Identifying Dietary Timing of Organic Trace Minerals to Reduce the Incidence of Osteomyelitis Lameness in Broiler Chickens Using the Aerosol Transmission Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Research and Facility
2.2. Experimental Design
2.3. The BCO Challenge Model
2.4. Lameness Assessment
2.5. Bacterial Identification
2.6. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ingredient Name | Starter Diet (%) | Finisher Diet (%) | ||
|---|---|---|---|---|
| Diet A 1 | Diet B 2 | Diet A 1 | Diet B 2 | |
| Corn, ground, 8% | 57.12 | 57.12 | 64.555 | 64.55 |
| Soybean meal, 48% | 36.76 | 36.76 | 29.15 | 29.15 |
| Poultry oil | 2.50 | 2.50 | 3.17 | 3.17 |
| DL-Met | 0.34 | 0.34 | 0.31 | 0.31 |
| L-Lys HCl | 0.20 | 0.20 | 0.21 | 0.21 |
| L-Thr | 0.11 | 0.11 | 0.09 | 0.09 |
| Limestone | 1.09 | 1.09 | 0.99 | 0.99 |
| Dicalcium phosphate | 1.04 | 1.04 | 0.72 | 0.72 |
| NaCl | 0.41 | 0.41 | 0.42 | 0.42 |
| Na bicarbonate | 0.08 | 0.08 | 0.07 | 0.07 |
| Phytase 3 | 0.03 | 0.03 | 0.03 | 0.03 |
| Choline chloride, 60% | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin premix 4 | 0.06 | 0.06 | 0.03 | 0.03 |
| Salinamycin Na 5 | 0.05 | 0.05 | 0.05 | 0.05 |
| Mineral premix 6 | 0.10 | 0.04 | 0.10 | 0.04 |
| Availa® ZMC 7 | - | 0.15 | - | 0.15 |
| Inert Filler, sand | 0.07 | - | 0.07 | - |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated Composition, % unless otherwise noted 8 | ||||
| ME, kcal/kg | 3050 | 3050 | 3175 | 3175 |
| CP (%) | 22.34 | 22.34 | 19.21 | 19.21 |
| Lys, % digestible | 1.22 | 1.22 | 1.05 | 1.05 |
| Met + Cys, % digestible | 0.92 | 0.92 | 0.82 | 0.82 |
| Thr, % digestible | 0.82 | 0.82 | 0.69 | 0.69 |
| Ca, % total | 0.90 | 0.90 | 0.76 | 0.76 |
| P, % available | 0.45 | 0.45 | 0.38 | 0.38 |
| Na, % total | 0.21 | 0.21 | 0.21 | 0.21 |
| Zn, mg/kg of diet total | 100 | 100 | 100 | 100 |
| Mn, mg/kg of diet total | 100 | 100 | 100 | 100 |
| Cu, mg/kg of diet total | 10 | 11 | 10 | 11 |
| Treatments | Lame Count (wk) | BW (kg) 2 |
|---|---|---|
| Avg 1 | Avg 1 | |
| Diet A—BCO challenge source | 43.0 a ± 2.0 | 4.08 a ± 0.25 |
| Diet A—Negative control | 39.0 a ± 1.0 | 4.64 b ± 0.29 |
| Diet B | 20.2 b ± 1.7 | 4.90 c ± 0.15 |
| Diet C | 37.6 a ± 0.9 | 4.88 c ± 0.08 |
| Diet D | 23.3 b ± 0.7 | 4.94 c ± 0.17 |
References
- McNamee, P.T.; Smyth, J.A. Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: A review. In Avian Pathology; Carfax Publishing Company: Centerville, UT, USA, 2000; Volume 29, pp. 253–270. [Google Scholar] [CrossRef]
- Wideman, R.F. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: A review. In Poultry Science; Oxford University Press: Oxford, UK, 2016; Volume 95, pp. 325–344. [Google Scholar] [CrossRef]
- Bradshaw, R.H.; Kirkden, R.D.; Broom, D.M. A review of the aetiology and pathology of leg weakness in broilers in relation to welfare. Avian Poult. Biol. Rev. 2002, 13, 45–104. [Google Scholar] [CrossRef]
