Replacement of Corn with Different Levels of Wheat Impacted the Growth Performance, Intestinal Development, and Cecal Microbiota of Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Birds, Diets, and Management
2.3. Sample Collection
2.4. Intestinal and Liver Morphology Determination
2.5. Serum Biochemistry
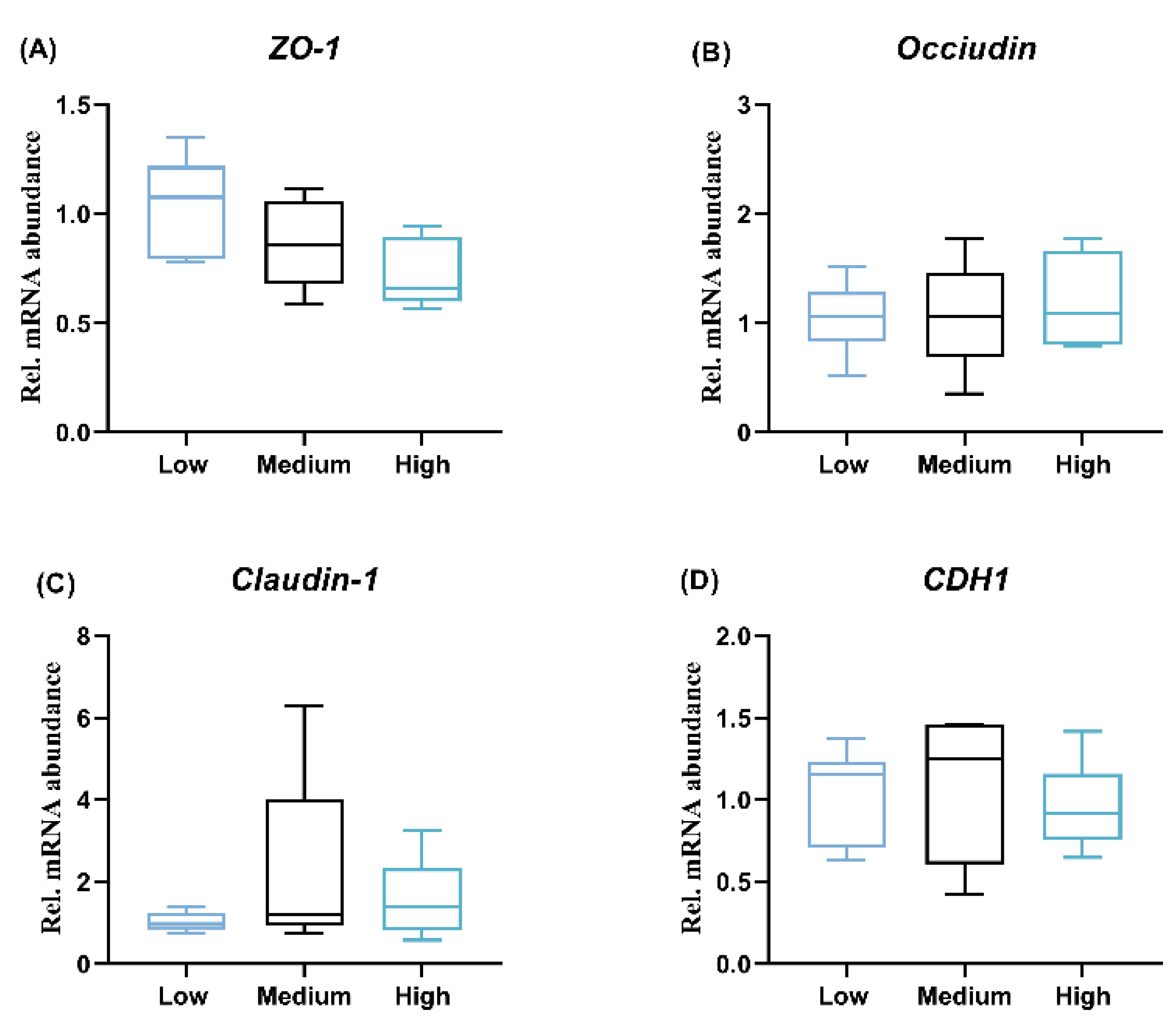
2.6. Ileum Gene Expression
2.7. Cecal Microbiota Analysis through 16S RNA
2.8. Statistical Analyses
3. Results
3.1. Growth Performance
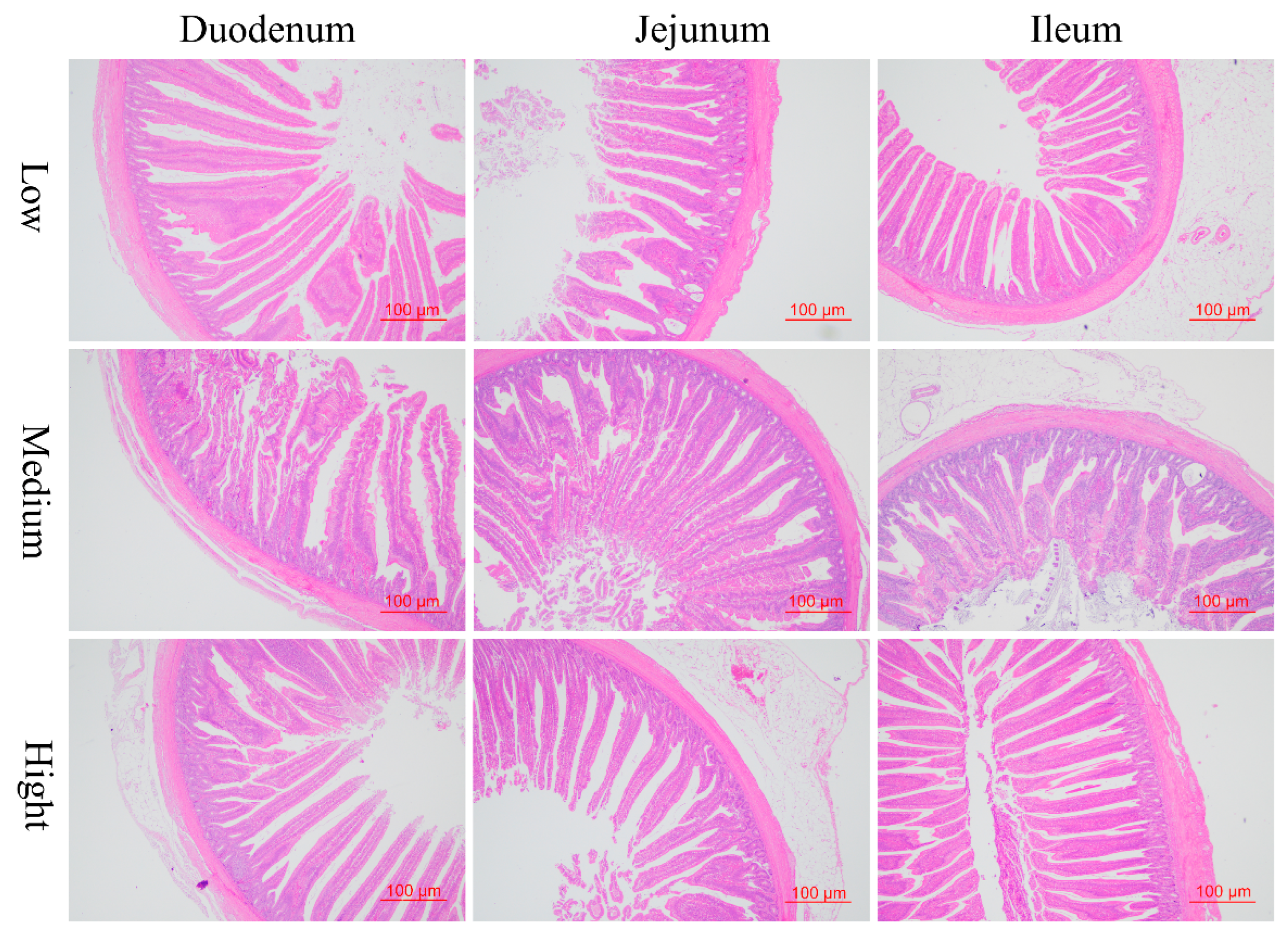
3.2. Alterations in the Gastrointestinal Tract
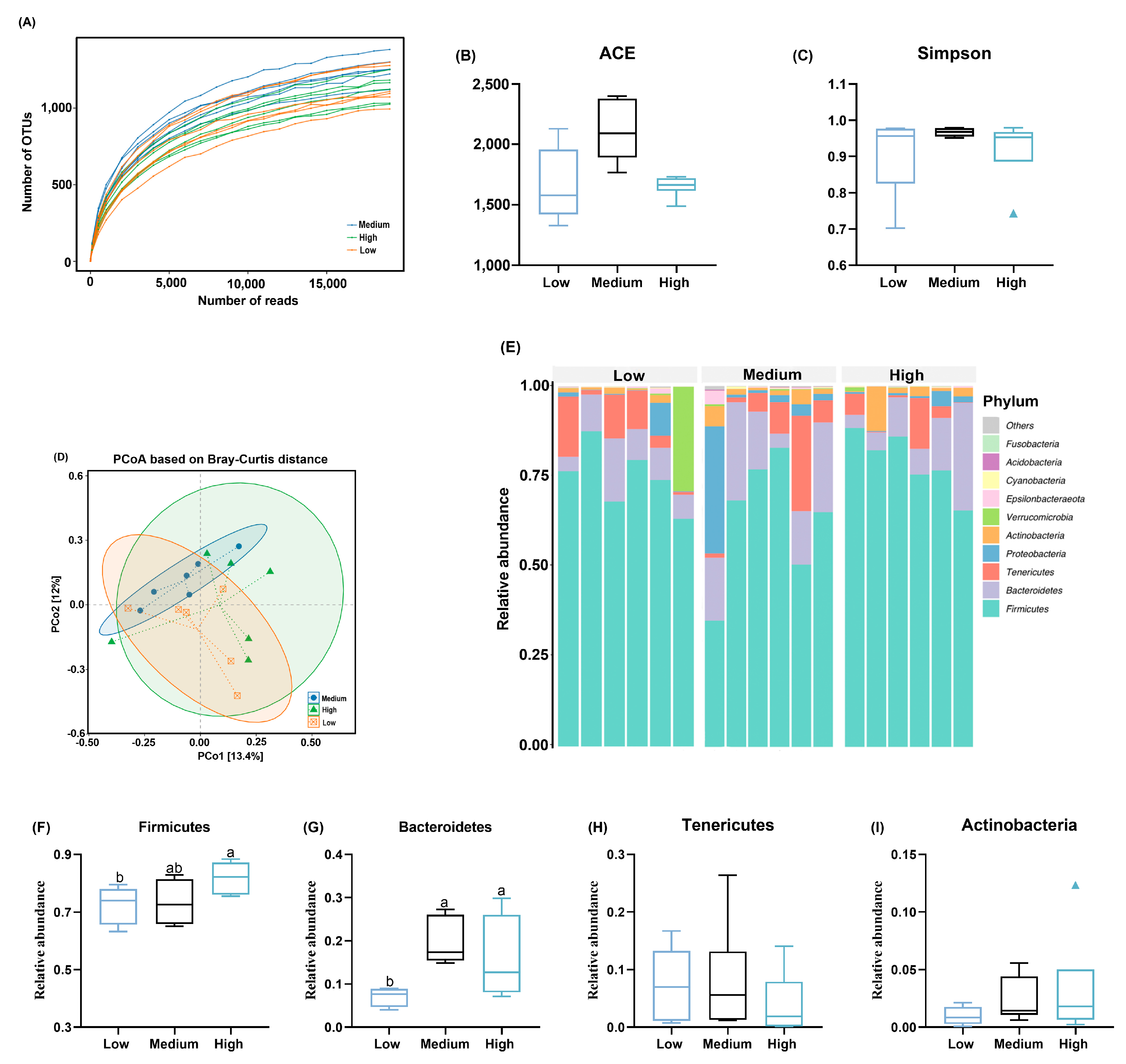
3.3. Cecal Microbiota Composition
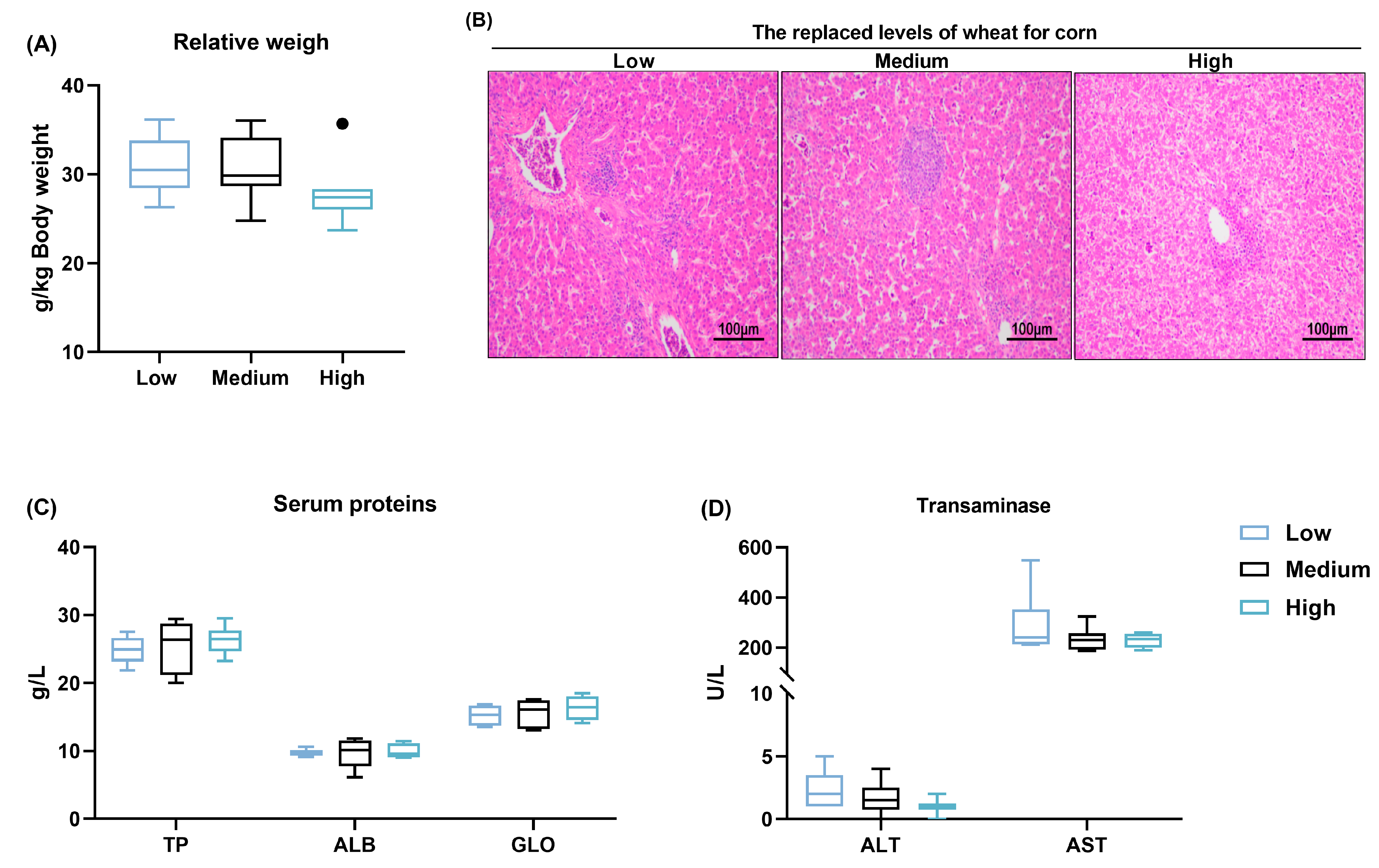
3.4. Liver Health
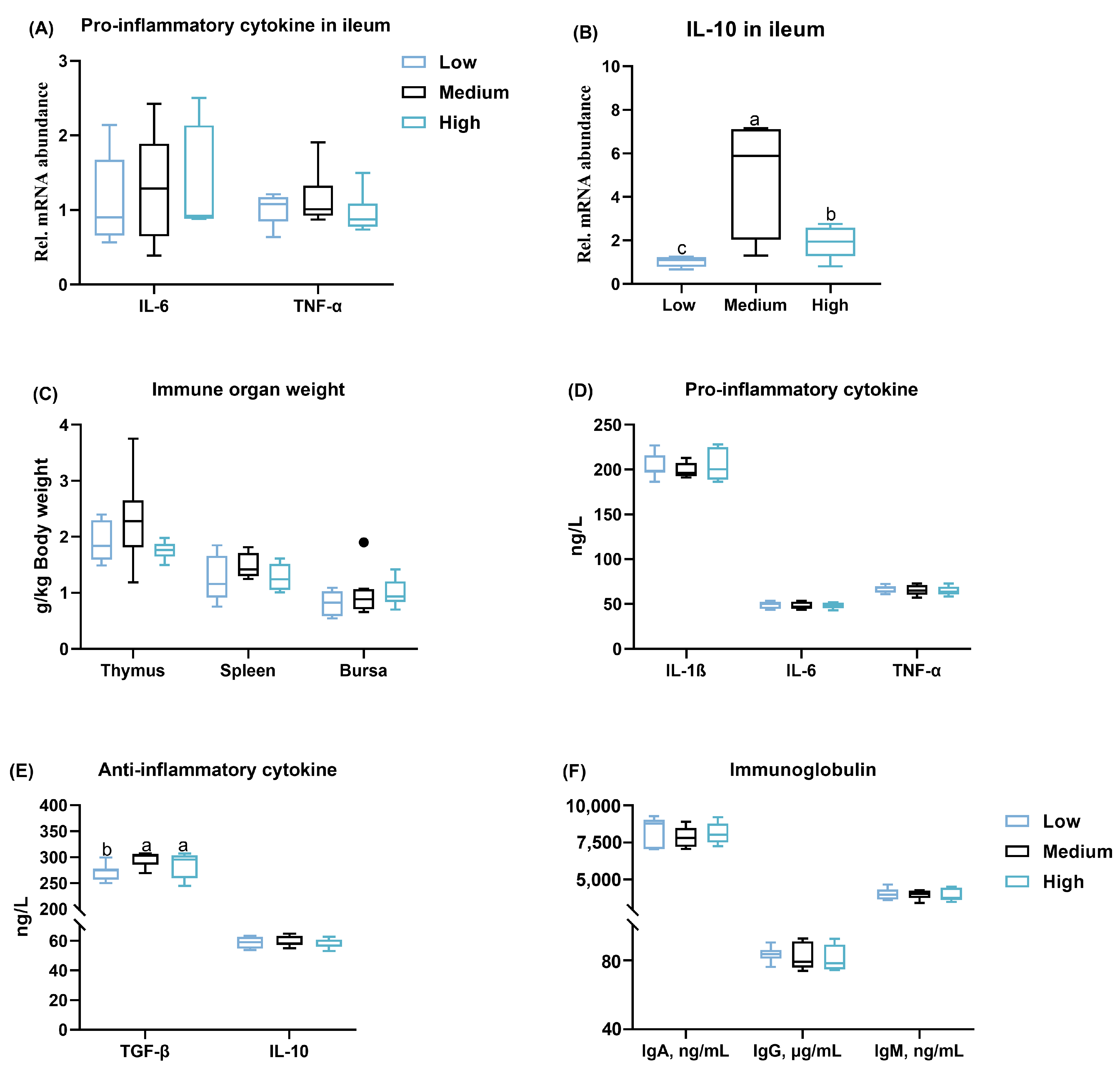
3.5. Inflammatory Status
3.6. Serum Glycolipid Profile and Bone Turnover
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- An, S.H.; Sung, J.Y.; Kang, H.K.; Kong, C. Additivity of ileal amino acid digestibility in diets containing corn, soybean meal, and corn distillers dried grains with solubles for male broilers. Animals 2020, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Smeets, N.; Nuyens, F.; Van Campenhout, L.; Delezie, E.; Niewold, T.A. Interactions between the concentration of non-starch polysaccharides in wheat and the addition of an enzyme mixture in a broiler digestibility and performance trial. Poult. Sci. 2018, 97, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.R.; Rose, S.P.; Mackenzie, A.M.; Mansbridge, S.C.; Bedford, M.R.; Lovegrove, A.; Pirgozliev, V.R. Wheat sample affects growth performance and the apparent metabolisable energy value for broiler chickens. Br. Poult. Sci. 2019, 60, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Arabshahi, H.; Ghasemi, H.A.; Hajkhodadadi, I.; Khaltabadi Farahani, A.H. Effects of multicarbohydrase and butyrate glycerides on productive performance, nutrient digestibility, gut morphology, and ileal microbiota in late-phase laying hens fed corn- or wheat-based diets. Poult. Sci. 2021, 100, 101066. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.; Pahm, A.; Roth, J. Feeding wheat to pigs. Swine Focus 2010, 2, 1–8. [Google Scholar]
