits
2.3. Hematoxylin–Eosin (HE) Staining
2.4. Transcriptome Sequencing
2.5. Gene Expressions
2.6. Statistical Analysis
3. Results
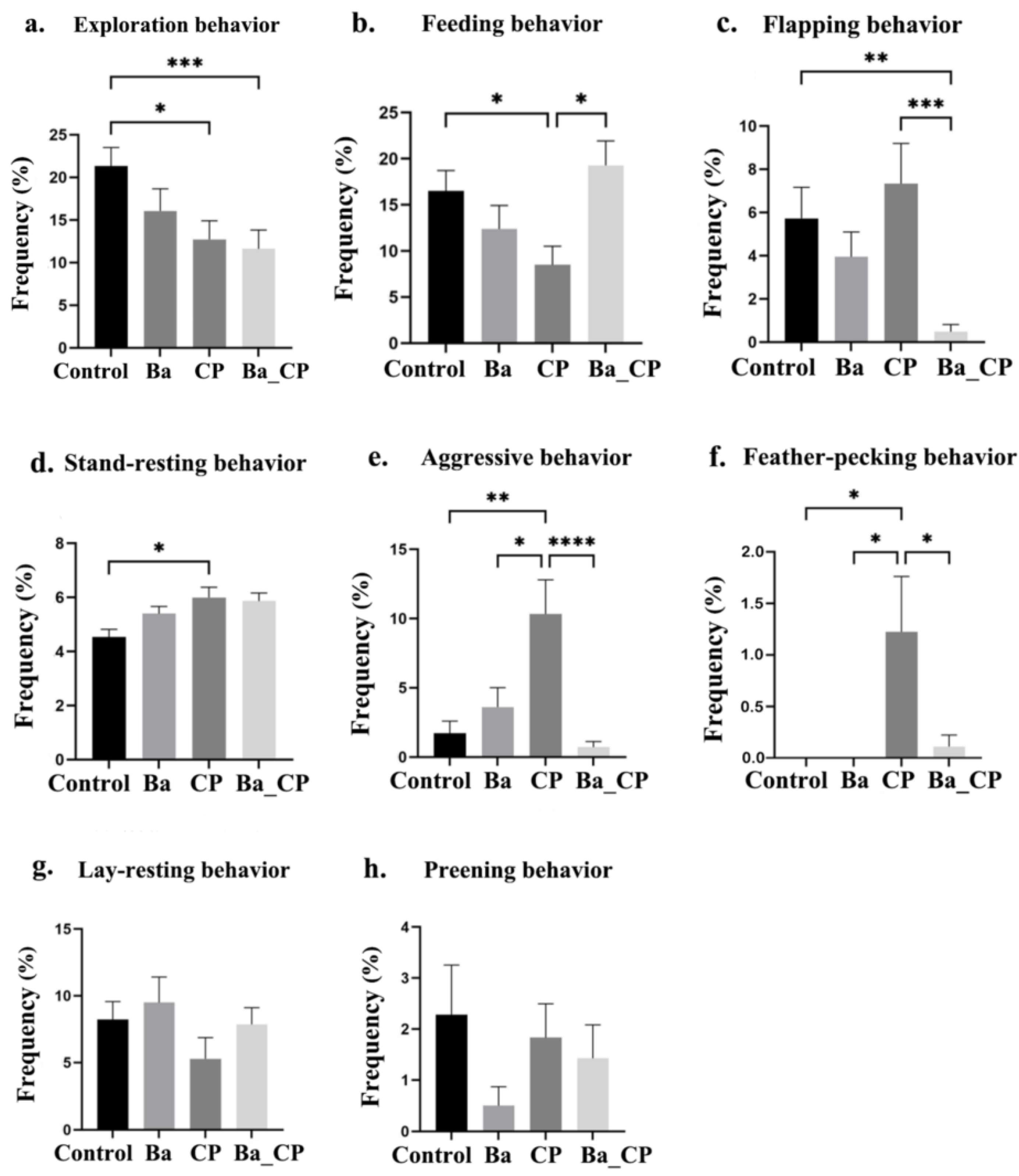
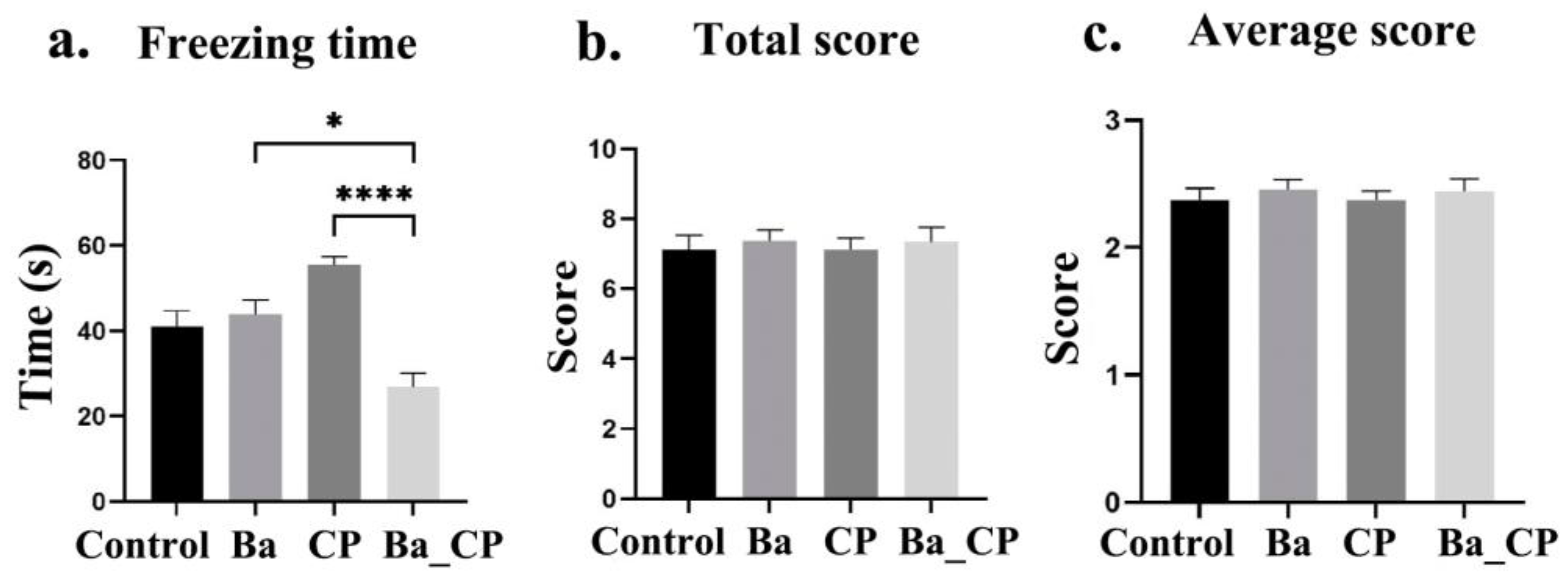
3.1. Behavioral Traits
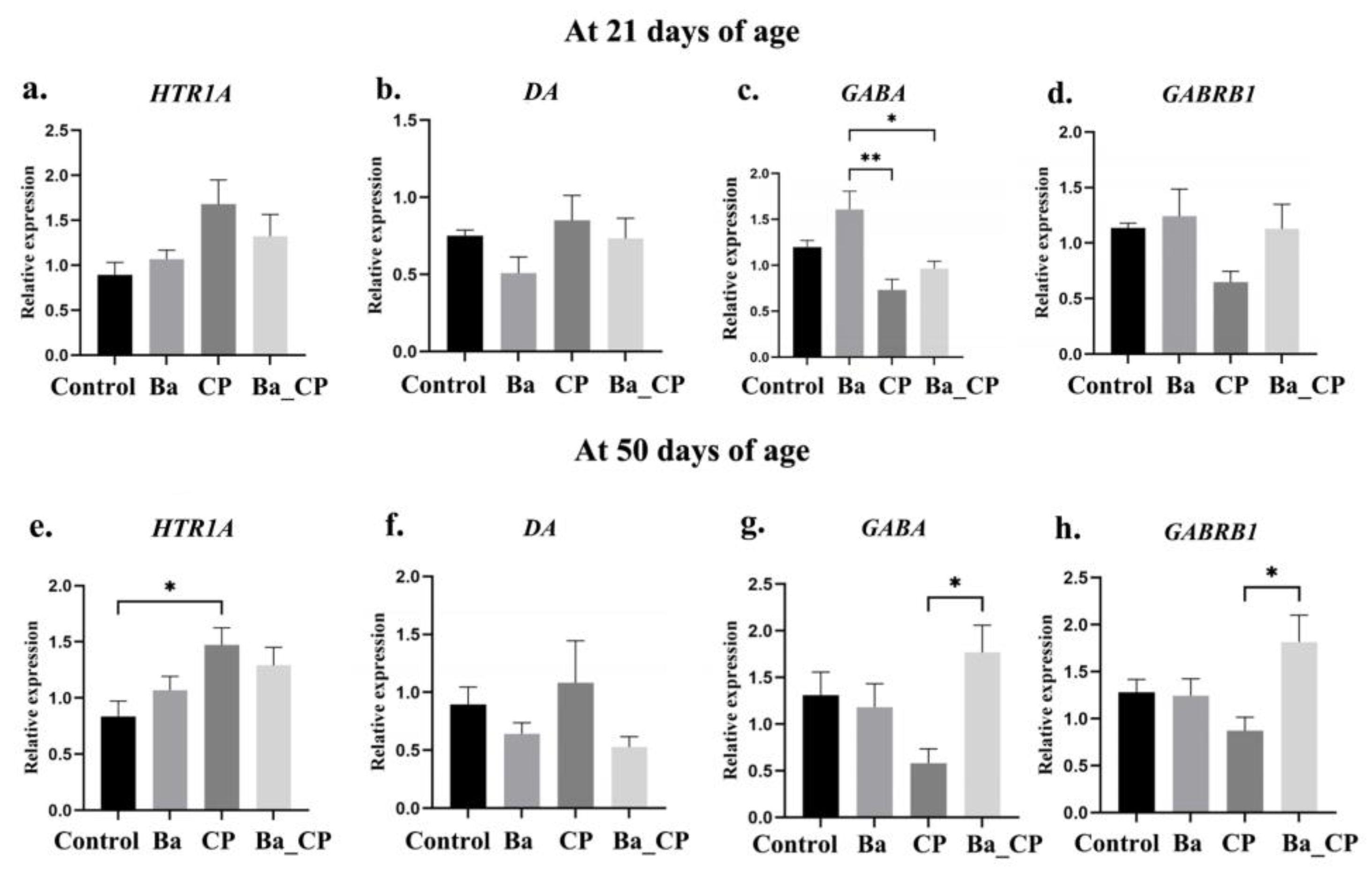
3.2. Gene Expression
3.3. Hematoxylin–Eosin Staining
3.4. mRNA Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milton, A.A.P.; Momin, A.G.; Gandhale, P.N.; Das, S.; Ghatak, S.; Priya, G.B.; Firake, D.M.; Srinivas, K.; Momin, K.M.; Hussain, Z.; et al. Prevalence, toxinotyping, antimicrobial susceptibility and biofilm-forming ability of Clostridium perfringens isolated from free-living rodents and shrews. Anaerobe 2022, 77, 102618. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.G. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 1996, 9, 216–234. [Google Scholar] [CrossRef]
- Thiemann, R.A.; Thornton, J.K.; Stayer, P.A.; Riley, E.; Clark, R.; Armour, N.; Pulido-Landínez, M. A Novel Presentation of Clostridium perfringens in Young Broilers. Avian Dis. 2022, 66, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- García-Vela, S.; Ben Said, L.; Soltani, S.; Guerbaa, R.; Fernández-Fernández, R.; Ben Yahia, H.; Ben Slama, K.; Torres, C.; Fliss, I. Targeting Enterococci with Antimicrobial Activity against Clostridium perfringens from Poultry. Antibiotics 2023, 12, 231. [Google Scholar] [CrossRef]
- Fernandes da Costa, S.P.; Mot, D.; Geeraerts, S.; Bokori-Brown, M.; Van Immerseel, F.; Titball, R.W. Variable protection against experimental broiler necrotic enteritis after immunization with the C-terminal fragment of Clostridium perfringens alpha-toxin and a non-toxic NetB variant. Avian Pathol. 2016, 45, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Gharib-Naseri, K.; Kheravii, S.; Keerqin, C.; Swick, R.A.; Choct, M.; Wu, S.B. Differential expression of intestinal genes in necrotic enteritis challenged broiler chickens with 2 different Clostridium perfringens strains. Poult. Sci. 2021, 100, 100886. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Lillehoj, H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019, 98, 188–198. [Google Scholar] [CrossRef]
- Cooper, K.K.; Songer, J.G.; Uzal, F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Investig. 2013, 25, 314–327. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Bai, S.; Wang, J.; Zeng, Q.; Peng, H.; Lv, H.; Mu, Y.; Xuan, Y.; Li, S.; et al. Extract of Scutellaria baicalensis and Lonicerae flos improves growth performance, antioxidant capacity, and intestinal barrier of yellow-feather broiler chickens against Clostridium perfringens. Poult. Sci. 2024, 103, 103718. [Google Scholar] [CrossRef]
