Assessment of Suitable Habitat of the Demoiselle Crane (Anthropoides virgo) in the Wake of Climate Change: A Study of Its Wintering Refugees in Pakistan
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
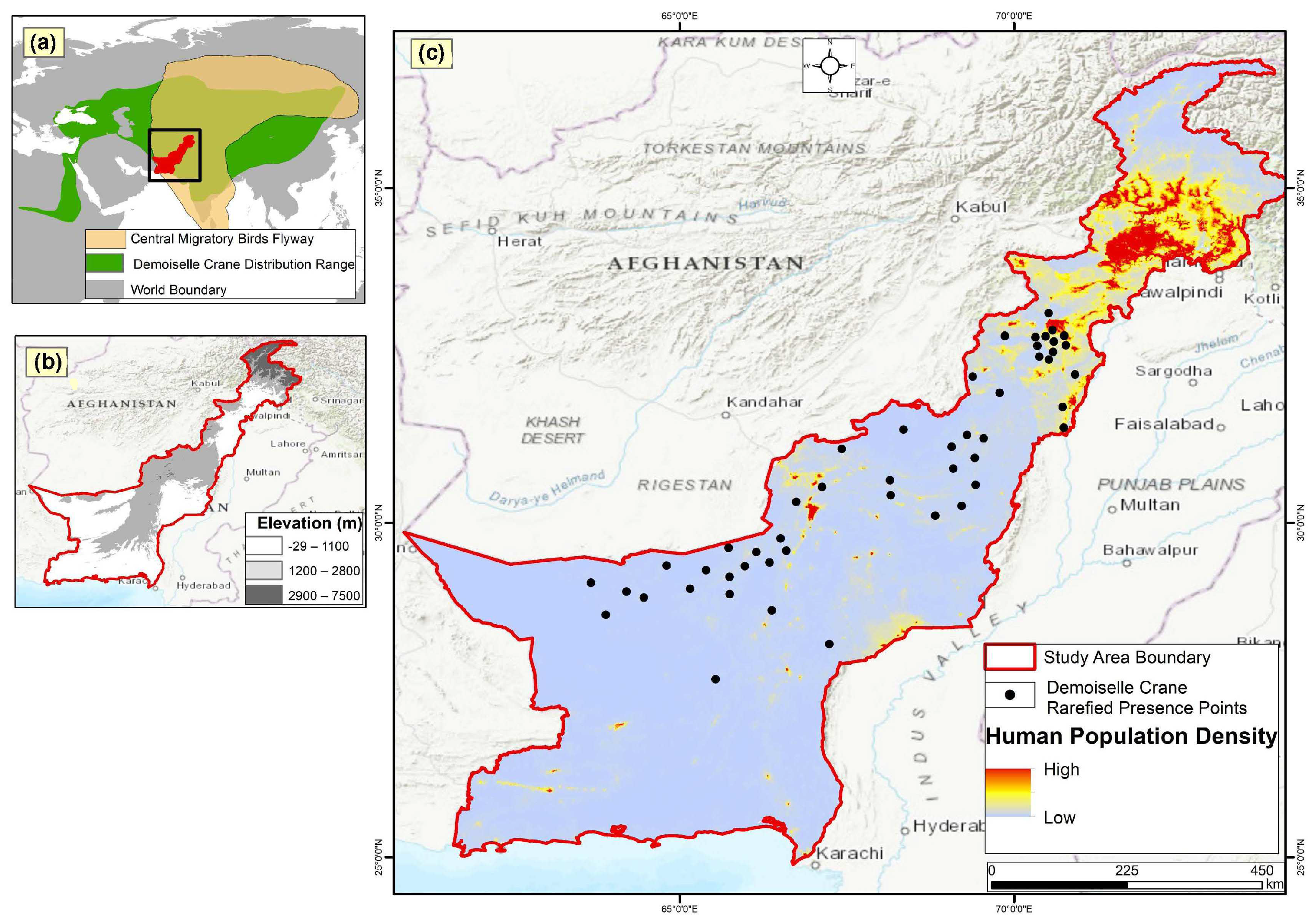
2.1. Study Area and Our Species of Interest
2.2. Occurrence/Presence Data
2.3. Preprocessing of Occurrence/Presence Data
2.4. Procurement, and Pretreatment of Predictor Variables
2.5. Preprocessing of Current Climatic Data
2.6. Future Projection Data
2.7. Construction of Maximum Entropy (MaxEnt) Model
2.8. Division of Potential Suitable Growing Areas for Demoiselle Crane
3. Results
3.1. MaxEnt Model Prediction Evaluation
3.2. Predictor Variables Defining the Habitat Suitability of Demoiselle Crane
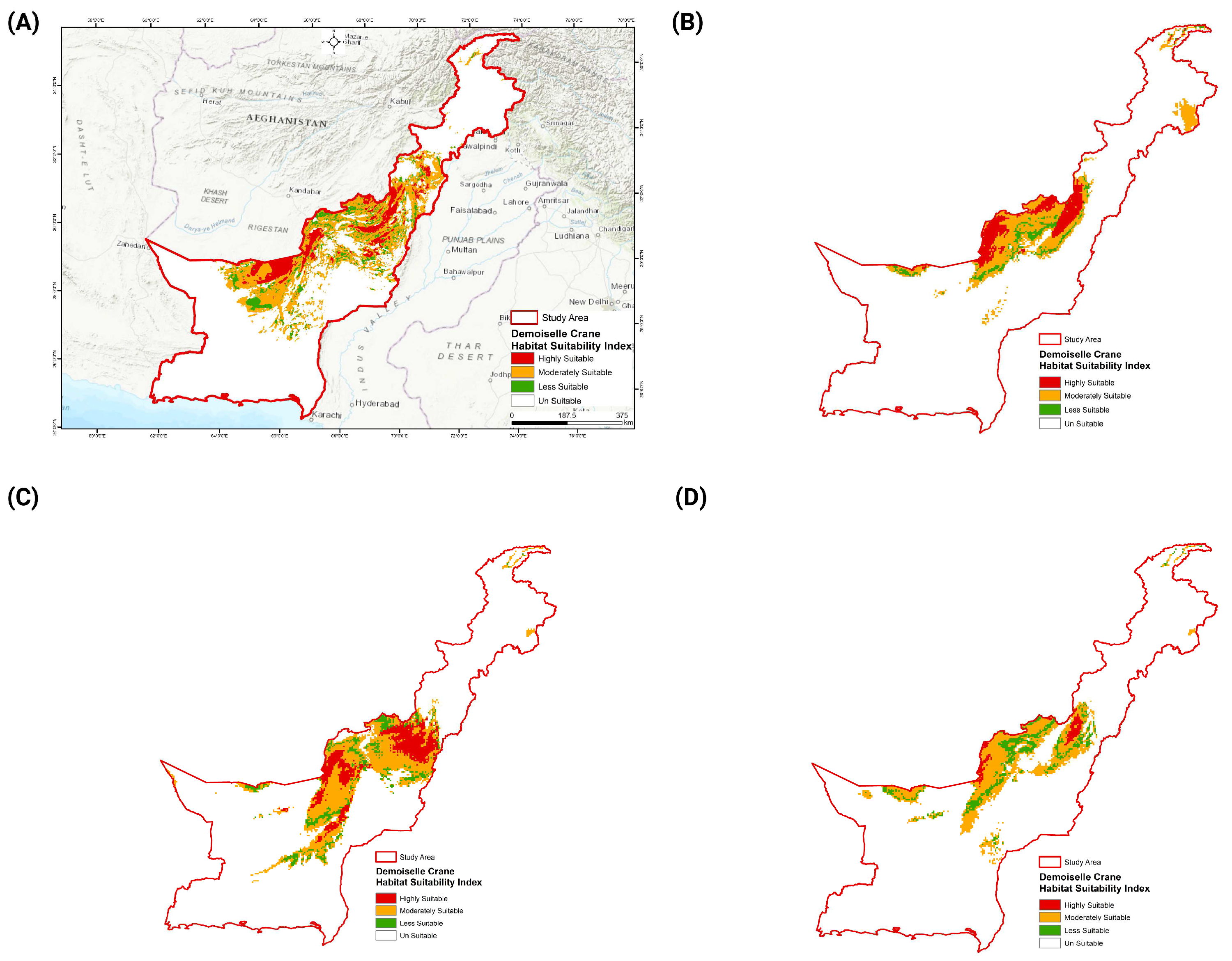
3.3. Future Distribution of Demoiselle Crane Habitat
3.4. Movement of the Demoiselle Crane’s Suitable Habitat
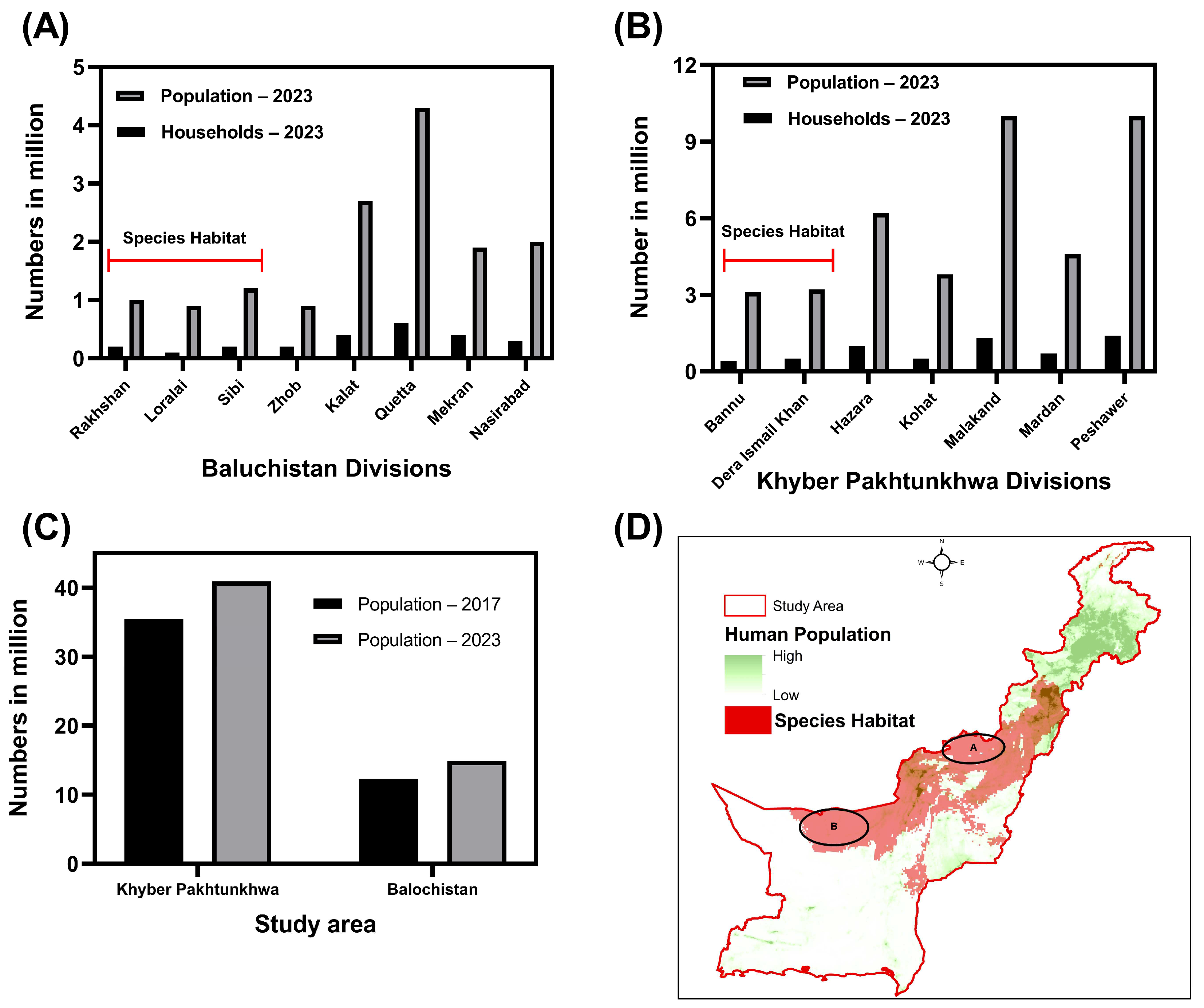
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.M. Climate change is impacting nutritional security from seafood. Nat. Clim. Change 2023, 13, 1166–1167. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Biaou, S.; Gouwakinnou, G.N.; Noulèkoun, F.; Salako, K.V.; Kpoviwanou, J.M.R.H.; Houehanou, T.D.; Biaou, H.S.S. Incorporating intraspecific variation into species distribution models improves climate change analyses of a widespread West African tree species (Pterocarpus erinaceus Poir, Fabaceae). Glob. Ecol. Conserv. 2023, 45, e02538. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xu, C. Study on Taiwania cryptomerioides under climate change: MaxEnt modeling for predicting the potential geographical distribution. Glob. Ecol. Conserv. 2020, 24, e01313. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T. Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 2006, 87, 2773–2786. [Google Scholar] [CrossRef]
- Barrett, M.A.; Brown, J.L.; Junge, R.E.; Yoder, A.D. Climate change, predictive modeling and lemur health: Assessing impacts of changing climate on health and conservation in Madagascar. Biol. Conserv. 2013, 157, 409–422. [Google Scholar] [CrossRef]
- Kellermann, V.; Van Heerwaarden, B.; Sgrò, C.M.; Hoffmann, A.A. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 2009, 325, 1244–1246. [Google Scholar] [CrossRef]
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. MaxEnt modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cuena-Lombraña, A.; Fois, M.; Fenu, G.; Cogoni, D.; Bacchetta, G. The impact of climatic variations on the reproductive success of Gentiana lutea L. in a Mediterranean mountain area. Int. J. Biometeorol. 2018, 62, 1283–1295. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Soroye, P.; Newbold, T.; Kerr, J. Climate change contributes to widespread declines among bumble bees across continents. Science 2020, 367, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef]
- Spooner, F.E.; Pearson, R.G.; Freeman, R. Rapid warming is associated with population decline among terrestrial birds and mammals globally. Glob. Change Biol. 2018, 24, 4521–4531. [Google Scholar] [CrossRef]
- Suggitt, A.J.; Wheatley, C.J.; Aucott, P.; Beale, C.M.; Fox, R.; Hill, J.K.; Isaac, N.J.; Martay, B.; Southall, H.; Thomas, C.D. Linking climate warming and land conversion to species’ range changes across Great Britain. Nat. Commun. 2023, 14, 6759. [Google Scholar] [CrossRef]
- Moraitis, M.L.; Valavanis, V.D.; Karakassis, I. Modelling the effects of climate change on the distribution of benthic indicator species in the Eastern Mediterranean Sea. Sci. Total Environ. 2019, 667, 16–24. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Wilson, K.L.; Skinner, M.A.; Lotze, H.K. Projected 21st-century distribution of canopy-forming seaweeds in the Northwest Atlantic with climate change. Divers. Distrib. 2019, 25, 582–602. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Cao, L.; Yin, H.; Xu, M.; Wang, Z.; Liu, Y.; Wang, X.; Deng, Y. Habitat environments impacted the gut microbiome of long-distance migratory swan geese but central species conserved. Sci. Rep. 2018, 8, 13314. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Varela, S.; Ji, X. The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ 2016, 4, e2185. