The Honey Bee Colony’s Criterion for Candidate Selection: “Ongoing” or “One-Shot”?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Colony without Nepotism
2.2. Number of Capped Queen Cells and Newly Emerged Queens
2.3. Nursing Behavior of Worker and Physiological Indicators of Queens
2.4. Gene Expression Analysis
3. Results
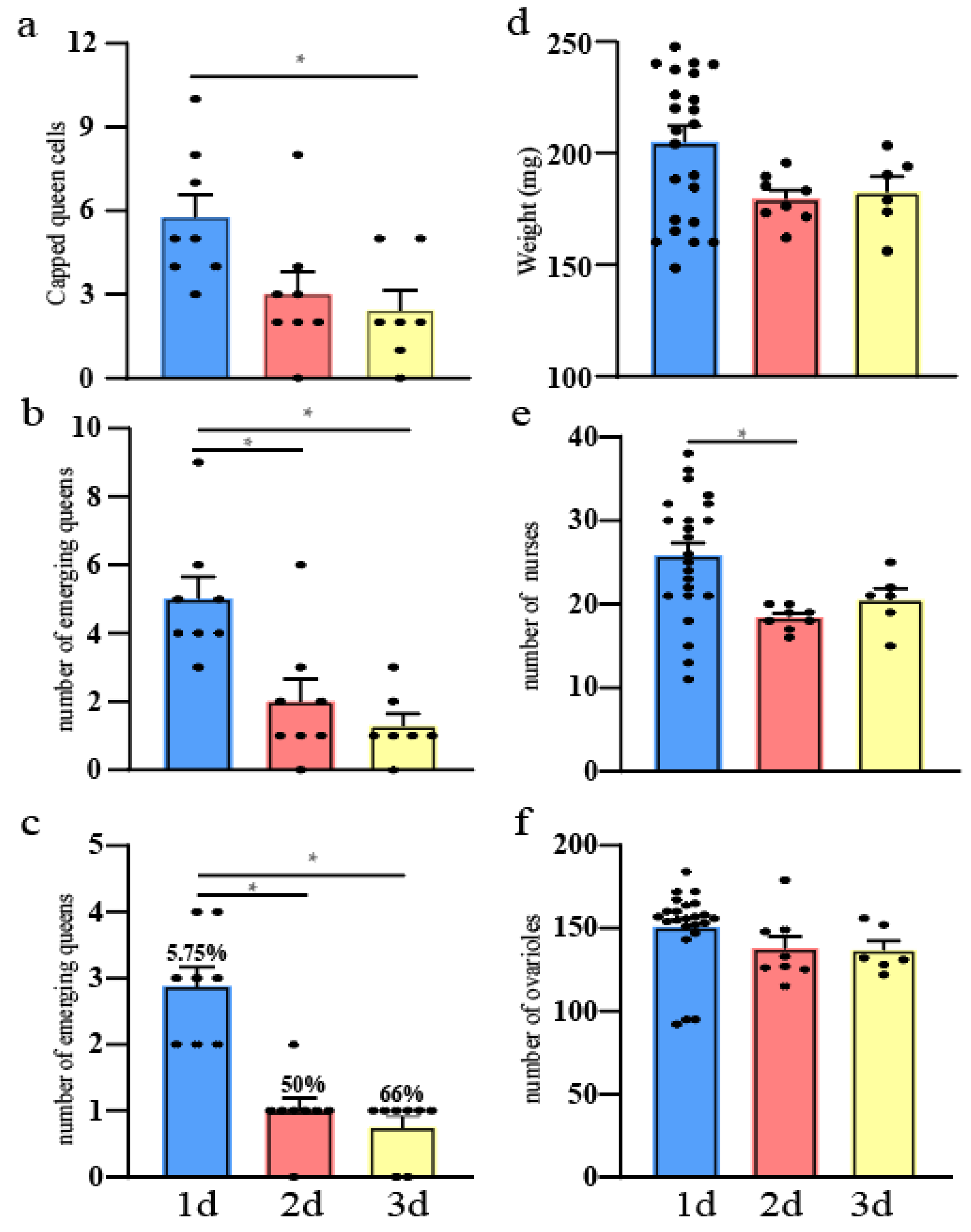
3.1. Number of Capped Queen Cells
3.2. Number of Newly Emerged Queens
3.3. Quantity Statistics of Newly Emerged Queens on the First Day
3.4. Queen Birth Weight
3.5. Nursing Behavior and Ovariole Numbers of New Queens
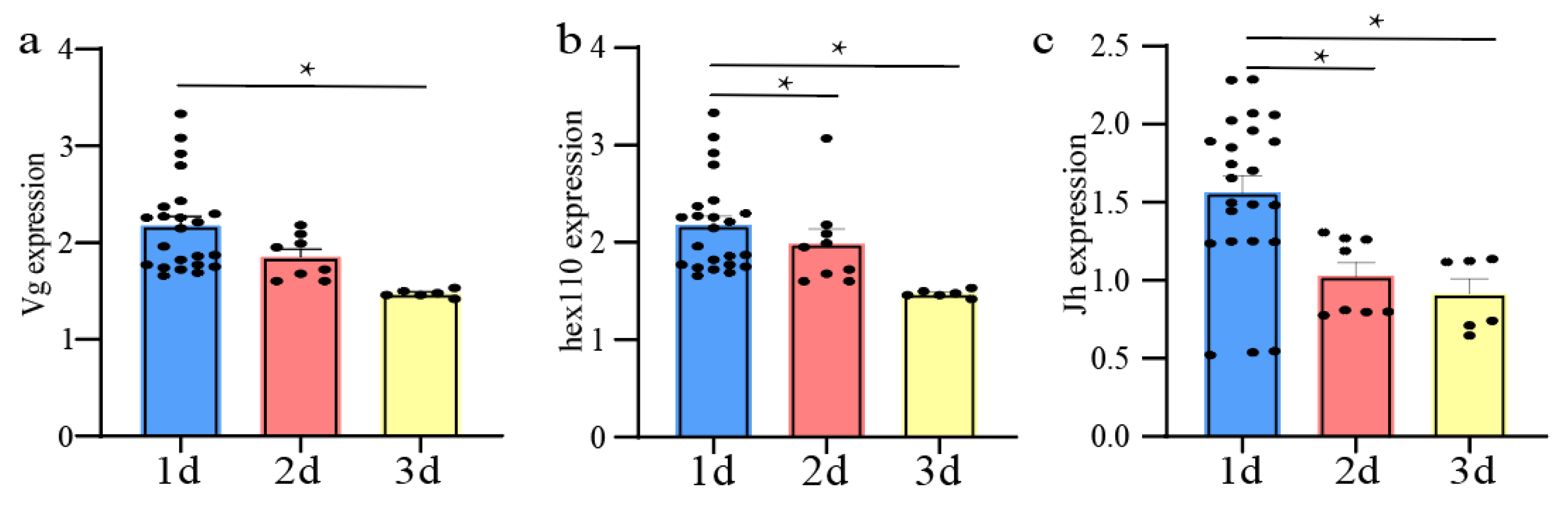
3.6. Expression of Genes Related to Ovarian Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huber, F. Nouvelles Observations sur les Abeilles, I and II (New Observation on Bees, I and II); CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 1926. [Google Scholar]
- Ribbands, C.R. The Behaviour and Social Life of Honeybees; Research Bees: London, UK, 1953. [Google Scholar]
- Crozier, R.H.; Pamilo, P. Genetic Intrigues. (Book Reviews: Evolution of Social Insect Colonies. Sex Allocation and Kin Selection.). Science 1996, 274, 1477–1478. [Google Scholar]
- Page, R.E., Jr. Sperm utilization in social insects. Annu. Rev. Entomol. 1986, 31, 297–320. [Google Scholar] [CrossRef]
- Strassmann, J. The rarity of multiple mating by females in the social Hymenoptera. Insectes Sociaux 2001, 48, 1–13. [Google Scholar] [CrossRef]
- Meixner, M.; Moritz, R. Clique formation of super-sister honeybee workers (Apis mellifera) in experimental groups. Insectes Sociaux 2004, 51, 43–47. [Google Scholar] [CrossRef]
- Getz, W.M.; Smith, K.B. Genetic kin recognition: Honey bees discriminate between full and half sisters. Nature 1983, 302, 147–148. [Google Scholar] [CrossRef]
- Makert, G.R.; Paxton, R.J.; Hartfelder, K. Ovariole number—A predictor of differential reproductive success among worker subfamilies in queenless honeybee (Apis mellifera L.) colonies. Behav. Ecol. Sociobiol. 2006, 60, 815–825. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Gilley, D.C.; Seeley, T.D. Levels of selection in a social insect: A review of conflict and cooperation during honey bee (Apis mellifera) queen replacement. Behav. Ecol. Sociobiol. 2004, 55, 513–523. [Google Scholar] [CrossRef]