- Knowles, T.G.; Kestin, S.C.; Haslam, S.M.; Brown, S.N.; Green, L.E.; Butterworth, A.; Pope, S.J.; Pfeiffer, D.; Nicol, C.J. Leg disorders in broiler chickens: Prevalence, risk factors and prevention. PLoS ONE 2008, 3, e1545. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Núñez, I.; Moore, A.F.; Gino Lorenzoni, A. Incidence of bacterial chondronecrosis with osteomyelitis (Femoral head necrosis) induced by a model of skeletal stress and its correlation with subclinical necrotic enteritis. Microorganisms 2020, 8, 205. [Google Scholar] [CrossRef]
- Choppa VS, R.; Kim, W.K. A Review on Pathophysiology, and Molecular Mechanisms of Bacterial Chondronecrosis and Osteomyelitis in Commercial Broilers. Biomolecules 2023, 13, 1032. [Google Scholar] [CrossRef]
- Thorp, B.H.; Waddington, D. Relationships between the bone pathologies, ash and mineral content of long bones in 35-day-old broiler chickens. Res. Vet. Sci. 1997, 62, 67–73. [Google Scholar] [CrossRef]
- Prisby, R.; Menezes, T.; Campbell, J.; Benson, T.; Samraj, E.; Pevzner, I.; Wideman, R.F. Kinetic examination of femoral bone modeling in broilers. Poult. Sci. 2014, 93, 1122–1129. [Google Scholar] [CrossRef]
- Wideman, R.F.; Al-Rubaye, A.; Gilley, A.; Reynolds, D.; Lester, H.; Yoho, D.; Hughes, J.M.; Pevzner, I. Susceptibility of 4 commercial broiler crosses to lameness attributable to bacterial chondronecrosis with osteomyelitis. Poult. Sci. 2013, 92, 2311–2325. [Google Scholar] [CrossRef]
- Al-Rubaye AA, K.; Couger, M.B.; Ojha, S.; Pummill, J.F.; Koon, J.A.; Wideman, R.F.; Rhoads, D.D. Genome analysis of staphylococcus agnetis, an agent of lameness in broiler chickens. PLoS ONE 2015, 10, e0143336. [Google Scholar] [CrossRef]
- Alrubaye, A.A.K.; Ekesi, N.S.; Hasan, A.; Elkins, E.; Ojha, S.; Zaki, S.; Dridi, S.; Wideman, R.F.; Rebollo, M.A.; Rhoads, D.D. Chondronecrosis with osteomyelitis in broilers: Further defining lameness-inducing models with wire or litter flooring to evaluate protection with organic trace minerals. Poult. Sci. 2020, 99, 5422–5429. [Google Scholar] [CrossRef]
- Wideman, R.F.; Prisby, R.D. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: A translational model for the pathogenesis of femoral head necrosis. Front. Endocrinol. 2013, 3, 40620. [Google Scholar] [CrossRef]
- Siegel, P.B.; Barger, K.; Siewerdt, F. Limb Health in Broiler Breeding: History Using Genetics to Improve Welfare. J. Appl. Poult. Res. 2019, 28, 785–790. [Google Scholar] [CrossRef]
- Nääs, I.A.; Paz IC, L.A.; Baracho, M.S.; Menezes, A.G.; Bueno, L.G.F.; Almeida, I.C.L.; Moura, D.J. Impact of lameness on broiler well-being. J. Appl. Poult. Res. 2009, 18, 432–439. [Google Scholar] [CrossRef]
- de Jong, I.C.; Berg, C.; Butterworth, A.; Estevez, I. Scientific report updating the EFSA opinions on the welfare of broilers and broiler breeders. Support. Publ. 2012, 9, 295E. [Google Scholar] [CrossRef]
- Al-Rubaye, A.A.K. Molecular Diagnosis of Metabolic Fast Growth Related Diseases in Broiler. ProQuest Dissertations Publishing. 2013. Available online: https://scholarworks.uark.edu/etd (accessed on 18 April 2024).