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.N.; Maitland, B.M.; Pandit, L.K.; Poesch, M.S.; Enders, E.C. Climate change risks, extinction debt, and conservation implications for a threatened freshwater fish: Carmine shiner (Notropis percobromus). Sci. Total Environ. 2017, 598, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Boyd, C.; Grünbaum, D.; Hunt, G.L., Jr.; Punt, A.E.; Weimerskirch, H.; Bertrand, S. Effectiveness of social information used by seabirds searching for unpredictable and ephemeral prey. Behav. Ecol. 2016, 27, 1223–1234. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Bar-Massada, A.; Ives, A.R.; Butsic, V. A mathematical partitioning of the effects of habitat loss and habitat degradation on species abundance. Landsc. Ecol. 2019, 34, 9–15. [Google Scholar] [CrossRef]
- Wiegand, T.; Revilla, E.; Moloney, K.A. Effects of habitat loss and fragmentation on population dynamics. Conserv. Biol. 2005, 19, 108–121. [Google Scholar] [CrossRef]
- Bogich, T.L.; Barker, G.M.; Mahlfeld, K.; Climo, F.; Green, R.; Balmford, A. Fragmentation, grazing and the species–area relationship. Ecography 2012, 35, 224–231. [Google Scholar] [CrossRef]
- Heinrichs, J.A.; Bender, D.J.; Schumaker, N.H. Habitat degradation and loss as key drivers of regional population extinction. Ecol. Model. 2016, 335, 64–73. [Google Scholar] [CrossRef]
- Aguayo, J.; Elegbede, F.; Husson, C.; Saintonge, F.X.; Marçais, B. Modeling climate impact on an emerging disease, the Phytophthora alni-induced alder decline. Glob. Change Biol. 2014, 20, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Halstead, N.T.; Raffel, T.R. Modelling the future distribution of the amphibian chytrid fungus: The influence of climate and human-associated factors. J. Appl. Ecol. 2011, 48, 174–176. [Google Scholar] [CrossRef]
- Kinezaki, N.; Kawasaki, K.; Shigesada, N. The effect of the spatial configuration of habitat fragmentation on invasive spread. Theor. Popul. Biol. 2010, 78, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.R.; Deb, P.; Singha, H.; Chakdar, B.; Medhi, M. Predicting the probable distribution and threat of invasive Mimosa diplotricha Suavalle and Mikania micrantha Kunth in a protected tropical grassland. Ecol. Eng. 2016, 97, 23–31. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Michalski, F.; Peres, C.A. Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol. Conserv. 2005, 124, 383–396. [Google Scholar] [CrossRef]
- Sih, A.; Jonsson, B.G.; Luikart, G. Habitat loss: Ecological, evolutionary and genetic consequences. Trends Ecol. Evol. 2000, 15, 132–134. [Google Scholar] [CrossRef]
- Liang, J.; Peng, Y.; Zhu, Z.; Li, X.; Xing, W.; Li, X.; Yan, M.; Yuan, Y. Impacts of changing climate on the distribution of migratory birds in China: Habitat change and population centroid shift. Ecol. Indic. 2021, 127, 107729. [Google Scholar] [CrossRef]
- Jetz, W.; Wilcove, D.S.; Dobson, A.P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007, 5, e157. [Google Scholar] [CrossRef]
- Saino, N.; Ambrosini, R.; Rubolini, D.; von Hardenberg, J.; Provenzale, A.; Hüppop, K.; Hüppop, O.; Lehikoinen, A.; Lehikoinen, E.; Rainio, K. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B Biol. Sci. 2011, 278, 835–842. [Google Scholar] [CrossRef]
- Russell, D.J.; Wanless, S.; Collingham, Y.C.; Anderson, B.J.; Beale, C.; Reid, J.B.; Huntley, B.; Hamer, K.C. Beyond climate envelopes: Bio-climate modelling accords with observed 25-year changes in seabird populations of the British Isles. Divers. Distrib. 2015, 21, 211–222. [Google Scholar] [CrossRef]
- Van Doren, B.M. How migratory birds might have tracked past climate change. Proc. Natl. Acad. Sci. USA 2022, 119, e2121738119. [Google Scholar] [CrossRef]
- Roberts, C.P.; Allen, C.R.; Angeler, D.G.; Twidwell, D. Shifting avian spatial regimes in a changing climate. Nat. Clim. Change 2019, 9, 562–566. [Google Scholar] [CrossRef]
- Newton, I. Obligate and facultative migration in birds: Ecological aspects. J. Ornithol. 2012, 153, 171–180. [Google Scholar] [CrossRef]
- Dufour, P.; de Franceschi, C.; Doniol-Valcroze, P.; Jiguet, F.; Guéguen, M.; Renaud, J.; Lavergne, S.; Crochet, P.-A. A new westward migration route in an Asian passerine bird. Curr. Biol. 2021, 31, 5590–5596.e5594. [Google Scholar] [CrossRef]