- Goodisman, M.A.; Kovacs, J.L.; Hoffman, E.A. Lack of conflict during queen production in the social wasp Vespula maculifrons. Mol. Ecol. 2007, 16, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Noonan, K.C. Recognition of queen larvae by worker honey bees (Apis mellifera). Ethology 1986, 73, 295–306. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, Y.B.; Barron, A.B.; Zeng, Z.J. Transcriptomic, morphological, and developmental comparison of adult honey bee queens (Apis mellifera) reared from eggs or worker larvae of differing ages. J. Econ. Entomol. 2020, 113, 2581–2587. [Google Scholar] [CrossRef]
- Kraus, F.B.; Moritz, R.F. Extreme polyandry in social Hymenoptera: Evolutionary causes and consequences for colony organisation. In Animal Behaviour: Evolution and Mechanisms; Springer: Berlin/Heidelberg, Germany, 2010; pp. 413–439. [Google Scholar]
- Schneider, S.; DeGrandi-Hoffman, G. The influence of worker behavior and paternity on the development and emergence of honey bee queens. Insectes Sociaux 2002, 49, 306–314. [Google Scholar] [CrossRef]
- Fell, R.; Morse, R. Emergency queen cell production in the honey bee colony. Insectes Sociaux 1984, 31, 221–237. [Google Scholar] [CrossRef]
- Hatch, S.; Tarpy, D.; Fletcher, D. Worker regulation of emergency queen rearing in honey bee colonies and the resultant variation in queen quality. Insectes Sociaux 1999, 46, 372–377. [Google Scholar] [CrossRef]
- Sagili, R.R.; Metz, B.N.; Lucas, H.M.; Chakrabarti, P.; Breece, C.R. Honey bees consider larval nutritional status rather than genetic relatedness when selecting larvae for emergency queen rearing. Sci. Rep. 2018, 8, 7679. [Google Scholar] [CrossRef] [PubMed]
- Margarita, O.; Osnat, M.; Abraham, H. Choosing the best: Honeybee workers can assess reproductive quality of the queen through pheromonal signalling in simultaneous choice assays. Apidologie 2020, 51, 291–306. [Google Scholar] [CrossRef]
- Rangel, J.; Keller, J.; Tarpy, D. The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth. Insectes Sociaux 2013, 60, 65–73. [Google Scholar] [CrossRef]
- Dong, S.; Lin, T.; Nieh, J.C.; Tan, K. Social signal learning of the waggle dance in honey bees. Science 2023, 379, 1015–1018. [Google Scholar] [CrossRef]
- Allen, M.D. The behaviour of honeybees preparing to swarm. Br. J. Anim. Behav. 1956, 4, 14–22. [Google Scholar] [CrossRef]
- Caron, D.M.; Greve, C.W. Destruction of queen cells placed in queenright Apis mellifera colonies. Ann. Entomol. Soc. Am. 1979, 72, 405–407. [Google Scholar] [CrossRef]
