Trace Elements in Stenella coeruleoalba: Assessment of Marine Environmental Pollution and Dolphin Health Status
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sampling
2.3. Sample Preparation
2.4. Analysis in Inductively Coupled Plasma-Mass Spectometry (ICP-MS)
2.5. Validation Method
2.6. Assessment of Metals Pollution and Dolphin Health Status
2.7. Statistical Analyses
3. Results

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.L.; Salvador, S.M.; Liu, Y.P.; Sequeira, M. Heavy metals in the tissues of common dolphins Delphinus delphis stranded on the Portuguese coast. Sci. Total Environ. 2001, 273, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.L.; Mayer-Pinto, M.; Crowe, T.P. Chemical contaminant effects on marine eco system functioning. J. Appl. Ecol. 2015, 52, 140–149. [Google Scholar] [CrossRef]
- McKinley, A.; Johnston, E.L. Impacts of contaminant sources on marine fish abundance and species richness: Are view and meta-analysis of evidence from the field. Mar. Ecol. Prog. Ser. 2010, 420, 175–191. [Google Scholar] [CrossRef]
- Delgado-Suarez, I.; Lozano-Bilbao, E.; Hardisson, A.; Paz, S.; Gutierrez, A.J. Metal and trace element concentrations in cetaceans world wide: A review. Mar. Pollut. Bull. 2023, 192, 115010. [Google Scholar] [CrossRef] [PubMed]
- Ferrantelli, V.; Giangrosso, G.; Cicero, A.; Naccari, C.; Macaluso, A.; Galvano, F.; D’Orazio, N.; Arcadipane, G.E.; Naccari, F. Evaluation of mercury levels in Pangasius and Cod filet traded in Sicily (Italy). Food Addit. Contam. Part A 2012, 29, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Calò, M.; Naccari, F. Heavy metals in liver and muscle of bluefin tuna (Thunnus thinnus) caught in the straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Tanaka, M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: A review and synthesis. Mar. Pollut. Bull. 2004, 48, 624–649. [Google Scholar] [CrossRef] [PubMed]
- Pachés, M.; Martínez-Guijarro, R.; Romero, I.; Aguado, D. Assessment of Metal Pollution and Its Environmental Impact on Spanish Mediterranean Coastal Ecosystems. J. Mar. Sci. Eng. 2023, 11, 89. [Google Scholar] [CrossRef]
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; DuránValsero, J.J.; MoralesGarcía, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; Del Valls, A. Assessment and Review of Heavy Metals Pollution in Sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- EA Report No 7/2018 European Waters Assessment of Status and Pressures 2018; Report2013; EEA, European Environment Agency: Copenhagen, Denmark, 2018; ISBN 978-92-9213-947-6.
- Polizzi, P.; Romero, M.B.; Chiodi Boudet, L.; Dolagaratz Carricavur, A.; Gerpe, M. What do small cetaceans tell us about trace elements pollution on the Argentinean coast? Franciscana dolphin as a biomonitor. Sci. Total Environ. 2024, 906, 167428. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a frame work for community action in the field of marine environmental policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, 164, 19–40.