- Alrubaye, A.A.K.; Ekesi, N.S.; Hasan, A.; Koltes, D.A.; Wideman, R.F.; Rhoads, D.D. Chondronecrosis with osteomyelitis in broilers: Further defining a bacterial challenge model using standard litter flooring and protection with probiotics. Poult. Sci. 2020, 99, 6474–6480. [Google Scholar] [CrossRef] [PubMed]
- Ekesi, N.S.; Dolka, B.; Alrubaye AA, K.; Rhoads, D.D. Analysis of genomes of bacterial isolates from lameness outbreaks in broilers. Poult. Sci. 2021, 100, 101148. [Google Scholar] [CrossRef] [PubMed]
- Shwani, A.; Zuo, B.; Alrubaye, A.; Zhao, J.; Rhoads, D.D. A Simple, Inexpensive Alkaline Method for Bacterial DNA Extraction from Environmental Samples for PCR Surveillance and Microbiome Analyses. Appl. Sci. 2023, 14, 141. [Google Scholar] [CrossRef]
- tom Dieck, H.; Döring, F.; Roth, H.-P.; Daniel, H. Changes in rat hepatic gene expression in response to zinc deficiency as assessed by DNA arrays. J. Nutr. 2003, 133, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.T. Nutritional Modulation of Immune Function in Broilers 1. Poult. Sci. 2004, 83, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Glüer, C.-C.; Schrezenmeir, J. The Journal of Nutrition Effects of Probiotics and Prebiotics Prebiotics, Probiotics, and Synbiotics Affect Mineral Absorption, Bone Mineral Content, and Bone Structure 1–3. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef]
- Sirri, F.; Maiorano, G.; Tavaniello, S.; Chen, J.; Petracci, M.; Meluzzi, A. Effect of different levels of dietary zinc, manganese, and copper from organic or inorganic sources on performance, bacterial chondronecrosis, intramuscular collagen characteristics, and occurrence of meat quality defects of broiler chickens. Poult. Sci. 2016, 95, 1813–1824. [Google Scholar] [CrossRef]
- Burin, A.M.; Fernandes NL, M.; Snak, A.; Fireman, A.; Horn, D.; Fernandes, J.I.M. Arginine and manganese supplementation on the immune competence of broilers immune stimulated with vaccine against Salmonella Enteritidis. Poult. Sci. 2019, 98, 2160–2168. [Google Scholar] [CrossRef]
- Mcknight, L.L.; Page, G.; Han, Y. Effect of Replacing In-Feed Antibiotics with Synergistic Organic Acids, with or without Trace Mineral and/or Water Acidification, on Growth Performance and Health of Broiler Chickens Under a Clostridium perfringens Type A Challenge. Avian Dis. 2020, 64, 374–378. [Google Scholar] [CrossRef]
- Pimentel, J.L.; Cook, M.E.; Greger, J.L. Immune Response of Chicks Fed Various Levels of Zinc 1. Poult. Sci. 1991, 70, 947–954. [Google Scholar] [CrossRef]