- Schneider, S.; Painter-Kurt, S.; DeGrandi-Hoffman, G. The role of the vibration signal during queen competition in colonies of the honeybee, Apis mellifera. Anim. Behav. 2001, 61, 1173–1180. [Google Scholar] [CrossRef]
- Morse, R.; McDonald, J. The treatment of capped queen cells by honeybees. J. Apic. Res. 1965, 4, 31–34. [Google Scholar] [CrossRef]
- Rehman, N.U.; Anjum, S.I.; Qureshi, N.A.; Khan, M.H.; Albasher, G.; Kaleem, M.; Kamal, A. The effect of larval age, and wet and dry grafting, on the rearing of queen bees using the Doolittle grafting method. Entomol. Res. 2024, 54, e12700. [Google Scholar] [CrossRef]
- Ucak Koc, A.; Karacaoglu, M.; Uygun, M.; Bakır, Z.B.; Keser, B. Effect of harvesting time and the number of queen cell cups on royal jelly composition. J. Apic. Res. 2023, 62, 478–484. [Google Scholar] [CrossRef]
- Kocher, S.D.; Grozinger, C.M. Cooperation, conflict, and the evolution of queen pheromones. J. Chem. Ecol. 2011, 37, 1263–1275. [Google Scholar] [CrossRef]
- Long, K.; Cao, T.; Keller, J.; Tarpy, D.; Shin, M.; Schneider, S. Levels of selection shaping caste interactions during queen replacement in the honey bee, Apis mellifera. Insectes Sociaux 2017, 64, 227–240. [Google Scholar] [CrossRef]
- Rangel, J.; Böröczky, K.; Schal, C.; Tarpy, D.R. Honey bee (Apis mellifera) queen reproductive potential affects queen mandibular gland pheromone composition and worker retinue response. PLoS ONE 2016, 11, e0156027. [Google Scholar] [CrossRef]
- Traynor, K.S.; Le Conte, Y.; Page, R.E. Queen and young larval pheromones impact nursing and reproductive physiology of honey bee (Apis mellifera) workers. Behav. Ecol. Sociobiol. 2014, 68, 2059–2073. [Google Scholar] [CrossRef]
- Tarpy, D.; Keller, J.; Caren, J.; Delaney, D. Experimentally induced variation in the physical reproductive potential and mating success in honey bee queens. Insectes Sociaux 2011, 58, 569–574. [Google Scholar] [CrossRef]
- Woyke, J. Correlations between the age at which honeybee brood was grafted, characteristics of the resultant queens, and results of insemination. J. Apic. Res. 1971, 10, 45–55. [Google Scholar] [CrossRef]
- Delaney, D.A.; Keller, J.J.; Caren, J.R.; Tarpy, D.R. The physical, insemination, and reproductive quality of honey bee queens (Apis mellifera L.). Apidologie 2011, 42, 1–13. [Google Scholar] [CrossRef]