- Bossart, G.D. Marine Mammals as Sentinel Species for Oceans and Human Health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kimura, O.; Hisamichi, Y.; Minoshima, Y.; Haraguchi, H.; Kakumoto, C.; Kobayashi, Y. Distribution of total mercury, methylmercury and selenium in podof killer whales (Orcinus Orca) stranded in the northern area of Japan: Comparison of mature females with calves. Environ. Pollut. 2006, 144, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Machovscky, G.; vonHaeften, G.; Romero, M.A.; Rodríguez, D.H.; Gerpe, M.S. Linking cadmium and mercury accumulation to nutritional intake in common dolphins (Delphinus delphis) from Patagonia, Argentina. Environ. Pollut. 2020, 263, 114480. [Google Scholar] [CrossRef] [PubMed]
- Stockin, K.A.; Amiot, C.; Meynier, L.; Purvin, C.; Machovsky-Capuska, G.E. Understanding common dolphin and Australasian gannet feeding associations from nutritional and ethological perspectives. ICES J. Mar. Sci. 2022, 79, 2032–2042. [Google Scholar] [CrossRef]
- Ringelstein, J.; Pusineri, C.; Hassani, S.; Meynier, L.; Nicolas, R.; Ridoux, V. Food and feeding ecology of the striped dolphin, Stenella coeruleoalba, in the oceanic waters of the north-east Atlantic. J. Mar. Biol. Assoc. U. K. 2006, 86, 909–918. [Google Scholar] [CrossRef]
- Queiros, Q.; Fromentin, J.M.; Astruc, G.; Bauer, R.K.; Saraux, C. Dolphin predation pressure on pelagic and demersal fish in the north western Mediterranean Sea. Mar. Ecol. Prog. Ser. 2018, 603, 13–27. [Google Scholar] [CrossRef]
- Rojo-Nieto, E.; Fernandez-Maldonado, C. Assessing trace elements in striped dolphins from the Strait of Gibraltar: Clues to link the bioaccumulation in the western most Mediterranean Sea area and nearest Atlantic Ocean. Chemosphere 2017, 170, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Shoham-Frider, E.; Goffman, O.; Harlavan, Y.; Kress, N.; Morick, D.; Roditi-Elasar, M.; Shefer, E.; Kerem, D. Trace elements in striped dolphins (Stenella coeruleoalba) from the Eastern Mediterranean: A 10-year sperspective. Mar. Pollut. Bull. 2016, 109, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Naccari, C.; Cicero, N.; Ferrantelli, V.; Giangrosso, G.; Vella, A.; Macaluso, A.; Naccari, F.; Dugo, G. Toxic Metals in Pelagic, Benthic and Demersal Fish Species from Mediterranean FAO Zone 37. Bull. Environ. Contam. Toxicol. 2015, 95, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Kojadinovica, J.; Potierd, M.; LeCorrea, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Afandi, I.; Talba, S.; Benhra, A.; Benbrahim, S.; Chfiri, R.; Labonne, M.; Masski, H.; Laë, R.; De Morais, L.T.; Bekkali, M.; et al. Trace metal distribution in pelagic fish species from the north-west African coast (Morocco). Int. Aquat. Res. 2018, 10, 191–205. [Google Scholar] [CrossRef]