- Linden, J.R.; Flores, C.; Schmidt, E.F.; Uzal, F.A.; Michel, A.O.; Valenzuela, M.; Dobrow, S.; Vartanian, T. Clostridium perfringens epsilon toxin induces blood brain barrier permeability via caveolae-dependent transcytosis and requires expression of MAL. PLoS Pathog. 2019, 15, e1008014. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S. Role of Clostridium perfringens Necrotic Enteritis B-like Toxin in Disease Pathogenesis. Vaccines 2021, 10, 61. [Google Scholar] [CrossRef]
- Shamshirgaran, M.A.; Golchin, M.; Salehi, M.; Kheirandish, R. Evaluation the efficacy of oral immunization of broiler chickens with a recombinant Lactobacillus casei vaccine vector expressing the Carboxy-terminal fragment of α-toxin from Clostridium perfringens. BMC Vet. Res. 2023, 19, 13. [Google Scholar] [CrossRef]
- Oda, M.; Terao, Y.; Sakurai, J.; Nagahama, M. Membrane-Binding Mechanism of Clostridium perfringens Alpha-Toxin. Toxins 2015, 7, 5268–5275. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, J.Y.; Cheng, H.W. The Impact of Probiotic Bacillus subtilis on Injurious Behavior in Laying Hens. Animals 2022, 12, 870. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Zhao, Y.; Jin, Q.; Li, S. Protective Effects of Bacillus coagulans JA845 against D-Galactose/AlCl(3)-Induced Cognitive Decline, Oxidative Stress and Neuroinflammation. J. Microbiol. Biotechnol. 2022, 32, 212–219. [Google Scholar] [CrossRef]
- Wang, W.C.; Yan, F.F.; Hu, J.Y.; Amen, O.A.; Cheng, H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018, 96, 1654–1666. [Google Scholar] [CrossRef]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity through Hypothalamic-Pituitary-Adrenal Axis Modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar] [CrossRef] [PubMed]
- van Hierden, Y.M.; Koolhaas, J.M.; Korte, S.M. Chronic increase of dietary l-tryptophan decreases gentle feather pecking behaviour. Appl. Anim. Behav. Sci. 2004, 89, 71–84. [Google Scholar] [CrossRef]
- Mindus, C.; van Staaveren, N.; Bharwani, A.; Fuchs, D.; Gostner, J.M.; Kjaer, J.B.; Kunze, W.; Mian, M.F.; Shoveller, A.K.; Forsythe, P.; et al. Ingestion of Lactobacillus rhamnosus modulates chronic stress-induced feather pecking in chickens. Sci. Rep. 2021, 11, 17119. [Google Scholar] [CrossRef] [PubMed]