- Schaefer, H.C.; Jetz, W.; Böhning-Gaese, K. Impact of climate change on migratory birds: Community reassembly versus adaptation. Glob. Ecol. Biogeogr. 2008, 17, 38–49. [Google Scholar] [CrossRef]
- Keller, V.; Herrando, S.; Voříšek, P.; Franch, M.; Kipson, M.; Milanesi, P.; Martí, D.; Anton, M.; Klvaňová, A.; Kalyakin, M.V. European Breeding Bird Atlas 2: Distribution, Abundance and Change; European Bird Census Council & Lynx Edicions: Barcelona, Spain, 2020. [Google Scholar]
- Ilyashenko, E.; Mudrik, E.A.; Andryushchenko, Y.A.; Belik, V.; Belyalov, O.; Wikelski, M.; Gavrilov, A.; Goroshko, O.A.; Guguyeva, E.V.; Korepov, M. Migrations of the Demoiselle Crane (Anthropoides virgo, Gruiformes): Remote Tracking along Flyways and at Wintering Grounds. Biol. Bull. 2022, 49, 863–888. [Google Scholar] [CrossRef]
- Belik, V.; Guguyeva, E.; Vetrov, V.; Milobog, Y.V. The Demoiselle crane in the northwestern Caspian lowland: Distribution, number, and breeding success. Cranes Eurasia (Biol. Distrib. Migr. Manag.) 2011, 4, 157–174. [Google Scholar]
- Farooq, M.; Ahmad, A.; Ghalib, S. The Cranes of Pakistan; World Wide Fund for Nature: Lahore, Pakistan, 1993; pp. 1–54. [Google Scholar]
- Eckstein, D.; Künzel, V.; Schäfer, L. Global climate risk index 2019: Who suffers most from extreme weather events. Weather-related loss events in 2017, 36. Available online: https://www.burmalibrary.org/sites/burmalibrary.org/files/obl/GLOBAL-CLIMATE-RISK-INDEX-2019-en.pdf (accessed on 25 October 2023).
- Eckstein, D.; Künzel, V.; Schäfer, L.; Winges, M. Global Climate Risk Index 2020; Germanwatch: Bonn, Germany, 2019; pp. 1–50. [Google Scholar]
- Rehman, A.; Jingdong, L.; Du, Y.; Khatoon, R.; Wagan, S.A.; Nisar, S.K. Flood disaster in Pakistan and its impact on agriculture growth (a review). Environ. Dev. Econ. 2016, 6, 39–42. [Google Scholar]
- Looney, R. Economic impacts of the floods in Pakistan. In Pakistan in National and Regional Change; Routledge: London, UK, 2016; pp. 53–69. [Google Scholar]
- Ahmad, Z.; Hafeez, M.; Ahmad, I. Hydrology of mountainous areas in the upper Indus Basin, Northern Pakistan with the perspective of climate change. Environ. Monit. Assess. 2012, 184, 5255–5274. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Naz, R.; Roohi, R. Glacial lake outburst flood hazards in Hindukush, Karakoram and Himalayan Ranges of Pakistan: Implications and risk analysis. Geomat. Nat. Hazards Risk 2012, 3, 113–132. [Google Scholar] [CrossRef]
- Joshi, S.; Jasra, W.; Ismail, M.; Shrestha, R.; Yi, S.; Wu, N. Herders’ perceptions of and responses to climate change in Northern Pakistan. Environ. Manag. 2013, 52, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Tahir, A.A.; Chevallier, P.; Arnaud, Y.; Ashraf, M.; Bhatti, M.T. Snow cover trend and hydrological characteristics of the Astore River basin (Western Himalayas) and its comparison to the Hunza basin (Karakoram region). Sci. Total Environ. 2015, 505, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Patwardhan, S.; Kumar, K.K.; Ashok, K.; Krishnan, R. Projected climate change in the Hindu Kush–Himalayan region by using the high-resolution regional climate model PRECIS. Mt. Res. Dev. 2013, 33, 142–151. [Google Scholar] [CrossRef]
- Xu, J.; Grumbine, R.E.; Shrestha, A.; Eriksson, M.; Yang, X.; Wang, Y.; Wilkes, A. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conserv. Biol. 2009, 23, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Hussain, M.; Murtaza, G.; Shaheen, F.A.; Zafar, F. Ecological concerns of migratory birds in Pakistan: A review. Punjab Univ. J. Zool. 2018, 33, 69–76. [Google Scholar] [CrossRef]
- Prange, H. Crane Research and Protection in Europe; Martin-Luther-Universität Halle-Wittenberg: Halle, Germany, 1995. [Google Scholar]
- Ullah, I.; Sun, X.; WU, Q.-M.; Deng, W.; Rajpar, M.; Majeed, A.; Ditta, A. Determining the relative abundance of, habitat preferences of and occurences of gastrointestinal parasites in common crane and demoiselle crane inhabiting three distinct habitats. Appl. Ecol. Environ. Res. 2023, 21, 451–465. [Google Scholar] [CrossRef]
- Mian, A. Crane migration through Balochistan. A preliminary report. J. Bombay Nat. Hist. Soc 1981, 86, 98–100. [Google Scholar]