- Veluri, S.; Gonzalez-Ortiz, G.; Bedford, M.R.; Olukosi, O.A. Interactive effects of a stimbiotic supplementation and wheat bran inclusion in corn- or wheat-based diets on growth performance, ileal digestibility, and expression of nutrient transporters of broilers chickens. Poult. Sci. 2024, 103, 103178. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, N.W.; Lærke, H.N.; Bach Knudsen, K.E.; Stein, H.H. Carbohydrate composition and in vitro digestibility of dry matter and nonstarch polysaccharides in corn, sorghum, and wheat and coproducts from these grains. J. Anim. Sci. 2015, 93, 1103–1113. [Google Scholar] [CrossRef]
- Bedford, M.R.; Schulze, H. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 1998, 11, 91–114. [Google Scholar] [CrossRef]
- Chen, L.; Gao, L.X.; Huang, Q.H.; Zhong, R.Q.; Zhang, L.L.; Tang, X.F.; Zhang, H.F. Viscous and fermentable nonstarch polysaccharides affect intestinal nutrient and energy flow and hindgut fermentation in growing pigs. J. Anim. Sci. 2017, 95, 5054–5063. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, R.; Gao, L.; Liu, Z.; Chen, L.; Zhang, H. Effects of Optimal Carbohydrase Mixtures on Nutrient Digestibility and Digestible Energy of Corn- and Wheat-Based Diets in Growing Pigs. Animals 2020, 10, 1846. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.; Hesselman, K.; Aman, P. The influence of wheat bran and sugar-beet pulp on the digestibility of dietary components in a cereal-based pig diet. J. Nutr. 1986, 116, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Hong, J.S.; Fang, L.H.; Do, S.H.; Kim, B.O.; Kim, Y.Y. Effects of wheat supplementation levels on growth performance, blood profiles, nutrient digestibility, and pork quality in growing-finishing pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 1150–1159. [Google Scholar] [CrossRef]
- Ghiasvand, A.R.; Khatibjoo, A.; Mohammadi, Y.; Akbari Gharaei, M.; Shirzadi, H. Effect of fennel essential oil on performance, serum biochemistry, immunity, ileum morphology and microbial population, and meat quality of broiler chickens fed corn or wheat-based diet. Br. Poult. Sci. 2021, 62, 562–572. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Ravindran, V. Growth performance, nutrient utilization, and digesta characteristics in broiler chickens fed corn or wheat diets without or with supplemental xylanase. Poult. Sci. 2014, 93, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Choct, M.; Hughes, R.J.; Wang, J.; Bedford, M.R.; Morgan, A.J.; Annison, G. Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br. Poult. Sci. 1996, 37, 609–621. [Google Scholar] [CrossRef]