- Umalkar, S.S.; Jadhav, V.V.; Paul, P.; Reche, A. Modern Anchorage Systems in Orthodontics. Cureus 2022, 14, e31476. [Google Scholar] [CrossRef] [PubMed]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Chen, S.; Yan, C.; Xiang, H.; Xiao, J.; Liu, J.; Zhang, H.; Wang, J.; Liu, H.; Zhang, X.; Ou, M.; et al. Transcriptome changes underlie alterations in behavioral traits in different types of chicken. J. Anim. Sci. 2020, 98, skaa167. [Google Scholar] [CrossRef]
- Favati, A.; Leimar, O.; Løvlie, H. Personality predicts social dominance in male domestic fowl. PLoS ONE 2014, 9, e103535. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Grigg, E.K.; Chou, J.; Parker, E.; Gatesy-Davis, A.; Clarkson, S.T.; Hart, L.A. Stress-Related Behaviors in Companion Dogs Exposed to Common Household Noises, and Owners’ Interpretations of Their Dogs’ Behaviors. Front. Vet. Sci. 2021, 8, 760845. [Google Scholar] [CrossRef]
- Remonato Franco, B.; Shynkaruk, T.; Crowe, T.; Fancher, B.; French, N.; Gillingham, S.; Schwean-Lardner, K. Light color and the commercial broiler: Effect on behavior, fear, and stress. Poult. Sci. 2022, 101, 102052. [Google Scholar] [CrossRef] [PubMed]
- Mignon-Grasteau, S.; Chantry-Darmon, C.; Boscher, M.Y.; Sellier, N.; Le Bihan-Duval, E.; Bertin, A. Genetic Determinism of Fearfulness, General Activity and Feeding Behavior in Chickens and Its Relationship with Digestive Efficiency. Behav. Genet. 2017, 47, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.L.M.; Dickson, E.J.; Lee, C. Application of open field, tonic immobility, and attention bias tests to hens with different ranging patterns. PeerJ 2019, 7, e8122. [Google Scholar] [CrossRef] [PubMed]
- Martinho, R.; Correia, G.; Seixas, R.; Oliveira, A.; Silva, S.; Serrão, P.; Fernandes-Lopes, C.; Costa, C.; Moreira-Rodrigues, M. Treatment with Nepicastat Decreases Contextual Traumatic Memories Persistence in Post-traumatic Stress Disorder. Front. Mol. Neurosci. 2021, 14, 745219. [Google Scholar] [CrossRef] [PubMed]
- Matrisciano, F.; Pinna, G. PPAR-α Hypermethylation in the Hippocampus of Mice Exposed to Social Isolation Stress Is Associated with Enhanced Neuroinflammation and Aggressive Behavior. Int. J. Mol. Sci. 2021, 22, 10678. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, L.; Jensen, P. Effects of stress during commercial hatching on growth, egg production and feather pecking in laying hens. PLoS ONE 2022, 17, e0262307. [Google Scholar] [CrossRef]