- Nawaz, M. Migratory cranes in Pakistan. Tigerpaper (FAO/RAPA) 1984, 11. [Google Scholar]
- Khan, A. Habitat Status and Hunting Pressure on Migratory Cranes in Pakistan and Assessment of Lake Ab-i-Estada in Afghanistan with Proposed Conservation Plans for Selected Wetlands; University of Wisconsin—Madison: Madison, WI, USA, 2004. [Google Scholar]
- Chaudhry, Q.U.Z. Climate Change Profile of Pakistan; Asian Development Bank: Mandaluyong, Philippines, 2017. [Google Scholar]
- Ali, Z.; Khan, A. Captive Breeding and Multiple Clutching Techniques of Captive Cranes in Bannu and Lakki Marwat, NWFP; The Minisitry of Environment’s Pakistan Wetlands Programme: Bannu, Pakistan, 2007; p. 42. [Google Scholar]
- Bari, F.; Rehman, E.U.; Kabir, M.; Ahmad, S. An Extension to the Known Wintering Range of the Steppe Eagle Aquila nipalensis, in the Poonch and Jhelum Valleys, Azad Jammu and Kashmir, Pakistan. Ardeola 2020, 67, 415–422. [Google Scholar] [CrossRef]
- Baig, M.B.; Al-Subaiee, F.S. Biodiversity in Pakistan: Key issues. Biodiversity 2009, 10, 20–29. [Google Scholar] [CrossRef]
- Khan, B.; Ali, Z. Assessment of birds’ fauna, occurrence status, diversity indices and ecological threats at ManglaDam, AJK from 2011 to 2014. J. Anim. Plant Sci. 2014, 25, 397–403. [Google Scholar]
- Khan, B.; Ablimit, A.; Khan, G.; Jasra, A.W.; Ali, H.; Ali, R.; Ahmad, E.; Ismail, M. Abundance, distribution and conservation status of Siberian ibex, Marco Polo and Blue sheep in Karakoram-Pamir mountain area. J. King Saud Univ. Sci. 2016, 28, 216–225. [Google Scholar] [CrossRef]
- Tahir, A.A.; Chevallier, P.; Arnaud, Y.; Neppel, L.; Ahmad, B. Modeling snowmelt-runoff under climate scenarios in the Hunza River basin, Karakoram Range, Northern Pakistan. J. Hydrol. 2011, 409, 104–117. [Google Scholar] [CrossRef]
- Tehsin, R. Migrating Demoiselle Cranes. Tigerpaper 1988, 14, 26–27. [Google Scholar]
- Khattak, R.H.; Xin, Z.; Ahmad, S.; Ur Rehman, E.; Roberts, N.J. An Avi-Faunal Inventory of Miangan Tarakai Game Reserve: A Future Destination for Eco-Tourists. Pak. J. Life Soc. Sci. 2019, 17, 39–45. [Google Scholar]
- Ahmad, A.; Shah, S.; Harris, J. The future of cranes in Pakistan with special reference to NWFP. In Proceedings of the 1987 International Crane Workshop, Quiqihar, China, 1–10 May 1987; pp. 335–339. [Google Scholar]
- Meine, C.; Archibald, G. The Cranes: Status Survey and Conservation Action Plan; IUcN: Gland, Switzerland, 1996. [Google Scholar]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- White, P.C.; Jennings, N.V.; Renwick, A.R.; Barker, N.H. Questionnaires in ecology: A review of past use and recommendations for best practice. J. Appl. Ecol. 2005, 42, 421–430. [Google Scholar] [CrossRef]
- Shima, A.L.; Berger, L.; Skerratt, L.F. Conservation and health of Lumholtz’s tree-kangaroo (Dendrolagus lumholtzi). Aust. Mammal. 2018, 41, 57–64. [Google Scholar] [CrossRef]
- Lunney, D.; Matthews, A. The contribution of the community to defining the distribution of a vulnerable species, the spotted-tailed quoll, Dasyurus maculatus. Wildl. Res. 2001, 28, 537–545. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Beauchamp, G.; Jiang, Z. Flock size and human disturbance affect vigilance of endangered red-crowned cranes (Grus japonensis). Biol. Conserv. 2011, 144, 101–105. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, W.; Luan, X.; Zhuang, H.; Khan, T.U.; Zhang, G.; Wu, S. Use of historical data to assess the impact of climate change and anthropogenic disturbance on the black-billed capercaillie (Tetrao urogalloides) in northeast China. Glob. Ecol. Conserv. 2020, 22, e00972. [Google Scholar] [CrossRef]
- Wanghe, K.; Guo, X.; Hu, F.; Ahmad, S.; Jin, X.; Khan, T.U.; Xiao, Y.; Luan, X. Spatial coincidence between mining activities and protected areas of giant panda habitat: The geographic overlaps and implications for conservation. Biol. Conserv. 2020, 247, 108600. [Google Scholar] [CrossRef]
- Ahmad, S.; Yang, L.; Khan, T.U.; Wanghe, K.; Li, M.; Luan, X. Using an ensemble modelling approach to predict the potential distribution of Himalayan gray goral (Naemorhedus goral bedfordi) in Pakistan. Glob. Ecol. Conserv. 2020, 21, e00845. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, X.; Dong, Y.; Yan, S.; Song, Y.; Zhou, L. Significant differences in the gut bacterial communities of Hooded Crane (Grus monacha) in different seasons at a stopover site on the flyway. Animals 2020, 10, 701. [Google Scholar] [CrossRef]