- Masey O’Neill, H.V.; Smith, J.A.; Bedford, M.R. Multicarbohydrase Enzymes for Non-ruminants. Asian-Australas J. Anim. Sci. 2014, 27, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Hetland, H.; Svihus, B.; Olaisen, V. Effect of feeding whole cereals on performance, starch digestibility and duodenal particle size distribution in broiler chickens. Br. Poult. Sci. 2002, 43, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Parsaie, S.; Shariatmadari, F.; Zamiri, M.J.; Khajeh, K. Influence of wheat-based diets supplemented with xylanase, bile acid and antibiotics on performance, digestive tract measurements and gut morphology of broilers compared with a maize-based diet. Br. Poult. Sci. 2007, 48, 594–600. [Google Scholar] [CrossRef]
- Biesek, J.; Banaszak, M.; Grabowicz, M.; Wlazlak, S.; Adamski, M. Production efficiency and utility features of broiler ducks fed with feed thinned with wheat grain. Animals 2022, 12, 3427. [Google Scholar] [CrossRef]
- Bennett, C.D.; Classen, H.L.; Riddell, C. Feeding broiler chickens wheat and barley diets containing whole, ground and pelleted grain. Poult. Sci. 2002, 81, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Pirgozliev, V.R.; Birch, C.L.; Rose, S.P.; Kettlewell, P.S.; Bedford, M.R. Chemical composition and the nutritive quality of different wheat cultivars for broiler chickens. Br. Poult. Sci. 2003, 44, 464–475. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yun, D.H.; Lee, J.H.; Kwon, Y.B.; Lee, Y.M.; Lee, D.H.; Kim, D.K. Extract of Triticum aestivum sprouts suppresses acetaminophen-induced hepatotoxicity in mice by inhibiting oxidative stress. Molecules 2021, 26, 6336. [Google Scholar] [CrossRef]
- Pozzo, L.; Vizzarri, F.; Ciardi, M.; Nardoia, M.; Palazzo, M.; Casamassima, D.; Longo, V. The effects of fermented wheat powder (Lisosan G) on the blood lipids and oxidative status of healthy rabbits. Food Chem. Toxicol. 2015, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.X.; Zeng, Z.; Qian, Y.Z.; Qiu, J.; Wang, K.; Wang, Y.; Ji, B.-P.; Zhou, F. Wheat Flour, Enriched with γ-Oryzanol, phytosterol, and ferulic acid, alleviates lipid and glucose metabolism in high-fat-fructose-fed rats. Nutrients 2019, 11, 1697. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, H.; Wang, X.; Li, W.; Wang, Y.; Yan, F.; Kang, X.; Li, Z.; Han, R. Clinical assessment of growth performance, bone morphometry, bone quality, and serum indicators in broilers affected by valgus-varus deformity. Poult. Sci. 2019, 98, 4433–4440. [Google Scholar] [CrossRef]