- Lopez-Berenguer, G.; Penalver, J.; Martínez-Lopez, E. A critical review about neurotoxic effects in marine mammals of mercury and other trace elements. Chemosphere 2020, 246, 125688. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.L.; Hall, A.J. Mercury in cetaceans: Exposure, bioaccumulation and toxicity. Sci. Total Environ. 2019, 694, 133683. [Google Scholar] [CrossRef] [PubMed]
- Lemos, L.S.; de Moura, J.F.; Hauser-Davis, R.A.; Campos, R.C.; Siciliano, S. Small cetaceans found stranded or accidentally captured in south eastern Brazil: Bioindicators of essential and non-essential trace elements in the environment. Ecotoxicol. Environ. Saf. 2013, 97, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Machovsky-Capuska, G.E.; Miller, M.G.; Silva, F.R.; Amiot, C.; Stockin, K.A.; Senior, A.M.; Schuckard, R.; Melville, D.; Raubenheimer, D. The nutritional nexus: Linking niche, habitat variability and prey composition in a generalist marine predator. J. Anim. Ecol. 2018, 87, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Gajdosechova, Z.; Brownlow, A.; Cottin, N.T.; Fernandes, M.; Read, F.L.; Urgast, D.S.; Raab, A.; Feldmann, J.; Krupp, E.M. Possible link between Hg and Cd accumulation in the brain of long-finned pilot whales (Globicephala melas). Sci. Total Environ. 2016, 545, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Brizio, P.; Chiaravalle, E.; Abete, M.C. Sperm whales (Physetermacro cephalus), found stranded along the adriatic coast (Southern Italy, Mediterranean Sea), as bioindicators of essential and non-essential trace elements in the environment. Ecol. Indic. 2015, 58, 418–425. [Google Scholar] [CrossRef]
- Gomez-Campos, E.; Borrell, A.; Aguilar, A. Assessment of nutritional condition indices a cross reproductive states in the striped dolphin (Stenella coeruleoalba). J. Exp. Mar. Biol. Ecol. 2011, 405, 18–24. [Google Scholar] [CrossRef]
- Saleem, M.; Iqbal, J.; Shi, Z.; Garrett, S.H.; Shah, M.H. Distribution and Bioaccumulation of Essential and Toxic Metals in Tissues of Thaila (Catla catla) froma Natural Lake, Pakistan and Its Possible Health Impact on Consumers. J. Mar. Sci. Eng. 2022, 10, 933. [Google Scholar] [CrossRef]