- De Luis, M.; Bartolomé, C.; García Cardo, Ó.; Álvarez-Jiménez, J. Gypsophila bermejoi G. López: A possible case of speciation repressed by bioclimatic factors. PLoS ONE 2018, 13, e0190536. [Google Scholar] [CrossRef]
- Smeraldo, S.; Bosso, L.; Salinas-Ramos, V.B.; Ancillotto, L.; Sánchez-Cordero, V.; Gazaryan, S.; Russo, D. Generalists yet different: Distributional responses to climate change may vary in opportunistic bat species sharing similar ecological traits. Mammal Rev. 2021, 51, 571–584. [Google Scholar] [CrossRef]
- Kaeslin, E.; Redmond, I.; Dudley, N. Wildlife in a Changing Climate; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012. [Google Scholar]
- Yang, X.-Q.; Kushwaha, S.; Saran, S.; Xu, J.; Roy, P. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Molloy, S.W.; Davis, R.A.; Van Etten, E.J. Species distribution modelling using bioclimatic variables to determine the impacts of a changing climate on the western ringtail possum (Pseudocheirus occidentals; Pseudocheiridae). Environ. Conserv. 2014, 41, 176–186. [Google Scholar] [CrossRef]
- Yi, Y.-j.; Zhou, Y.; Cai, Y.-p.; Yang, W.; Li, Z.-w.; Zhao, X. The influence of climate change on an endangered riparian plant species: The root of riparian Homonoia. Ecol. Indic. 2018, 92, 40–50. [Google Scholar] [CrossRef]
- Gelviz-Gelvez, S.M.; Pavón, N.P.; Illoldi-Rangel, P.; Ballesteros-Barrera, C. Ecological niche modeling under climate change to select shrubs for ecological restoration in Central Mexico. Ecol. Eng. 2015, 74, 302–309. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Hayat, M.A.; Wang, H. Modeling the present and future distribution of arbovirus vectors Aedes aegypti and Aedes albopictus under climate change scenarios in Mainland China. Sci. Total Environ. 2019, 664, 203–214. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Swanepoel, L.H.; Lindsey, P.; Somers, M.J.; Van Hoven, W.; Dalerum, F. Extent and fragmentation of suitable leopard habitat in South Africa. Anim. Conserv. 2013, 16, 41–50. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Márcia Barbosa, A.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F. The representative concentration pathways: An overview. Clim. Change 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, B.; Li, D.; Garber, P.A.; Zhu, P.; Xiang, Z.; Grueter, C.C.; Liu, Z.; Li, M. Climate change, grazing, and collecting accelerate habitat contraction in an endangered primate. Biol. Conserv. 2019, 231, 88–97. [Google Scholar] [CrossRef]
- Bellouin, N.; Collins, W.; Culverwell, I.; Halloran, P.; Hardiman, S.; Hinton, T.; Jones, C.; McDonald, R.; McLaren, A.; O’Connor, F. The HadGEM2 family of met office unified model climate configurations. Geosci. Model Dev. 2011, 4, 723–757. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. A J. R. Meteorol. Soc. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Waheed, M.; Arshad, F.; Majeed, M.; Haq, S.M.; Aziz, R.; Bussmann, R.W.; Ali, K.; Subhan, F.; Jones, D.A.; Zaitouny, A. Potential distribution of a noxious weed (Solanum viarum Du-nal), current status, and future invasion risk based on MaxEnt modeling. Geol. Ecol. Landsc. Feburary 2023, 1–16. [Google Scholar] [CrossRef]
- Gull, E.; Fareen, A.; Mahmood, T.; Bodlah, I.; Rashid, A.; Khalid, A.; Mahmood, S. Modeling potential distribution of newly recorded ant, Brachyponera nigrita using Maxent under climate change in Pothwar region, Pakistan. PLoS ONE 2022, 17, e0262451. [Google Scholar]
- Zahoor, B.; Songer, M.; Liu, X.; Huang, Q.; Dai, Y. Identifying stable and overlapping habitats for a predator (common leopard) and prey species (Himalayan grey goral & Himalayan grey langur) in northern Pakistan. Glob. Ecol. Conserv. 2023, 43, e02418. [Google Scholar]
- Phillips, A.J.; Vidafar, P.; Burns, A.C.; McGlashan, E.M.; Anderson, C.; Rajaratnam, S.M.; Lockley, S.W.; Cain, S.W. High sensitivity and interindividual variability in the response of the human circadian system to evening light. Proc. Natl. Acad. Sci. USA 2019, 116, 12019–12024. [Google Scholar] [CrossRef]