- Kajarabille, N.; Díaz-Castro, J.; Hijano, S.; López-Frías, M.; López-Aliaga, I.; Ochoa, J.J. A new insight to bone turnover: Role of ω-3 polyunsaturated fatty acids. Sci. World J. 2013, 2013, 589641. [Google Scholar] [CrossRef]
- During, A. Osteoporosis: A role for lipids. Biochimie 2020, 178, 49–55. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Nandha, N.K.; Kiarie, E.; Nyachoti, C.M.; Khafipour, E. Impact of combined beta-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poult. Sci. 2016, 95, 528–540. [Google Scholar] [CrossRef]
- McCafferty, K.W.; Bedford, M.R.; Kerr, B.J.; Dozier, W.A. Effects of age and supplemental xylanase in corn- and wheat-based diets on cecal volatile fatty acid concentrations of broilers. Poult. Sci. 2019, 98, 4787–4800. [Google Scholar] [CrossRef]
- Selle, P.H.; Macelline, S.P.; Greenhalgh, S.; Chrystal, P.V.; Liu, S.Y. Identifying the shortfalls of crude protein-reduced, wheat-based broiler diets. Anim. Nutr. 2022, 11, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Bedford, M.R.; Wu, S.B.; Morgan, N.K. Soluble non-starch polysaccharide modulates broiler gastrointestinal tract environment. Poult. Sci. 2021, 100, 101183. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.F.; Khoddami, A.; Chrystal, P.V.; Sorbara, J.-O.B.; Cowieson, A.J.; Selle, P.H.; Liu, S.Y. Starch digestibility and energy utilisation of maize- and wheat-based diets is superior to sorghum-based diets in broiler chickens offered diets supplemented with phytase and xylanase. Anim. Feed. Sci. Tech. 2020, 264, 114475. [Google Scholar] [CrossRef]
- Awad, W.A.; Böhm, J.; Razzazi-Fazeli, E.; Zentek, J. Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2006, 90, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.D.; Golestani, G.; Yaghobfar, A.; Khadem, A.; Pashazanussi, H. Effects of supplementing a multienzyme to broiler diets containing a high level of wheat or canola meal on intestinal morphology and performance of chicks. J. Appl. Poult. Res. 2013, 22, 671–679. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z.; Zhang, Y. Effects of the partial substitution of corn with wheat or barley on the growth performance, blood antioxidant capacity, intestinal health and fecal microbial composition of growing pigs. Antioxidants 2022, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Yaghobfar, A.; Kalantar, M. Effect of non-starch polysaccharide (NSP) of wheat and barley