- Łuczynska, J.; Paszczyk, B.; Łuczynski, M.J. Fish as a bioindicator of heavy metals pollution in aquatic eco system of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol. Environ. Saf. 2018, 153, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jamil, T.; Lias, K.; Norsila, D.; Syafinaz, N.S. Assessment of heavy metal contamination in squid (Loligo spp.) Tissues of Kedah-perlis waters. Malaysia. Malays. J. Anal. Sci. 2014, 18, 195–203. [Google Scholar]
- Datta, S.N.; Kaur, V.I.; Dhawan, A.; Jassal, G. Estimation of length-weight relationship and condition factor of spotted snake head Channa punctata (Bloch) under different feeding regimes. SpringerPlus 2013, 2, 436. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Fernandez, P.; Webster, L.; Chouvelon, T.; Bustamante, P.; Ferreira, M.; González, A.F.; López, A.; Moffat, C.F.; Pierce, G.J.; Read, F.L. An assessment of contaminant concentrations in toothed whale species of the NW Iberian Peninsula: Part II. Trace element concentrations. Sci. Total Environ. 2014, 484, 206–217. [Google Scholar] [CrossRef] [PubMed]
- ATSDR (2003)—Toxicological Profile for Selenium; Agency for Toxic Substances and Disease Registry (ATSDR); Public Health Service, Department of Health and Human Services: Atlanta, GA, USA, 2003.
- Abdel-Khalek, A.A.; Elhaddad, E.; Mamdouh, S.; Mohamed-Assem, S.M. Assessment of Metal Pollution around Sabal Drainage in River Nile and its Impacts on Bioaccumulation Level, Metals Correlation and Human Risk Hazard using Oreochromis niloticus as a Bioindicator. Turk. J. Fish. Aquat. Sci. 2016, 16, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Egbal, O.A.; Mohammed, E.A.; Afra, A.A.; Esam, M.K.R. Lenght-weight relationships and condition factors of five fresh water species in Roseires Reservoir, Sudan. Eur. J. Phys. Agric. Sci. 2017, 5, 26–33. [Google Scholar]
- Hao, Y.; Miao, X.; Song, M.; Zhang, H. The Bioaccumulation and Health Risk Assessment of Metals among Two Most Consumed Species of Angling Fish (Cyprinus carpio and Pseudohemiculter dispar) in Liuzhou (China): Winter Should Be Treated as a Suitable Season for Fish Angling. Int. J. Environ. Res. Public Health 2022, 19, 1519. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Miao, X.; Hao, Y.; Xie, Z.; Zou, S.; Zhou, C. Health Risk Assessment of Metals (Cu, Pb, Zn, Cr, Cd, As, Hg, Se) in Angling Fish with Different Lengths Collected from Liuzhou, China. Int. J. Environ. Res. Public Health 2020, 17, 2192. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, M.; Kuzminova, N.; Chowaniak, M. Bioaccumulation of Na, Mg, Ca, K, and P in fish larvae of the genus Atherina L. collected in three bays in the region of Sevastopol. J. Elem. 2016, 21, 769–779. [Google Scholar] [CrossRef]