- Kidd, M.T.; Ferket, P.R.; Qureshi, M.A. Zinc metabolism with special reference to its role in immunity. World’s Poult. Sci. J. 1996, 52, 309–324. [Google Scholar] [CrossRef]
- Caterson, B.; Flannery, C.R.; Hughes, C.E.; Little, C.B. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000, 19, 333–344. [Google Scholar] [CrossRef]
- Krane, S.M.; Inada, M. Matrix metalloproteinases and bone. Bone 2008, 43, 7–18. [Google Scholar] [CrossRef]
- Kwiecień, M. The effect of the level and form of Fe on the quality of femur bones in broiler chickens. Annales Universitatis Mariae Curie-Skłodowska. Sect. EE Zootech. 2013, 31, 24–34. [Google Scholar]
- Harris, E.D.; Rayton, J.K.; Balthrop, J.E.; DiSilvestro, R.A.; Garcia-de-Quevedo, M. Copper and the synthesis of elastin and collagen. Ciba Found. Symp. 1980, 79, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Nutritional factors in causation of osteoporosis. Ann. Chir. Gynaecol. 1988, 77, 176–179. [Google Scholar] [PubMed]
- Miggiano, G.A.; Gagliardi, L. Diet, nutrition and bone health. La Clin. Ter. 2005, 156, 47–56. [Google Scholar]
- Brink, E.J.; Beynen, A.C.; Dekker, P.R.; van Beresteijn, E.C.; van der Meer, R. Interaction of Calcium and Phosphate Decreases Ileal Magnesium Solubility and Apparent Magnesium Absorption in Rats. J. Nutr. 1992, 122, 580–586. [Google Scholar] [CrossRef]
- Underwood, E.J.; Suttle, N.F. The Mineral Nutrition of Livestock, 3rd ed.; CABI: Wallingford, UK, 1999; pp. ix+–614. [Google Scholar]
- Underwood, E.J. (Ed.) 2-Iron. In Trace Elements in Human and Animal Nutrition, 4th ed.; Academic Press: Cambridge, MA, USA, 1977; pp. 13–55. [Google Scholar]
- Son, E.W.; Lee, S.R.; Choi, H.S.; Koo, H.J.; Huh, J.E.; Kim, M.H.; Pyo, S. Effects of supplementation with higher levels of manganese and magnesium on immune function. Arch. Pharmacal Res. 2007, 30, 743–749. [Google Scholar] [CrossRef]
- Bao, Y.M.; Choct, M. Trace mineral nutrition for broiler chickens and prospects of application of organically complexed trace minerals: A review. Anim. Prod. Sci. 2009, 49, 269–282. [Google Scholar] [CrossRef]
- López-Alonso, M. Trace Minerals and Livestock: Not Too Much Not Too Little. ISRN Vet. Sci. 2012, 2012, 704825. [Google Scholar] [CrossRef]
- Saripinar, D.; Aksu, T.; Onel, S.E. Does Inclusion at Low Levels of Organically Complexed Minerals Versus Inorganic Forms Create a Weakness in Performance or Antioxidant Defense System in Broiler Diets? Int. J. Poult. Sci. 2012, 11, 666–672. [Google Scholar] [CrossRef]