supplemented with exogenous enzyme blend on growth performance, gut microbial, pancreatic enzyme activities, expression of glucose transporter (SGLT1) and mucin producer (MUC2) genes of broiler chickens. Rev. Bras. De Ciência Avícola 2017, 19, 629–638. [Google Scholar]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Engberg, R.M.; Hedemann, M.S.; Steenfeldt, S.; Jensen, B.B. Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult. Sci. 2004, 83, 925–938. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, J.; Peng, H.; Li, B.; Xu, J.; Song, X.; Yu, C.; Zhang, Z.; Du, X.; Bu, G.; et al. Research Note: The gut microbiota varies with dietary fiber levels in broilers. Poult. Sci. 2022, 101, 101922. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, S.; Zhu, Y.; Zhang, X.; Du, P.; Huang, Y.; Michiels, J.; Zeng, Q.; Chen, W. Dietary resistant starch from potato regulates bone mass by modulating gut microbiota and concomitant short-chain fatty acids production in meat ducks. Front. Nutr. 2022, 9, 860086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wang, Y.; Wei, B.; Wang, L.; Nguyen, M.T.; Lv, X.; Huang, Y.; Chen, W. Fermented calcium butyrate supplementation in post-peak laying hens improved ovarian function and tibia quality through the “gut-bone” axis. Anim. Nutr. 2024, 16, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Majdeddin, M.; Gaublomme, D.; Taminiau, B.; Boone, M.; Elewaut, D.; Daube, G.; Josipovic, I.; Zhang, K.; Michiels, J. 25-hydroxycholecalciferol reverses heat induced alterations in bone quality in finisher broilers associated with effects on intestinal integrity and inflammation. J. Anim. Sci. Biotechnol. 2021, 12, 104. [Google Scholar] [CrossRef]

| Items | Starter Diet (1–10 d) | Grower Diet (11 to 21 d) | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Ingredients, % | ||||
| Corn | 35.18 | 35.18 | 22.27 | 0.00 |
| Wheat | 15.00 | 15.00 | 30.00 | 55.77 |
| Flour | 15 | 15 | 15 | 15 |
| Soybean oil | 1.0 | 1.0 | 1.1 | 1.3 |
| Soybean meal (46%) | 20.2 | 20.2 | 18.0 | 14.2 |
| Peanut meal (50%) | 3.0 | 3.0 | 3.0 | 3.0 |
| Corn gluten meal (60%) | 3.0 | 3.0 | 3.0 | 3.0 |
| Meat meal | 2.2 | 2.2 | 2.2 | 2.2 |
| Sodium chloride | 0.2 | 0.2 | 0.2 | 0.2 |
| Limestone | 0.98 | 0.98 | 0.95 | 0.90 |
| Montmorillonite | 0.2 | 0.2 | 0.2 | 0.2 |
| Dicalcium phosphate | 1.60 | 1.60 | 1.57 | 1.55 |
| Choline (60%) | 0.1 | 0.1 | 0.1 | 0.1 |
| L-lysine HCl (70%) | 0.90 | 0.90 | 0.95 | 1.08 |