- Clausen, T. Na+–K+pump regulation and skeletal muscle contractility. Physiol. Rev. 2003, 83, 1269–1324. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, V.; Connolly, K.; Mc Eniery, C.; Wilkinson, I. Skin sodium and hypertension: A paradigm shift? Curr. Hypertens. Rep. 2018, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Saez, I.; Ribeiro Guevara, S.; Dellabianca, N.A.; Goodall, R.N.P.; Cappozzo, H.L. Heavy metals and essential elements in Commerson’s dolphins (Cephalorhynchus c. commersonii) from the south western South Atlantic Ocean. Environ Monit. Assess. 2013, 185, 5375–5386. [Google Scholar] [CrossRef] [PubMed]
- Seixas, T.G.; Kehrig, H.A.; Di Beneditto, A.P.M.; Souza, C.M.M.; Malm, O.; Moreira, I. Essential (Se, Cu) and non-essential (Ag, Hg, Cd) elements: What are their relationships in liver of Sotalia guianensis (Cetacea, Delphinidae)? Mar. Pollut. Bull. 2009, 58, 601–634. [Google Scholar] [CrossRef] [PubMed]
- Mouton, M.; Botha, A.; Thornton, M.; Mesjasz-Przybyłowicz, J.; Przybyłowicz, W.J. Elemental distribution patterns in the skins of false killer whales (Pseudorca crassidens) from a mass stranding in South Africa, analysed using micro-PIXENuclear. Instrum. Methods Phys. Res. B 2015, 363, 70–74. [Google Scholar] [CrossRef]
- Araneda, M.; Perez, E.P.; Gasca, L.E. White shrimp Penaeus vannamei culture in fresh water at three densities: Condition stat based on length and weight. Aquaculture 2008, 283, 13–18. [Google Scholar] [CrossRef]
- Tiago, M.S.; Joao, B.S.; Bruno, S.P.; Luciano, F.A.M. Length-weight relationship in ten fish species from the Nhamundá river, the Amazon Basin, Brazil. Acta Amaz. 2017, 47, 75–78. [Google Scholar]
- Ragheb, E. Length-weight relationship and well being factors of 33 fish species caught by gillnets from the Egyptian Mediterranean waters off Alexandria. Egypt. J. Aquat. Res. 2023, 49, 361–367. [Google Scholar] [CrossRef]
- Ralston, N.V.; Ralston, C.R.; Blackwell, J.L.; Raymond, L.J. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 2008, 29, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Kehrig, H.A.; Hauser-Davis, R.A.; Seixas, T.G.; Pinheiro, A.B.; Di Beneditto, A.P.M. Mercury species, selenium, metallothioneins and glutathione in two dolphins from the south eastern Brazilian coast: Mercury detoxification and physiological differences in diving capacity. Environ. Pollut. 2016, 213, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Marumoto, M.; Sakamoto, M.; Nakamura, M.; Marumoto, K.; Tsuruta, S. Organ-specifc accumulation of selenium and mercury in Indo-Pacific bottlenose dolphins (Tursiops aduncus). Acta Vet. Scand. 2022, 64, 1. [Google Scholar] [CrossRef] [PubMed]