- Abdallah, A.; El-Husseiny, O.; Abdel-Latif, K. Influence of some dietary organic mineral supplementations. Int. J. Poult. Sci. 2009, 8, 291–298. [Google Scholar] [CrossRef]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef]
- El-Husseiny, O.M.; Hashish, S.M.; Ali, R.A.; Arafa, S.A.; Abd El-Samee, L.D.; Olemy, A.A. Effects of Feeding Organic Zinc, Manganese and Copper on Broiler Growth, Carcass Characteristics, Bone Quality and Mineral Content in Bone, Liver and Excreta. Int. J. Poult. Sci. 2012, 11, 368–377. [Google Scholar] [CrossRef]
- Asnayanti, A.; Do AD, T.; Alharbi, K.; Alrubaye, A. Inducing experimental bacterial chondronecrosis with osteomyelitis lameness in broiler chickens using aerosol transmission model. Poult. Sci. 2024, 103, 103460. [Google Scholar] [CrossRef] [PubMed]
- Asnayanti, A.; Alharbi, K.; Do AD, T.; Al-Mitib, L.; Bühler, K.; Van der Klis, J.D.; Gonzalez, J.; Kidd, M.T.; Alrubaye, A.A.K. Early 1,25-dihydroxyvitamin D3-glycosides supplementation: An efficient feeding strategy against bacterial chondronecrosis with osteomyelitis lameness in broilers assessed by using an aerosol transmission model. J. Appl. Poult. Res. 2024, 100440. [Google Scholar] [CrossRef]
- Alharbi, K.; Ekesi, N.; Hasan, A.; Asnayanti, A.; Liu, J.; Murugesan, R.; Ramirez, S.; Rochell, S.; Kidd, M.T.; Alrubaye, A. Deoxynivalenol and fumonisin predispose broilers to bacterial chondronecrosis with osteomyelitis lameness. Poult. Sci. 2024, 103, 103598. [Google Scholar] [CrossRef] [PubMed]
- Kierończyk, B.; Rawski, M.; Józefiak, D.; Świątkiewicz, S. Infectious and non-infectious factors associated with leg disorders in poultry—A review. Ann. Anim. Sci. 2017, 17, 645–669. [Google Scholar] [CrossRef]
- Can, G.B. Healthy Bones for Broiler Chickens. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2022. [Google Scholar]
- Muszyński, S.; Tomaszewska, E.; Kwiecień, M.; Dobrowolski, P.; Tomczyk-Warunek, A. Subsequent somatic axis and bone tissue metabolism responses to a low-zinc diet with or without phytase inclusion in broiler chickens. PLoS ONE 2018, 13, e0191964. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, L.; Zhang, L.; Liao, X.; Li, S.; Luo, X. Evaluation of optimal dietary calcium level by bone characteristics and calcium metabolism-related gene expression of broilers from 22 to 42 d of age. J. Anim. Sci. 2022, 100, skac092. [Google Scholar] [CrossRef]