- Phillips, J.J.; Phillips, P.P. Handbook of Training Evaluation and Measurement Methods; Routledge: London, UK, 2016. [Google Scholar]
- Evcin, O.; Kucuk, O.; Akturk, E. Habitat suitability model with maximum entropy approach for European roe deer (Capreolus capreolus) in the Black Sea Region. Environ. Monit. Assess. 2019, 191, 669. [Google Scholar] [CrossRef] [PubMed]
- Friedman, K.; Shimony, A. Jaynes’s maximum entropy prescription and probability theory. J. Stat. Phys. 1971, 3, 381–384. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.; Li, J.; Peterson, A.T.; Graham, C.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Bosso, L.; Rebelo, H.; Garonna, A.P.; Russo, D. Modelling geographic distribution and detecting conservation gaps in Italy for the threatened beetle Rosalia alpina. J. Nat. Conserv. 2013, 21, 72–80. [Google Scholar] [CrossRef]
- Fois, M.; Fenu, G.; Lombrana, A.C.; Cogoni, D.; Bacchetta, G. A practical method to speed up the discovery of unknown populations using Species Distribution Models. J. Nat. Conserv. 2015, 24, 42–48. [Google Scholar] [CrossRef]
- Vasconcelos, T.S.; Rodríguez, M.Á.; Hawkins, B.A. Species distribution modelling as a macroecological tool: A case study using New World amphibians. Ecography 2012, 35, 539–548. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 94–98. [Google Scholar]
- Harte, J.; Zillio, T.; Conlisk, E.; Smith, A.B. Maximum entropy and the state-variable approach to macroecology. Ecology 2008, 89, 2700–2711. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; Liu, H.Y.; Zhang, Y.; Lu, C.H. Comparative analysis of the gut microbiota of hornbill and toucan in captivity. Microbiologyopen 2019, 8, e00786. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, X.; Liu, S.; Zhang, J. The major factors influencing distribution of three species of Dendrobium: Analysis of potential ecologically suitable distributions. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100275. [Google Scholar] [CrossRef]
- Rahmati, O.; Golkarian, A.; Biggs, T.; Keesstra, S.; Mohammadi, F.; Daliakopoulos, I.N. Land subsidence hazard modeling: Machine learning to identify predictors and the role of human activities. J. Environ. Manag. 2019, 236, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Clemente, P.; Calvache, M.; Antunes, P.; Santos, R.; Cerdeira, J.O.; Martins, M.J. Combining social media photographs and species distribution models to map cultural ecosystem services: The case of a Natural Park in Portugal. Ecol. Indic. 2019, 96, 59–68. [Google Scholar] [CrossRef]
- Hoveka, L.; Bezeng, B.; Yessoufou, K.; Boatwright, J.; Van der Bank, M. Effects of climate change on the future distributions of the top five freshwater invasive plants in South Africa. South Afr. J. Bot. 2016, 102, 33–38. [Google Scholar] [CrossRef]
- Hundessa, S.; Li, S.; Li Liu, D.; Guo, J.; Guo, Y.; Zhang, W.; Williams, G. Projecting environmental suitable areas for malaria transmission in China under climate change scenarios. Environ. Res. 2018, 162, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, R.; Mazzoni, S.; Bryn, A.; Bakkestuen, V. Opportunities for improved distribution modelling practice via a strict maximum likelihood interpretation of MaxEnt. Ecography 2015, 38, 172–183. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.; Wu, Y.; Liu, H.; Li, Y.; Chen, H.; Zhang, Y.; Zhao, Y. Integrating Maxent model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, 106472. [Google Scholar] [CrossRef]
- Walden-Schreiner, C.; Leung, Y.-F.; Kuhn, T.; Newburger, T.; Tsai, W.-L. Environmental and managerial factors associated with pack stock distribution in high elevation meadows: Case study from Yosemite National Park. J. Environ. Manag. 2017, 193, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Yi, Y.-j.; Cheng, X.; Yang, Z.-F.; Zhang, S.-H. Maxent modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Aryal, A.; Shrestha, U.B.; Ji, W.; Ale, S.B.; Shrestha, S.; Ingty, T.; Maraseni, T.; Cockfield, G.; Raubenheimer, D. Predicting the distributions of predator (snow leopard) and prey (blue sheep) under climate change in the Himalaya. Ecol. Evol. 2016, 6, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.D.S.; Nevin, O.T.; Smith, D.; Convery, I. Environmental niche overlap between snow leopard and four prey species in Kazakhstan. Ecol. Inform. 2018, 48, 97–103. [Google Scholar] [CrossRef]