- Baptista, G.; Kehrig, H.A.; Di Beneditto, A.P.M.; Hauser-Davis, R.A.; Almeida, M.G.; Carlos, E.; Rezende, C.E.; Siciliano, S.; de Moura, J.F.; Moreira, I. Mercury, selenium and stable isotopes in four small cetaceans from the South eastern Brazilian coast: Influence of feeding strategy. Environ. Pollut. 2016, 218, 1298–1307. [Google Scholar] [CrossRef] [PubMed]

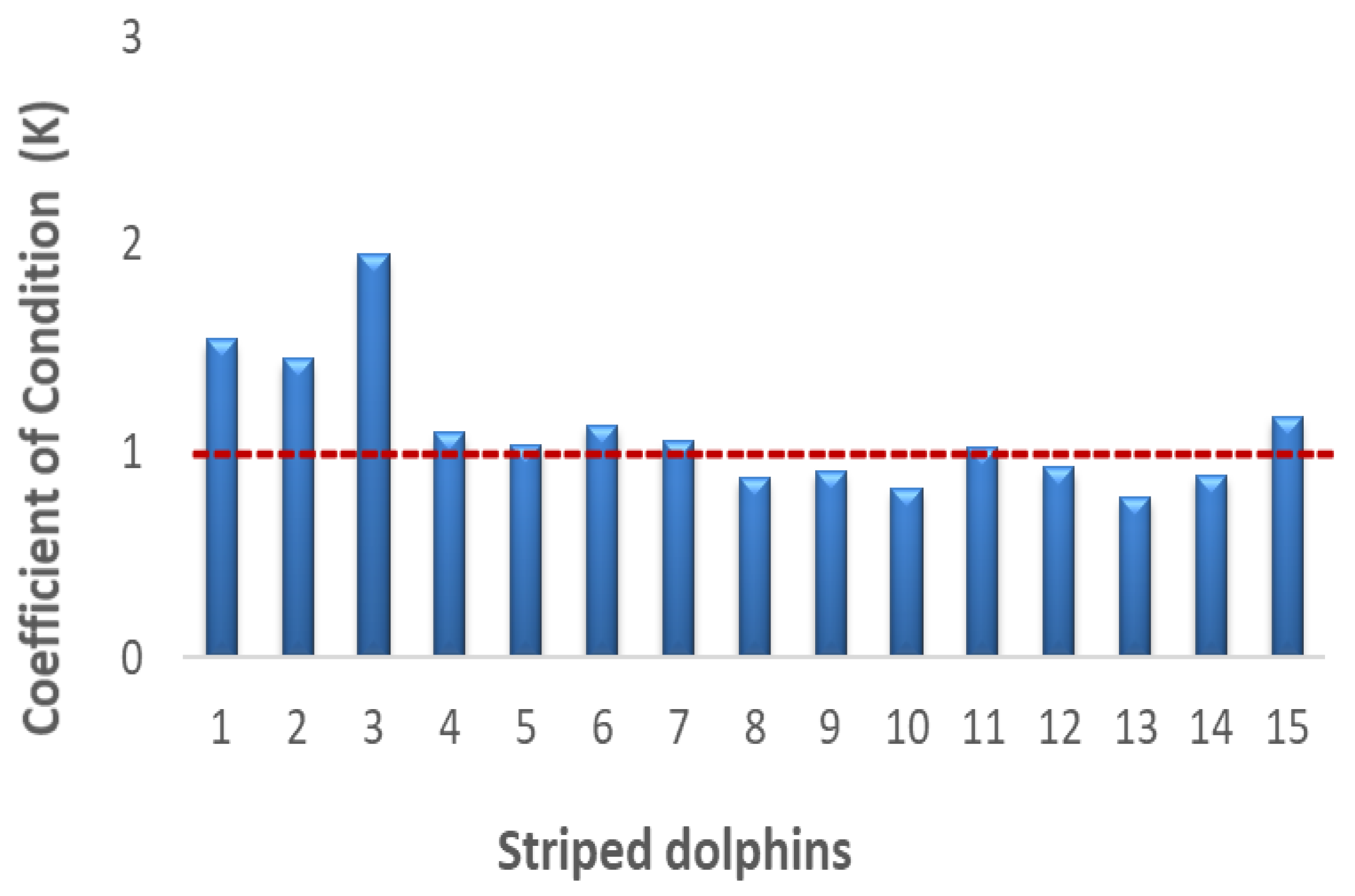
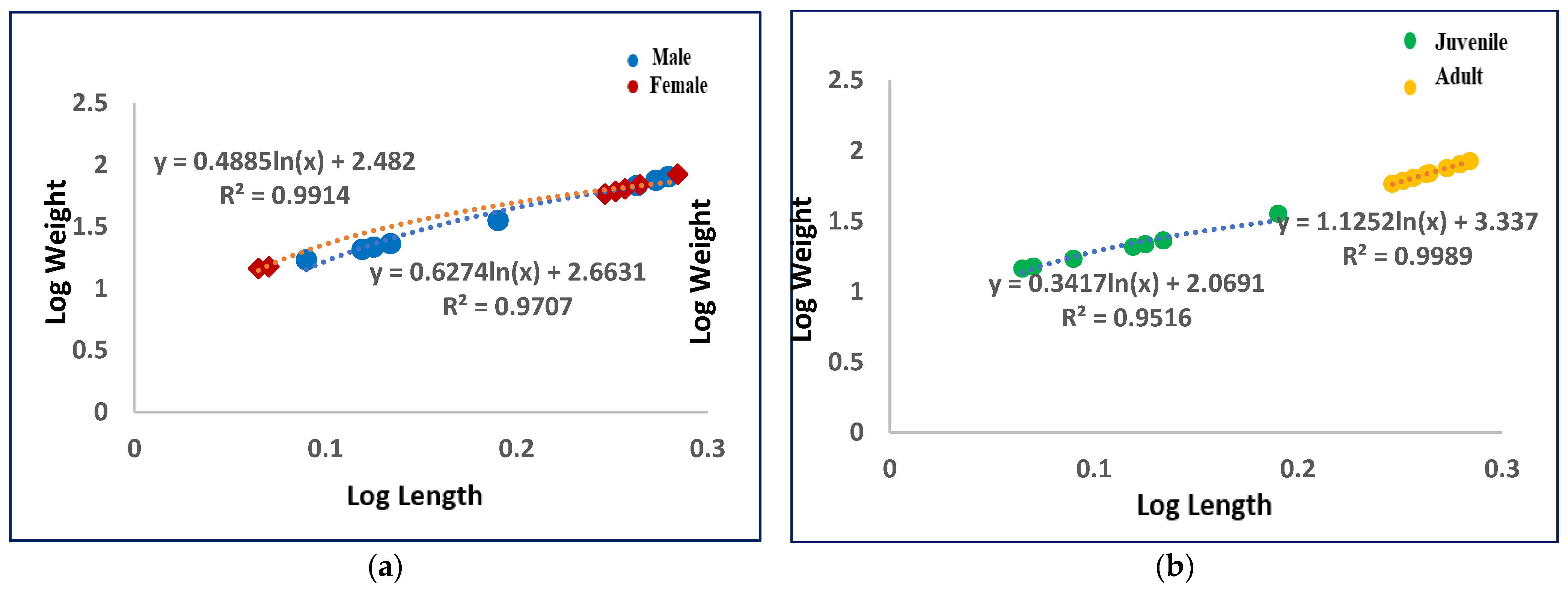
| Sample | Sex | Weight (kg) | Length (m) | Developmental Stage | Collection Site | a State of Beached Carcasses |
|---|---|---|---|---|---|---|
| 1 | F | 14.5 | 0.98 | Juveniles | Siracusa | 2 |
| 2 | M | 35.5 | 1.35 | Juveniles | Messina | 2 |
| 3 | M | 20.7 | 1.02 | Juveniles | Palermo | 2 |
| 4 | M | 17 | 1.16 | Juveniles | Siracusa | 2 |
| 5 | M | 21.6 | 1.28 | Juveniles | Messina | 3 |
| 6 | F | 15 | 1.10 | Juveniles | Messina | 2 |
| 7 | M | 23 | 1.30 | Juveniles | Palermo | 2 |
| 8 | F | 58 | 1.88 | Adult | Siracusa | 3 |
| 9 | M | 80 | 2.07 | Adult | Siracusa | 2 |
| 10 | F | 61 | 1.95 | Adult | Messina | 2 |
| 11 | F | 69 | 1.89 | Adult | Palermo | 3 |
| 12 | F | 64 | 1.91 | Adult | Palermo | 2 |
| 13 | F | 84 | 2.21 | Adult | Messina | 2 |
| 14 | M | 75 | 2.04 | Adult | Siracusa | 3 |
| 15 | M | 68 | 1.80 | Adult | Siracusa | 2 |
| Element | Linearity (R2) | LOD a (ng/g) | LOQ b (ng/g) | DOLT-5 c (µg/g) | Observed Values (µg/g) | Recovery (Range and %) | RSD d (%) | |
|---|---|---|---|---|---|---|---|---|
| Hg | 0.997 | 0.022 | 0.042 | 0.44 ± 0.18 | 0.33 ± 0.08 | 97–102% | (99.8) | 1.122 |
| Pb | 1.001 | 0.146 | 1.038 | 0.162 ± 0.032 | 0.154 ± 0.012 | 96–123% | (99.7) | 0.418 |
| Cd | 0.998 | 0.022 | 0.031 | 14.5 ± 0.6 | 14.1 ± 0.43 | 98–134% | (99.9) | 1.775 |
| As | 0.999 | 0.014 | 0.021 | 34.6 ± 2.4 | 33.8 ± 0.32 | 95–122% | (99.9) | 1.273 |
| Ni | 0.991 | 0.041 | 0.184 | 1.71 ± 0.56 | 1.62 ± 0.53 | 92–133% | (98.6) | 4.32 |
| Cr | 0.999 | 0.021 | 0.199 | - | - | 92–136% | (99.1) | 1.410 |
| Se | 0.999 | 0.019 | 0.063 | 8.3 ± 1.8 | 7.75 ± 0.68 | 98–184% | (98.9) | 1.454 |
| Co | 0.999 | 0.006 | 0.026 | 0.267 ± 0.026 | 0.19 ± 0.06 | 96–149% | (98.2) | 1.048 |
| Mn | 0.996 | 0.104 | 0.162 | 105.3 ± 5.4 | 100.7 ± 6.72 | 95–118% | (99.3) | 2.521 |