- Staes, N.; Sherwood, C.C.; Freeman, H.; Brosnan, S.F.; Schapiro, S.J.; Hopkins, W.D.; Bradley, B.J. Serotonin Receptor 1A Variation Is Associated with Anxiety and Agonistic Behavior in Chimpanzees. Mol. Biol. Evol. 2019, 36, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Özkan, S.; Yalçın, S.; Bayraktar, Ö.H.; Bilgen, G.; Dayıoğlu, M.; Bolhuis, J.E.; Rodenburg, T.B. Effects of incubation lighting with green or white light on brown layers: Hatching performance, feather pecking and hypothalamic expressions of genes related with photoreception, serotonin, and stress systems. Poult. Sci. 2022, 101, 102114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, C.; Xue, G.; Lei, X.; Li, J.; Moyzis, R.K.; Dong, Q.; Lin, C. The GABRB1 gene is associated with thalamus volume and modulates the association between thalamus volume and intelligence. NeuroImage 2014, 102 Pt 2, 756–763. [Google Scholar] [CrossRef]
- Evenseth, L.S.M.; Gabrielsen, M.; Sylte, I. The GABA(B) Receptor-Structure, Ligand Binding and Drug Development. Molecules 2020, 25, 3093. [Google Scholar] [CrossRef]
- Li, Z.; Cogswell, M.; Hixson, K.; Brooks-Kayal, A.R.; Russek, S.J. Nuclear Respiratory Factor 1 (NRF-1) Controls the Activity Dependent Transcription of the GABA-A Receptor Beta 1 Subunit Gene in Neurons. Front. Mol. Neurosci. 2018, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.C.; McClane, B.A.; Uzal, F.A. New insights into Clostridium perfringens epsilon toxin activation and action on the brain during enterotoxemia. Anaerobe 2016, 41, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Kang, J.; Kang, L.; Gao, S.; Yang, H.; Ji, B.; Li, P.; Liu, J.; Xin, W.; Wang, J. Immunization with a novel Clostridium perfringens epsilon toxin mutant rETX(Y196E)-C confers strong protection in mice. Sci. Rep. 2016, 6, 24162. [Google Scholar] [CrossRef]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. Enterotoxic Clostridia:Clostridium perfringens Enteric Diseases. Microbiol. Spectr. 2018, 6, 1128. [Google Scholar] [CrossRef] [PubMed]
- Rajanala, K.; Kumar, N.; Chamallamudi, M.R. Modulation of Gut-Brain Axis by Probiotics: A Promising Anti-depressant Approach. Curr. Neuropharmacol. 2021, 19, 990–1006. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Hu, A.; Shu, X.; Huang, W.; Liu, J.; Wang, B.; Zhang, R.; Yue, M.; Yang, C. Lactobacillus plantarum-derived postbiotics prevent Salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front. Nutr. 2022, 9, 946096. [Google Scholar] [CrossRef]