- Sony, R.; Sen, S.; Kumar, S.; Sen, M.; Jayahari, K. Niche models inform the effects of climate change on the endangered Nilgiri Tahr (Nilgiritragus hylocrius) populations in the southern Western Ghats, India. Ecol. Eng. 2018, 120, 355–363. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, Z.; Tang, S. Impacts of climate change on distributions and diversity of ungulates on the Tibetan Plateau. Ecol. Appl. 2015, 25, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Stabach, J.A.; Laporte, N.; Olupot, W. Modeling habitat suitability for Grey Crowned-cranes (Balearica regulorum gibbericeps) throughout Uganda. Int. J. Biodivers. Conserv. 2009, 1, 177–186. [Google Scholar]
- Ali, H.; Din, J.U.; Bosso, L.; Hameed, S.; Kabir, M.; Younas, M.; Nawaz, M.A. Expanding or shrinking? range shifts in wild ungulates under climate change in Pamir-Karakoram mountains, Pakistan. PLoS ONE 2021, 16, e0260031. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khattak, R.H.; Teng, L.; Kaneez, K.; Liu, Z. Factors Affecting Habitat Selection of Endangered Steppe Eagle (Aquila nipalensis) in Pakistan: Implications for Raptors Conservation. Diversity 2022, 14, 1135. [Google Scholar] [CrossRef]
- Xu, F.; Yang, W.; Ma, M.; Blank, D.A. Vigilance of the demoiselle crane antropoides virgo: The effects of group size, human disturbance, and predation vulnerability. Pak. J. Zool. 2021, 53, 371. [Google Scholar] [CrossRef]
- Volkov, S.; Grinchenko, O.; Sviridova, T. The effects of weather and climate changes on the timing of autumn migration of the common crane (Grus grus) in the north of Moscow region. Biol. Bull. 2016, 43, 1203–1211. [Google Scholar] [CrossRef]
- Orellana Macías, J.M.; Bautista-Sopelana, L.M.; Merchán, D.; Causapé Valenzuela, J.A.; Alonso López, J.C. Shifts in crane migration phenology associated with climate change in southwestern Europe. ACE-ÉCO. 2020, 15, 16. [Google Scholar] [CrossRef]
- Swanberg, P. Studies on the influence of weather on migrating cranes (Grus grus) in Sweden. Aquila 1987, 93, 203–212. [Google Scholar]
- Andryushchenko, Y.A. Revision of the range of the Azov-Black Sea population of the Demoiselle Crane. Cranes Eurasia Biol. Distrib. Captiv. Breed. 2015, 5, 153–165. [Google Scholar]
- Sarwar, M.; Hussain, I.; Khan, A.; Anwar, M. Diet composition of the Demoiselle crane (Anthropoides virgo) migrating through Lakki Marwat, Pakistan. Avian Biol. Res. 2013, 6, 269–274. [Google Scholar] [CrossRef]
- Dong, H.Y.; Lu, G.Y.; Zhong, X.Y.; Yang, X.J. Winter diet and food selection of the Black-necked Crane Grus nigricollis in Dashanbao, Yunnan, China. PeerJ 2016, 4, e1968. [Google Scholar] [CrossRef] [PubMed]
- Wamiti, S.W. Factors Affecting Population, Nesting Habits, and Conservation of Grey Crowned Crane (Balearica Reguloram, Bennett 1834) in Lake Ol’Bolossat Basin, Kenya. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2022. [Google Scholar]
- Zelelew, S.A.; Nowald, G.; Archibald, G.; Tadele, H.; Aticho, A.; Morrison, K.; Gutema, T.M. Distribution and population estimates of four crane species in Ethiopia: A global crane hotspot facing increasing threats. Scopus J. East Afr. Ornithol. 2020, 40, 1–17. [Google Scholar]
- Sarwar, M.; Hamid, A.; Hussain, I. Hunting Pressure on Migratory Demoiselle Cranes in Pakistan. Pak. J. Zool. 2022, 54, 471. [Google Scholar] [CrossRef]
- Cabrera-Cruz, S.A.; Smolinsky, J.A.; McCarthy, K.P.; Buler, J.J. Urban areas affect flight altitudes of nocturnally migrating birds. J. Anim. Ecol. 2019, 88, 1873–1887. [Google Scholar] [CrossRef]
- Lindström, Å.; Alerstam, T.; Andersson, A.; Bäckman, J.; Bahlenberg, P.; Bom, R.; Ekblom, R.; Klaassen, R.H.; Korniluk, M.; Sjöberg, S. Extreme altitude changes between night and day during marathon flights of great snipes. Curr. Biol. 2021, 31, 3433–3439.e3433. [Google Scholar] [CrossRef]
- Policy, C.C. Khyber Pakhtunkhwa Climate Change Policy. 2016. Available online: https://resourcecenter.nhnpakistan.org/phocadownload/government/policy-documents/Final_Climate_Change_Policy_for_KP.pdf (accessed on 25 October 2023).