- Dedej, S.; Hartfelder, K.; Aumeier, P.; Rosenkranz, P.; Engels, W. Caste determination is a sequential process: Effect of larval age at grafting on ovariole number, hind leg size and cephalic volatiles in the honey bee (Apis mellifera carnica). J. Apic. Res. 1998, 37, 183–190. [Google Scholar] [CrossRef]
- Tarpy, D.; Mayer, M. The effects of size and reproductive quality on the outcomes of duels between honey bee queens (Apis mellifera L.). Ethol. Ecol. Evol. 2009, 21, 147–153. [Google Scholar] [CrossRef]
- De Souza, D.A.; Hartfelder, K.H.; Tarpy, D.R. Effects of larval age at grafting and juvenile hormone on morphometry and reproductive quality parameters of in vitro reared honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2019, 112, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Bitondi, M.M.G. The HEX 110 hexamerin is a cytoplasmic and nucleolar protein in the ovaries of Apis mellifera. PLoS ONE 2016, 11, e0151035. [Google Scholar] [CrossRef]
- Tian, Y.; Qu, Y.; Dong, K.; He, S.; Jie, W.; Huang, J. Characterization and developmental expression patterns of four hexamerin genes in the bumble bee, Bombus terrestris (Hymenoptera: Apidae). J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef]

| Target Gene | Forward Primer (5’-3’) | Reverse Primer (5’-3’) |
|---|---|---|
| gapdh | GCTGGTTTCATCGATGGTTT | ACGATTTCGACCACCGTAAC |
| hex110 | AACGTGCCAGGCGCAGTTGT | TTCACCAGCATGGAGGTTCTGGA |
| jh | CACTGGCACCAGAGCCTGTC | GATTCCCATTGAACGAGCGA |
| vg | CGTGTTCCAGAGGACGTTGA | ACGCTCCTCAGGCTCAACTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Zhong, S.; Xu, T.; Chen, W.; Zeng, Z. The Honey Bee Colony’s Criterion for Candidate Selection: “Ongoing” or “One-Shot”? Animals 2024, 14, 1535. https://doi.org/10.3390/ani14111535
Pan L, Zhong S, Xu T, Chen W, Zeng Z. The Honey Bee Colony’s Criterion for Candidate Selection: “Ongoing” or “One-Shot”? Animals. 2024; 14(11):1535. https://doi.org/10.3390/ani14111535
Chicago/Turabian StylePan, Luxia, Shiqing Zhong, Tianyu Xu, Weixuan Chen, and Zhijiang Zeng. 2024. "The Honey Bee Colony’s Criterion for Candidate Selection: “Ongoing” or “One-Shot”?" Animals 14, no. 11: 1535. https://doi.org/10.3390/ani14111535
APA StylePan, L., Zhong, S., Xu, T., Chen, W., & Zeng, Z. (2024). The Honey Bee Colony’s Criterion for Candidate Selection: “Ongoing” or “One-Shot”? Animals, 14(11), 1535. https://doi.org/10.3390/ani14111535