- van Riet, J.; van de Werken, H.J.G.; Cuppen, E.; Eskens, F.; Tesselaar, M.; van Veenendaal, L.M.; Klümpen, H.J.; Dercksen, M.W.; Valk, G.D.; Lolkema, M.P.; et al. The genomic landscape of 85 advanced neuroendocrine neoplasms reveals subtype-heterogeneity and potential therapeutic targets. Nat. Commun. 2021, 12, 4612. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Shi, W. FAM84B acts as a tumor promoter in human glioma via affecting the Akt/GSK-3β/β-catenin pathway. BioFactors 2021, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Zhuang, X.; Lv, Y.; Zhang, Y.; Xu, J.; Gao, F.; Chen, D.; Wang, Y. FAM84B promotes the proliferation of glioma cells through the cell cycle pathways. World J. Surg. Oncol. 2022, 20, 368. [Google Scholar] [CrossRef]
- Tang, J.; Yang, Q.; Cui, Q.; Zhang, D.; Kong, D.; Liao, X.; Ren, J.; Gong, Y.; Wu, G. Weighted gene correlation network analysis identifies RSAD2, HERC5, and CCL8 as prognostic candidates for breast cancer. J. Cell. Physiol. 2020, 235, 394–407. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.; Cao, J.; Abula, M.; Yimingjiang, Y.; Feng, S. Weighted gene co-expression network analysis reveals that CXCL10, IRF7, MX1, RSAD2, and STAT1 are related to the chronic stage of spinal cord injury. Ann. Transl. Med. 2021, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A.; Mastrangelo, M.A.; Ture, S.K.; Smith, C.O.; Loelius, S.G.; Berg, R.A.; Shi, X.; Burke, R.M.; Spinelli, S.L.; Cameron, S.J.; et al. The choline transporter Slc44a2 controls platelet activation and thrombosis by regulating mitochondrial function. Nat. Commun. 2020, 11, 3479. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wan, L.; Ren, H.; Xie, Z.; Xie, L.; Huang, J.; Deng, X.; Xie, Z.; Luo, S.; Li, M.; et al. Screening of interferon-stimulated genes against avian reovirus infection and mechanistic exploration of the antiviral activity of IFIT5. Front. Microbiol. 2022, 13, 998505. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yin, Y.; Yang, H.L.; Yang, C.W.; Yu, C.L.; Wang, Y.; Yin, H.D.; Lian, T.; Peng, H.; Zhu, Q.; et al. mRNA expression and functional analysis of chicken IFIT5 after infected with Newcastle disease virus. Infect. Genet. Evol. 2020, 86, 104585. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Unno, K.; Truica, M.I.; Chalmers, Z.R.; Yoo, Y.A.; Vatapalli, R.; Sagar, V.; Yu, J.; Lysy, B.; Hussain, M.; et al. A Genome-Wide CRISPR Activation Screen Identifies PRRX2 as a Regulator of Enzalutamide Resistance in Prostate Cancer. Cancer Res. 2022, 82, 2110–2123. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, A.; Liu, X. PRRX2 predicts poor survival prognosis, and promotes malignant phenotype of lung adenocarcinoma via transcriptional activates PSMD1. Transl. Oncol. 2023, 27, 101586. [Google Scholar] [CrossRef] [PubMed]