| Cu | 0.995 | 0.152 | 1.495 | 35.0 ± 2.4 | 32.22 ± 0.86 | 98–111% | (99.1) | 2.753 |
| Zn | 0.990 | 0.012 | 0.721 | 105.3 ± 5.4 | 108.2 ± 8.04 | 97–110% | (99.5) | 4.052 |
| Fe | 0.999 | 0.014 | 0.493 | 1070 ± 80 | 998 ± 13.2 | 97–111% | (98.1) | 1.094 |
| Na | 0.998 | 0.181 | 1.722 | 9.900 ± 1600 | 9561± 87.4 | 98–110% | (99.7) | 1.111 |
| Ca | 0.997 | 0.153 | 1.586 | 550 ± 80 | 508 ± 67 | 97–115% | (98.2) | 1.542 |
| K | 0.999 | 0.168 | 1.778 | 14.400 ± 300 | 13.980 ± 450 | 98–118% | (99.1) | 2.018 |
| Mg | 0.998 | 0.144 | 1.825 | 940 ± 100 | 912 ± 65 | 97–125% | (99.3) | 1.756 |
| P | 0.997 | 0.211 | 1.967 | - | - | 98–132% | (99.1) | 1.438 |
| Cd | Pb | Hg | As | MPI | ||
|---|---|---|---|---|---|---|
| Mean | 0.029 | 0.25 | c 9.29 | 0.12 | ||
| LUNG | S.D | 0.01 | 0.01 | 1.62 | 0.008 | 0.0020 |
| Median | 0.03 | 0.17 | 6.28 | 0.101 | ||
| Mean | 0.076 | 0.17 | c 41.51 | 0.31 | ||
| SKIN | S.D | 0.004 | 0.006 | 2.15 | 0.007 | 0.0408 |
| Median | 0.07 | 0.126 | 38.92 | 0.229 | ||
| Mean | 0.049 | 0.21 | a 12.11 | 0.16 | ||
| MUSCLE | S.D | 0.07 | 0.05 | 1.02 | 0.004 | 0.0050 |
| Median | 0.041 | 0.152 | 9.84 | 0.048 | ||
| Mean | 0.54 | 0.26 | c 31.22 | 0.33 | ||
| LIVER | S.D | 0.03 | 0.01 | 1.24 | 0.08 | 0.3616 |
| Median | 0.26 | 0.16 | 11.17 | 0.24 |
| Cr | Ni | Fe | Co | Mn | Se | Zn | Cu | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | 10.98 | 0.81 | c 79.53 | 6.03 | 0.42 | 5.14 | 8.14 | 1.09 | |
| LUNG | S.D | 1.28 | 0.03 | 2.18 | 2.19 | 0.04 | 0.96 | 2.19 | 0.62 |
| Median | 9.06 | 0.65 | 79.22 | 7.16 | 0.21 | 4.81 | 5.18 | 1.03 | |
| Mean | 7.27 | 0.47 | 21.45 | 3.02 | 0.98 | 5.48 | 6.67 | 1.16 | |
| SKIN | S.D | 1.73 | 0.03 | 3.17 | 0.08 | 0.07 | 0.82 | 1.26 | 0.74 |
| Median | 7.07 | 0.45 | 18.37 | 2.68 | 0.083 | 2.39 | 10.34 | 0.97 | |
| Mean | 7.86 | 0.54 | 35.63 | 3.03 | 0.14 | 6.05 | 7.25 | 1.09 | |
| MUSCLE | S.D | 1.02 | 0.01 | 1.98 | 0.02 | 0.08 | 0.71 | 0.82 | 0.28 |
| Median | 5.07 | 0.46 | 32.56 | 2.01 | 0.14 | 2.21 | 5.79 | 1.02 | |
| Mean | 8.54 | 0.77 | c 82.24 | 6.54 | 0.84 | 11.59 | 8.29 | 2.35 | |
| LIVER | S.D | 1.02 | 0.03 | 1.18 | 1.27 | 0.02 | 1.92 | 0.5 | 0.81 |
| Median | 6.59 | 0.52 | 64.05 | 8.04 | 0.61 | 8.50 | 10.96 | 2.73 |
| Na | K | Ca | Mg | P | ||
|---|---|---|---|---|---|---|
| Mean | 534.54 | 388.23 | 411.32 | 59.78 | c 3296.55 | |
| LUNG | S.D | 42.35 | 43.30 | 37.29 | 11.82 | 51.28 |
| Median | 523.56 | 374.45 | 428.87 | 49.96 | 3311.01 | |
| Mean | 291.89 | c 344.03 | c 19.24 | c 30.21 | c 1989.84 | |