| DL-Methionine (99%) | 0.22 | 0.22 | 0.22 | 0.23 |
| L-Threonine | 0.25 | 0.25 | 0.27 | 0.30 |
| Broiler complex enzyme a | 0.30 | 0.30 | 0.30 | 0.30 |
| Sodium bicarbonate | 0.1 | 0.1 | 0.1 | 0.1 |
| Sodium butyrate (98%) | 0.04 | 0.04 | 0.04 | 0.04 |
| Premix b | 0.53 | 0.53 | 0.53 | 0.53 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Nutrient content, % | ||||
| AME, Kcal/Kg | 2950 | 2950 | 2950 | 2950 |
| Crude protein c | 21.32 | 21.31 | 21.35 | 21.44 |
| Dry Matter c | 86.82 | 86.82 | 87.23 | 87.94 |
| Crude fiber | 2.50 | 2.50 | 2.45 | 2.35 |
| Calcium c | 0.88 | 0.88 | 0.88 | 0.88 |
| Total phosphorus c | 0.67 | 0.67 | 0.68 | 0.68 |
| Available phosphorus | 0.45 | 0.45 | 0.45 | 0.45 |
| Digestible lysine c | 1.28 | 1.28 | 1.28 | 1.28 |
| Digestible Methionine c | 0.50 | 0.50 | 0.48 | 0.51 |
| Digestible tryptophan c | 0.19 | 0.20 | 0.20 | 0.21 |
| Digestible threonine c | 0.86 | 0.85 | 0.86 | 0.84 |
| Gene | Gene ID | Primer Sequences (5′→3′) | Product Length, bp |
|---|---|---|---|
| ZO-1 | NM_001256123.2 | F: GAACGTGAAGAGGGGGAAGG | 101 |
| R: TTGCCAGGCTCTCTCCAAAG | |||
| Occiduin | NM_205128.1 | F: CATGCTCATCGCCTCCATC | 256 |
| R: TAGCGCCAGATCTTACTGCG | |||
| Claudin | NM_001013611.2 | F: GGTATGGCAACAGAGTGGCT | 91 |
| R: CAGCCAATGAAGAGGGCTGA | |||
| CDH1 | NM_001039258.3 | F: AGCCAAGGGCCTGGATTATG | 157 |
| R: GATAGGGGGCACGAAGACAG | |||
| TNF-α | NM_204267.2 | F: AGTCAGGAGCGTTGACTTGG | 68 |
| R: GCAACAACCAGCTATGCACC | |||
| IL-10 | NM_001004414.4 | F: AGGAGCAAAGCCATCAAGCA | 251 |
| R: TAGCGGACCGAACGTTAAGC | |||
| IL-6 | NM_204628.2 | F: AGGGCCGTTCGCTATTTGAA | 72 |
| R: CAGAGGATTGTGCCCGAACT | |||
| β-actin | NM_205518.1 | F: GTCCACCGCAAATGCTTCTAA | 78 |
| R: TGCGCATTTATGGGTTTTGTT |
| Item | The Replaced Levels of Wheat for Corn | p-Value | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Body weight, kg/bird | ||||
| 10 d | 0.29 ± 0.01 | 0.29 ± 0.02 | 0.29 ± 0.01 | 0.995 |
| 21 d | 0.92 ± 0.03 b | 0.90 ± 0.04 b | 0.96 ± 0.02 a | 0.011 |
| Weight gain, kg/bird | ||||
| 10–21 d | 0.63 ± 0.02 b | 0.62 ± 0.04 b | 0.67 ± 0.01 a | 0.003 |
| Feed intake/bird | ||||
| 10–21 d | 0.89 ± 0.06 b | 0.91 ± 0.02 b | 0.99 ± 0.07 a | 0.004 |
| F:G kg/kg | ||||
| 10–21 d | 1.41 ± 0.06 | 1.48 ± 0.11 | 1.47 ± 0.11 | 0.271 |
| Mortality, % | ||||
| 10–21 d | 1.25 ± 2.31 | 0.63 ± 1.77 | 0.63 ± 1.77 | 0.766 |