- Asnaghi, L.; White, D.T.; Key, N.; Choi, J.; Mahale, A.; Alkatan, H.; Edward, D.P.; Elkhamary, S.M.; Al-Mesfer, S.; Maktabi, A.; et al. ACVR1C/SMAD2 signaling promotes invasion and growth in retinoblastoma. Oncogene 2019, 38, 2056–2075. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Khera, A.V.; Aragam, K.; Haas, M.; Chaffin, M.; Klarin, D.; Natarajan, P.; Bick, A.; Zekavat, S.M.; Nomura, A.; et al. DNA Sequence Variation in ACVR1C Encoding the Activin Receptor-like Kinase 7 Influences Body Fat Distribution and Protects Against Type 2 Diabetes. Diabetes 2019, 68, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Guo, D.; Tan, L.; Xiao, Z.; Shi, Y. NPSR1-AS1 activates the MAPK pathway to facilitate thyroid cancer cell malignant behaviors via recruiting ELAVL1 to stabilize NPSR1 mRNA. Cell Cycle 2022, 21, 439–449. [Google Scholar] [CrossRef]
- Tengvall, K.; Huang, J.; Hellström, C.; Kammer, P.; Biström, M.; Ayoglu, B.; Lima Bomfim, I.; Stridh, P.; Butt, J.; Brenner, N.; et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc. Natl. Acad. Sci. USA 2019, 116, 16955–16960. [Google Scholar] [CrossRef]
- Xu, S.; Hu, L.; Yang, L.; Wu, B.; Cao, Y.; Zhang, R.; Xu, X.; Ma, H.; Zhou, W.; Cheng, G.; et al. Galloway-Mowat Syndrome Type 3 Caused by OSGEP Gene Variants: A Case Report and Literature Review. Front. Pediatr. 2022, 10, 899991. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Gu, J.; Xin, J.; Liu, H.; Wu, Y.; Du, M.; Chu, H.; Liu, Y.; Zhang, Z. Genetic variants in choline metabolism pathway are associated with the risk of bladder cancer in the Chinese population. Arch. Toxicol. 2022, 96, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Alqhtani, A.H.; Swelum, A.A.; Salem, H.M.; Elbestawy, A.R.; Noreldin, A.E.; Babalghith, A.O.; Khafaga, A.F.; Hassan, M.I.; et al. The relationship among avian influenza, gut microbiota and chicken immunity: An updated overview. Poult. Sci. 2022, 101, 102021. [Google Scholar] [CrossRef]
- Davies, W.I.L.; Sghari, S.; Upton, B.A.; Nord, C.; Hahn, M.; Ahlgren, U.; Lang, R.A.; Gunhaga, L. Distinct Opsin 3 (Opn3) Expression in the Developing Nervous System during Mammalian Embryogenesis. eNeuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, Z.; Deng, H.; Hao, J.; Ding, R.; Zhao, M. TMEM213 as a novel prognostic and predictive biomarker for patients with lung adenocarcinoma after curative resection: A study based on bioinformatics analysis. J. Thorac. Dis. 2019, 11, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, T.; Tanimoto, K.; Yamaoka, E.; Kojima, M.; Kanawa, M.; Hirohashi, N.; Hiyama, E. Oncogenic Role of ADAM32 in Hepatoblastoma: A Potential Molecular Target for Therapy. Cancers 2022, 14, 4732. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, S.; Zhao, X.; Yuan, Y.; Zhang, B.; Guan, Y. Knockdown of Circular RNA Hsa_circ_0000714 Can Regulate RAB17 by Sponging miR-370-3p to Reduce Paclitaxel Resistance of Ovarian Cancer through CDK6/RB Pathway. OncoTargets Ther. 2020, 13, 13211–13224. [Google Scholar] [CrossRef] [PubMed]
- Allende, G.; Chávez-Reyes, J.; Guerrero-Alba, R.; Vázquez-León, P.; Marichal-Cancino, B.A. Advances in Neurobiology and Pharmacology of GPR12. Front. Pharmacol. 2020, 11, 628. [Google Scholar] [CrossRef]
- Kim, T.; Hunt, H.D.; Parcells, M.S.; van Santen, V.; Ewald, S.J. Two class I genes of the chicken MHC have different functions: BF1 is recognized by NK cells while BF2 is recognized by CTLs. Immunogenetics 2018, 70, 599–611. [Google Scholar] [CrossRef]
- Salas-Pérez, F.; Ramos-Lopez, O.; Mansego, M.L.; Milagro, F.I.; Santos, J.L.; Riezu-Boj, J.I.; Martínez, J.A. DNA methylation in genes of longevity-regulating pathways: Association with obesity and metabolic complications. Aging 2019, 11, 1874–1899. [Google Scholar] [CrossRef]
- Allen, E.A.; Baehrecke, E.H. Autophagy in animal development. Cell Death Differ. 2020, 27, 903–918. [Google Scholar] [CrossRef]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2019, 266, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Castro, F.; Zappettini, S.; Pressey, J.C.; Silva, C.G.; Russeau, M.; Gervasi, N.; Figueiredo, M.; Montmasson, C.; Renner, M.; Canas, P.M.; et al. Convergence of adenosine and GABA signaling for synapse stabilization during development. Science 2021, 374, eabk2055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lian, Y.; Li, J.; Zhang, Y.; Liu, Y.; Wang, X.; Ma, J.; Li, F. KangPiLao decoction modulates cognitive and emotional disorders in rats with central fatigue through the GABA/Glu pathway. Front. Pharmacol. 2022, 13, 939169. [Google Scholar] [CrossRef]
- Ibrahim, D.; Al-Khalaifah, H.S.; Abdelfattah-Hassan, A.; Eldoumani, H.; Khater, S.I.; Arisha, A.H.; Mohamed, S.A.M.; Ismail, T.A.; Tolba, S.A. Promising Role of Growth Hormone-Boosting Peptide in Regulating the Expression of Muscle-Specific Genes and Related MicroRNAs in Broiler Chickens. Animals 2021, 11, 1906. [Google Scholar] [CrossRef]

| Comparable Group | Upregulated Gene Name | Down-Regulated Gene Name | Pathways |
|---|---|---|---|