| SKIN | S.D | 23.29 | 35.29 | 1.23 | 7.18 | 38.25 |
| Median | 314.77 | 412.32 | 14.56 | 31.96 | 2075.93 | |
| Mean | 284.01 | 568.09 | a 90.13 | 103.87 | c 3834.72 | |
| MUSCLE | S.D | 41.08 | 21.28 | 26.21 | 32.16 | 54.27 |
| Median | 222.28 | 562.90 | 19.33 | 104.74 | 4036.19 | |
| Mean | c 311.07 | b 437.33 | c 32.38 | a 60.17 | c 3824.01 | |
| LIVER | S.D | 35.09 | 25.38 | 9.27 | 21.19 | 31.29 |
| Median | 298.17 | 426.82 | 18.62 | 30.68 | 3394.25 |
| Ratio | LUNG | MUSCLE | LIVER | SKIN |
|---|---|---|---|---|
| 66Zn/201Hg | 0.19 | 0.59 | 0.26 | 0.13 |
| 82Se/201Hg | 0.12 | 0.49 | 0.18 | 0.10 |
| 63Cu/201Hg | 0.03 | 0.09 | 0.07 | 0.02 |
| 66Zn/207Pb | 32.56 | 34.52 | 31.88 | 39.94 |
| 82Se/207Pb | 20.55 | 28.80 | 21.58 | 32.81 |
| 63Cu/207Pb | 4.38 | 5.22 | 9.03 | 6.97 |
| 66Zn/112Cd | 281.87 | 95.62 | 147.95 | 87.76 |
| 82Se/112Cd | 177.99 | 79.85 | 21.46 | 72.10 |
| 63Cu/112Cd | 37.97 | 16.69 | 37.99 | 47.61 |
| 66Zn/75As | 67.83 | 44.92 | 25.12 | 21.55 |
| 82Se/75As | 42.72 | 37.48 | 16.94 | 17.67 |
| 63Cu/75As | 9.14 | 6.78 | 7.11 | 3.75 |
| 66Zn/60Ni | 45.22 | 44.18 | 42.12 | 14.14 |
| 82Se/60Ni | 28.56 | 24.25 | 28.40 | 9.89 |
| 63Cu/60Ni | 6.09 | 6.79 | 11.93 | 2.46 |
| 66Zn/52Cr | 0.74 | 0.92 | 0.97 | 0.91 |
| 82Se/52Cr | 0.46 | 0.77 | 0.65 | 0.75 |
| 63Cu/52Cr | 0.02 | 0.14 | 0.27 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naccari, C.; Ferrantelli, V.; Cammilleri, G.; Ruga, S.; Castagna, F.; Bava, R.; Palma, E. Trace Elements in Stenella coeruleoalba: Assessment of Marine Environmental Pollution and Dolphin Health Status. Animals 2024, 14, 1514. https://doi.org/10.3390/ani14111514
Naccari C, Ferrantelli V, Cammilleri G, Ruga S, Castagna F, Bava R, Palma E. Trace Elements in Stenella coeruleoalba: Assessment of Marine Environmental Pollution and Dolphin Health Status. Animals. 2024; 14(11):1514. https://doi.org/10.3390/ani14111514
Chicago/Turabian StyleNaccari, Clara, Vincenzo Ferrantelli, Gaetano Cammilleri, Stefano Ruga, Fabio Castagna, Roberto Bava, and Ernesto Palma. 2024. "Trace Elements in Stenella coeruleoalba: Assessment of Marine Environmental Pollution and Dolphin Health Status" Animals 14, no. 11: 1514. https://doi.org/10.3390/ani14111514
APA StyleNaccari, C., Ferrantelli, V., Cammilleri, G., Ruga, S., Castagna, F., Bava, R., & Palma, E. (2024). Trace Elements in Stenella coeruleoalba: Assessment of Marine Environmental Pollution and Dolphin Health Status. Animals, 14(11), 1514. https://doi.org/10.3390/ani14111514