| Item | The Replaced Levels of Wheat for Corn | p-Value | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Body weight, kg | 0.91 ± 0.04 b | 0.92 ± 0.05 b | 0.99 ± 0.03 a | 0.003 |
| Absolute weight, g | ||||
| Glandular stomach | 6.14 ± 1.13 | 6.96 ± 1.65 | 6.95 ± 0.83 | 0.339 |
| Gizzard | 14.29 ± 2.33 | 14.83 ± 2.32 | 15.81 ± 2.18 | 0.416 |
| Duodenum | 9.54 ± 1.28 | 9.64 ± 1.85 | 9.41 ± 1.95 | 0.965 |
| Jejunum | 18.90 ± 3.88 | 17.95 ± 2.91 | 15.52 ± 3.78 | 0.171 |
| Ileum | 16.46 ± 5.00 | 14.47 ± 2.97 | 13.34 ± 4.86 | 0.372 |
| Ceca | 14.75 ± 3.15 | 11.35 ± 3.78 | 14.86 ± 2.37 | 0.314 |
| Relative weight, g/kg body weight | ||||
| Glandular stomach | 6.72 ± 1.06 | 7.58 ± 1.83 | 7.07 ± 0.89 | 0.441 |
| Gizzard | 15.69 ± 2.55 | 16.11 ± 2.41 | 16.10 ± 2.53 | 0.929 |
| Duodenum | 10.47 ± 1.37 | 10.47 ± 1.95 | 9.54 ± 1.89 | 0.484 |
| Jejunum | 20.83 ± 4.70 a | 19.50 ± 3.20 ab | 15.69 ± 3.37 b | 0.036 |
| Ileum | 18.09 ± 5.65 | 15.80 ± 3.56 | 13.44 ± 4.47 | 0.158 |
| Ceca | 16.14 ± 3.19 | 13.01 ± 4.48 | 15.11 ± 2.52 | 0.097 |
| Absolute length, cm | ||||
| Duodenum | 25.53 ± 1.98 | 25.50 ± 3.66 | 25.10 ± 1.93 | 0.937 |
| Jejunum | 54.73 ± 4.89 | 55.63 ± 6.59 | 55.74 ± 4.12 | 0.916 |
| Ileum | 47.50 ± 2.73 | 51.38 ± 5.58 | 49.58 ± 5.34 | 0.282 |
| Ceca | 26.03 ± 4.31 | 23.06 ± 4.99 | 27.45 ± 5.83 | 0.235 |
| Relative length, cm/kg body weight | ||||
| Duodenum | 28.06 ± 2.58 | 27.75 ± 4.22 | 25.48 ± 1.69 | 0.199 |
| Jejunum | 60.20 ± 6.66 | 60.55 ± 8.06 | 56.60 ± 3.95 | 0.414 |
| Ileum | 52.16 ± 2.94 | 55.85 ± 6.28 | 50.30 ± 4.76 | 0.089 |
| Ceca | 28.50 ± 4.12 | 25.13 ± 5.54 | 27.81 ± 5.53 | 0.393 |
| Intestinal Segment | The Replaced Levels of Wheat for Corn | p-Value | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Duodenum | ||||
| Villus height, μm | 1538.84 ± 79.69 | 1630.21 ± 89.03 | 1655.39 ± 208.03 | 0.128 |
| Crypt depth, μm | 232.99 ± 30.09 | 206.03 ± 45.84 | 194.76 ± 32.20 | 0.075 |
| Villus height to crypt depth | 6.70 ± 0.88 b | 8.28 ± 1.90 a | 8.78 ± 2.17 a | 0.035 |
| Muscular thickness, μm | 155.08 ± 19.41 | 163.21 ± 33.25 | 146.38 ± 12.43 | 0.389 |
| Jejunum | ||||
| Villus height, μm | 1288.43 ± 185.81 | 1403.04 ± 146.06 | 1296.74 ± 76.08 | 0.167 |
| Crypt depth, μm | 178.16 ± 38.81 ab | 160.49 ± 35.91 b | 232.87 ± 72.84 a | 0.012 |
| Villus height to crypt depth | 7.64 ± 2.37 ab | 9.21 ± 2.47 a | 6.06 ± 1.82 b | 0.015 |
| Muscular thickness, μm | 102.01 ± 18.17 b | 140.74 ± 23.97 a | 124.55 ± 27.70 ab | 0.004 |
| Ileum | ||||