- Fernandes Müller, I.J.; Ribeiro Vissotto, M.; Cardoso Bittencourt, L.; Riffel, T.E.; Lima Kaiser, F.; Palma Castro, S.; Hermes, G.R. Mineral supplementation: Effects on bone integrity and intestinal morphometry of broiler chickens challenged with Eimeria sp. Acta Vet. 2019, 69, 88–105. [Google Scholar] [CrossRef]


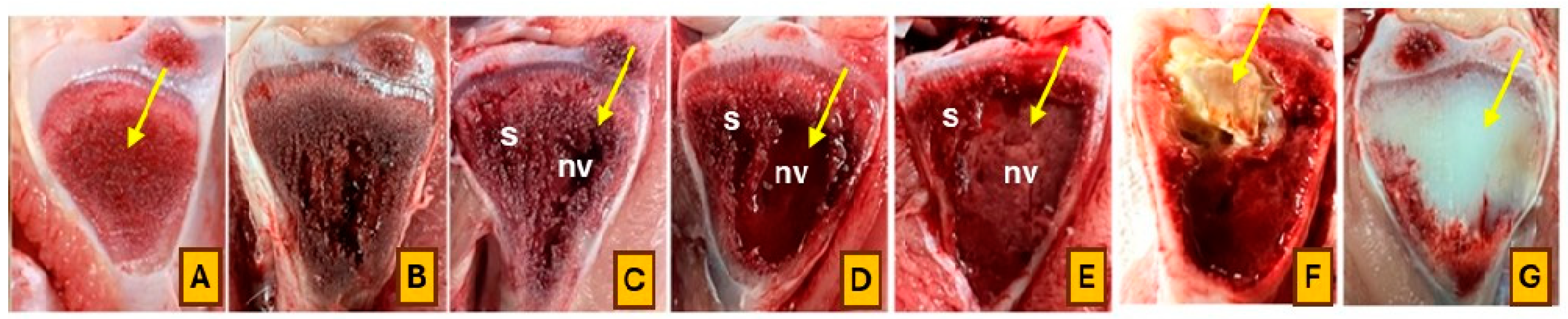




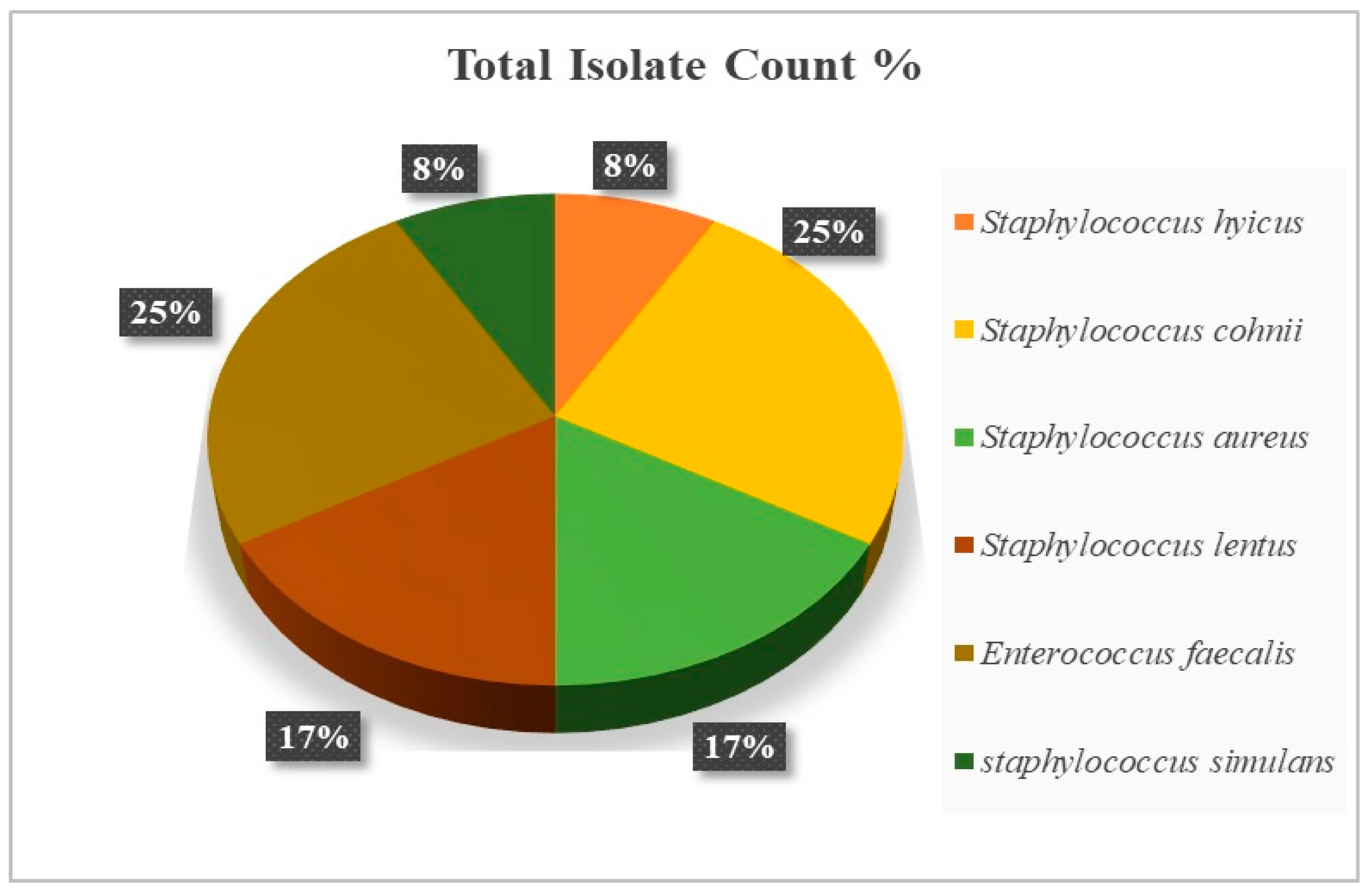
| Dietary Treatment | Description |
|---|---|
| Diet A in wire floor | BCO source group fed for 56 d |
| Diet A—Basal diet | Negative control diet fed for 56 d |
| Diet B—15% Availa® ZMC 1 | Feeding for 56 d |
| Diet C—Late feeding | Diet A on 1–28 d switched to Diet B on 29–56 d |
| Diet D—Early feeding | Diet B on 1–28 d switched to Diet A on 29–56 d |
| Day | BCO Source in Wire | Diet A—NC | Diet B | Diet C | Diet D |
|---|---|---|---|---|---|
| 42 | 36.00 a | 19.00 b | 7.00 c | 14.00 b | 5.00 c |
| 49 | 65.00 d | 38.00 e | 19.00 f | 40.00 e | 17.00 f |
| 56 | 83.00 g | 76.00 h | 37.00 j | 74.00 h | 45.00 i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, K.; Asnayanti, A.; Do, A.D.T.; Perera, R.; Al-Mitib, L.; Shwani, A.; Rebollo, M.A.; Kidd, M.T.; Alrubaye, A.A.K. Identifying Dietary Timing of Organic Trace Minerals to Reduce the Incidence of Osteomyelitis Lameness in Broiler Chickens Using the Aerosol Transmission Model. Animals 2024, 14, 1526. https://doi.org/10.3390/ani14111526
Alharbi K, Asnayanti A, Do ADT, Perera R, Al-Mitib L, Shwani A, Rebollo MA, Kidd MT, Alrubaye AAK. Identifying Dietary Timing of Organic Trace Minerals to Reduce the Incidence of Osteomyelitis Lameness in Broiler Chickens Using the Aerosol Transmission Model. Animals. 2024; 14(11):1526. https://doi.org/10.3390/ani14111526
Chicago/Turabian StyleAlharbi, Khawla, Andi Asnayanti, Anh Dang Trieu Do, Ruvindu Perera, Layla Al-Mitib, Abdulkarim Shwani, Marco A. Rebollo, Michael T. Kidd, and Adnan Ali Khalaf Alrubaye. 2024. "Identifying Dietary Timing of Organic Trace Minerals to Reduce the Incidence of Osteomyelitis Lameness in Broiler Chickens Using the Aerosol Transmission Model" Animals 14, no. 11: 1526. https://doi.org/10.3390/ani14111526
APA StyleAlharbi, K., Asnayanti, A., Do, A. D. T., Perera, R., Al-Mitib, L., Shwani, A., Rebollo, M. A., Kidd, M. T., & Alrubaye, A. A. K. (2024). Identifying Dietary Timing of Organic Trace Minerals to Reduce the Incidence of Osteomyelitis Lameness in Broiler Chickens Using the Aerosol Transmission Model. Animals, 14(11), 1526. https://doi.org/10.3390/ani14111526