| Control VS. CP | LRAT domain containing 2 Radical S-adenosyl methionine domain containing 2 Solute carrier family 44 member 2 Interferon induced protein with tetratricopeptide repeats 5 | Paired related homeobox 2 | Choline metabolism in cancer, Hepatitis C, and Influenza A |
| Control VS. Ba_CP | Activin A receptor type I G protein-coupled receptor 12 | Osialoglyco protein endopeptidase | Longevity regulating pathway–mammal, Autophagy–animal, Longevity regulating pathway–multiple species, GnRH secretion, Spinocerebellar ataxia, Cholinergic synapse, Glutamatergic synapse, Growth hormone synthesis, secretion and action |
| Ba VS. CP | Major histocompatibility complex class I antigen BF2 | / | Type I diabetes mellitus, Allograft rejection, Autoimmune thyroid disease, Viral myocarditis, Cellular senescence, Kaposi sarcoma-associated herpesvirus infection, and Viral carcinogenesis |
| Ba VS. Ba_CP | Activin A receptor type 1C Neuropeptide S receptor 1 Anoctamin 2 | Osialoglyco protein endopeptidase Small lysine rich protein 1 | Autophagy–animal, Longevity regulating pathway–mammal, cholinergic synapse, Glutamatergic synapse, Longevity regulating pathway–multiple species, Dopaminergic synapse, and GnRH secretion |
| CP VS. Ba_CP | Opsin 3 Transmembrane protein 213 | O-sialoglyco protein endopeptidase A disintegrin and metalloproteinase domain 32 Member RAS oncogene family | Cholinergic synapse, Spinocerebellar ataxia, Glutamatergic synapse, cAMP signaling pathway, GnRH secretion, Dopaminergic synapse, Circadian entrainment, Insulin secretion, Growth hormone synthesis, and secretion and action |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Liu, J.; Luo, S.; Xing, L.; Li, W.; Gong, L. The Effects of Bacillus amyloliquefaciens SC06 on Behavior and Brain Function in Broilers Infected by Clostridium perfringens. Animals 2024, 14, 1547. https://doi.org/10.3390/ani14111547
Chen S, Liu J, Luo S, Xing L, Li W, Gong L. The Effects of Bacillus amyloliquefaciens SC06 on Behavior and Brain Function in Broilers Infected by Clostridium perfringens. Animals. 2024; 14(11):1547. https://doi.org/10.3390/ani14111547
Chicago/Turabian StyleChen, Siyu, Jinling Liu, Shuyan Luo, Limin Xing, Weifen Li, and Li Gong. 2024. "The Effects of Bacillus amyloliquefaciens SC06 on Behavior and Brain Function in Broilers Infected by Clostridium perfringens" Animals 14, no. 11: 1547. https://doi.org/10.3390/ani14111547
APA StyleChen, S., Liu, J., Luo, S., Xing, L., Li, W., & Gong, L. (2024). The Effects of Bacillus amyloliquefaciens SC06 on Behavior and Brain Function in Broilers Infected by Clostridium perfringens. Animals, 14(11), 1547. https://doi.org/10.3390/ani14111547