| Villus height, μm | 887.09 ± 92.08 b | 990.26 ± 76.27 a | 1039.25 ± 96.87 a | 0.002 |
| Crypt depth, μm | 142.67 ± 39.66 b | 217.00 ± 66.97 a | 143.81 ± 28.65 b | 0.002 |
| Villus height to crypt depth | 6.68 ± 2.09 ab | 4.88 ± 1.24 b | 7.51 ± 1.77 a | 0.007 |
| Muscular thickness, μm | 140.77 ± 26.58 | 120.66 ± 19.07 | 131.75 ± 40.02 | 0.326 |
| Item | The Replaced Levels of Wheat for Corn | p-Value | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Glycolipid profile | ||||
| GLU, mmol/L | 13.76 ± 1.99 | 12.36 ± 1.08 | 12.83 ± 0.92 | 0.249 |
| CHO, mmol/L | 3.35 ± 0.39 | 3.28 ± 0.65 | 3.49 ± 0.69 | 0.829 |
| TG, mmol/L | 0.69 ± 0.30 | 0.67 ± 0.3 | 0.57 ± 0.19 | 0.727 |
| HDL-c, mmol/L | 2.23 ± 0.24 | 2.28 ± 0.41 | 2.27 ± 0.30 | 0.970 |
| LDL-c, mmol/L | 0.98 ± 0.24 | 0.87 ± 0.24 | 0.99 ± 0.33 | 0.702 |
| Bone turnover | ||||
| PINP, μg/L | 13.91 ± 1.08 | 13.95 ± 0.88 | 13.77 ± 1.07 | 0.929 |
| CTX, pg/mL | 195.6 ± 17.25 | 189 ± 14.10 | 195.59 ± 18.71 | 0.673 |
| ALP, U/L | 4990.17 ± 3106.61 | 4622.67 ± 1768.94 | 4852.17 ± 1752.32 | 0.962 |
| Ca, mmol/L | 2.47 ± 0.09 | 2.54 ± 0.19 | 2.51 ± 0.05 | 0.573 |
| P, mmol/L | 2.18 ± 0.06 | 2.22 ± 0.19 | 2.03 ± 0.16 | 0.097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, Z.; Wei, B.; Wang, L.; Zhang, Q.; Si, X.; Huang, Y.; Zhang, H.; Chen, W. Replacement of Corn with Different Levels of Wheat Impacted the Growth Performance, Intestinal Development, and Cecal Microbiota of Broilers. Animals 2024, 14, 1536. https://doi.org/10.3390/ani14111536
Liu L, Wang Z, Wei B, Wang L, Zhang Q, Si X, Huang Y, Zhang H, Chen W. Replacement of Corn with Different Levels of Wheat Impacted the Growth Performance, Intestinal Development, and Cecal Microbiota of Broilers. Animals. 2024; 14(11):1536. https://doi.org/10.3390/ani14111536
Chicago/Turabian StyleLiu, Luxin, Zilin Wang, Bin Wei, Leilei Wang, Qianqian Zhang, Xuemeng Si, Yanqun Huang, Huaiyong Zhang, and Wen Chen. 2024. "Replacement of Corn with Different Levels of Wheat Impacted the Growth Performance, Intestinal Development, and Cecal Microbiota of Broilers" Animals 14, no. 11: 1536. https://doi.org/10.3390/ani14111536
APA StyleLiu, L., Wang, Z., Wei, B., Wang, L., Zhang, Q., Si, X., Huang, Y., Zhang, H., & Chen, W. (2024). Replacement of Corn with Different Levels of Wheat Impacted the Growth Performance, Intestinal Development, and Cecal Microbiota of Broilers. Animals, 14(11), 1536. https://doi.org/10.3390/ani14111536