- Nilsson, L.; Persson, J.; Bunnefeld, N.; Månsson, J. Central place foraging in a human-dominated landscape: How do common cranes select feeding sites? J. Avian Biol. 2020, 51, 6. [Google Scholar] [CrossRef]
- GH, O. On the theory of central place foraging. Anal. Ecol. Syst. 1979, 157–177. [Google Scholar]
- Brown, J.S. Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 1988, 22, 37–47. [Google Scholar] [CrossRef]
- Tablado, Z.; Jenni, L. Determinants of uncertainty in wildlife responses to human disturbance. Biol. Rev. 2017, 92, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, D.; Weimerskirch, H. Scale-dependent habitat use in a long-ranging central place predator. J. Anim. Ecol. 2005, 74, 852–863. [Google Scholar] [CrossRef]
- Chudzińska, M.E.; van Beest, F.M.; Madsen, J.; Nabe-Nielsen, J. Using habitat selection theories to predict the spatiotemporal distribution of migratory birds during stopover—A case study of pink-footed geese Anser brachyrhynchus. Oikos 2015, 124, 851–860. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; van Beest, F.M.; Richard, E.; Uzal, A.; Medill, S.A.; McLoughlin, P.D. Density-dependent, central-place foraging in a grazing herbivore: Competition and tradeoffs in time allocation near water. Oikos 2015, 124, 1142–1150. [Google Scholar] [CrossRef]
- Olsson, O.; Brown, J.S.; Helf, K.L. A guide to central place effects in foraging. Theor. Popul. Biol. 2008, 74, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Kotler, B.P. Hazardous duty pay and the foraging cost of predation. Ecol. Lett. 2004, 7, 999–1014. [Google Scholar] [CrossRef]
- Jensen, G.H.j.; Tombre, I.M.; Madsen, J. Environmental factors affecting numbers of pink-footed geese Anser brachyrhynchus utilising an autumn stopover site. Wildl. Biol. 2016, 22, 183–193. [Google Scholar] [CrossRef]
- Salik, K.M.; Ishfaq, S.; Saeed, F.; Noel, E.; Syed, Q.-u.-A. Pakistan Country Situation Assessment. 2015. Available online: https://idl-bnc-idrc.dspacedirect.org/items/2828b909-4598-4cb3-b978-d85f6567420e (accessed on 25 October 2023).
- Mustafa, Z. Climate change and its impact with special focus in Pakistan. In Proceedings of the Pakistan Engineering Congress, Symposium, Lahore, Pakistan; 2011; p. 290. [Google Scholar]
- GFfDRaR, G. Vulnerability, Risk Reduction, and Adaptation to Climate Change; World Bank Group: Washington, DC, USA, 2011. [Google Scholar]
- Din, J.U.; Ali, H.; Ali, A.; Younus, M.; Mehmood, T.; Norma-Rashid, Y.; Nawaz, M.A. Pastoralist-predator interaction at the roof of the world: Conflict dynamics and implications for conservation. Ecol. Soc. 2017, 22, 32. [Google Scholar] [CrossRef]
- Karris, G.; Martinis, A.; Kabassi, K.; Dalakiari, A.; Korbetis, M. Changing social awareness of the illegal killing of migratory birds in the Ionian Islands, western Greece. J. Biol. Educ. 2020, 54, 162–175. [Google Scholar] [CrossRef]


| Predictor Variable | Percent (%) Contribution | Permutation Importance |
|---|---|---|
| Temperature seasonality (standard deviation ×100) | 29.6 | 19.1 |
| Annual mean temperature | 23.5 | 42.9 |
| Ruggedness index of the area | 18.1 | 2.6 |
| Human population density | 10.1 | 3.6 |
| Precipitation of wettest quarter | 7.2 | 2.6 |
| Min temperature of coldest month | 6.1 | 10.2 |
| Precipitation of coldest quarter | 3.1 | 7.7 |
| Land cover | 2.3 | 11.3 |
| Future Projection | Scenario | Habitat Categories | ||||
|---|---|---|---|---|---|---|
| Model | Year | Unsuitable | Minimally Suitable | Moderately Suitable | Highly Suitable | |
| Current | 267,539 | 28,865 | 134,068 | 27,911 | ||
| BCC-CSM1-1 | 2050 | RCP4.5_Change | 51 | −38 | −82 | −51 |
| RCP8.5 Change | 57 | −37 | −92 | −67 | ||
| 2070 | RCP4.5 Change | 49 | −25 | −81 | −60 | |
| RCP8.5 Change | 61 | −65 | −93 | −72 | ||
| CCSM4 | 2050 | RCP4.5 Change | 19 | 52 | −33 | −75 |
| RCP8.5 Change | 53 | −41 | −84 | −65 | ||
| 2070 | RCP4.5 Change | 59 | −64 | −89 | −71 | |
| RCP8.5 Change | 65 | −72 | −95 | −85 | ||
| HADGEM2-AO | 2050 | RCP4.5 Change | 39 | −31 | −73 | 12 |
| RCP8.5 Change | 35 | −41 | −65 | 24 | ||
| 2070 | RCP4.5 Change | 44 | −59 | −75 | −1 | |
| RCP8.5 Change | 49 | −33 | −83 | −37 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.U.; Ullah, I.; Hu, Y.; Liang, J.; Ahmad, S.; Omifolaji, J.K.; Hu, H. Assessment of Suitable Habitat of the Demoiselle Crane (Anthropoides virgo) in the Wake of Climate Change: A Study of Its Wintering Refugees in Pakistan. Animals 2024, 14, 1453. https://doi.org/10.3390/ani14101453
Khan TU, Ullah I, Hu Y, Liang J, Ahmad S, Omifolaji JK, Hu H. Assessment of Suitable Habitat of the Demoiselle Crane (Anthropoides virgo) in the Wake of Climate Change: A Study of Its Wintering Refugees in Pakistan. Animals. 2024; 14(10):1453. https://doi.org/10.3390/ani14101453
Chicago/Turabian StyleKhan, Tauheed Ullah, Inam Ullah, Yiming Hu, Jianchao Liang, Shahid Ahmad, James Kehinde Omifolaji, and Huijian Hu. 2024. "Assessment of Suitable Habitat of the Demoiselle Crane (Anthropoides virgo) in the Wake of Climate Change: A Study of Its Wintering Refugees in Pakistan" Animals 14, no. 10: 1453. https://doi.org/10.3390/ani14101453
APA StyleKhan, T. U., Ullah, I., Hu, Y., Liang, J., Ahmad, S., Omifolaji, J. K., & Hu, H. (2024). Assessment of Suitable Habitat of the Demoiselle Crane (Anthropoides virgo) in the Wake of Climate Change: A Study of Its Wintering Refugees in Pakistan. Animals, 14(10), 1453. https://doi.org/10.3390/ani14101453






