Convergent High O2 Affinity but Distinct ATP-Mediated Allosteric Regulation of Hemoglobins in Oviparous and Viviparous Eremias Lizards from the Qinghai-Tibet Plateau
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. RT-PCR and Sequencing of α and β Globins
2.4. Sequence Alignment and Phylogenetic Analysis
2.5. Hb Purification and Isoform Identification
2.6. Measuring O2 Equilibrium Curves
2.7. Homology Modeling and Molecular Dynamics Simulation of Tetrameric Hb
2.8. Statistical Analyses
3. Results
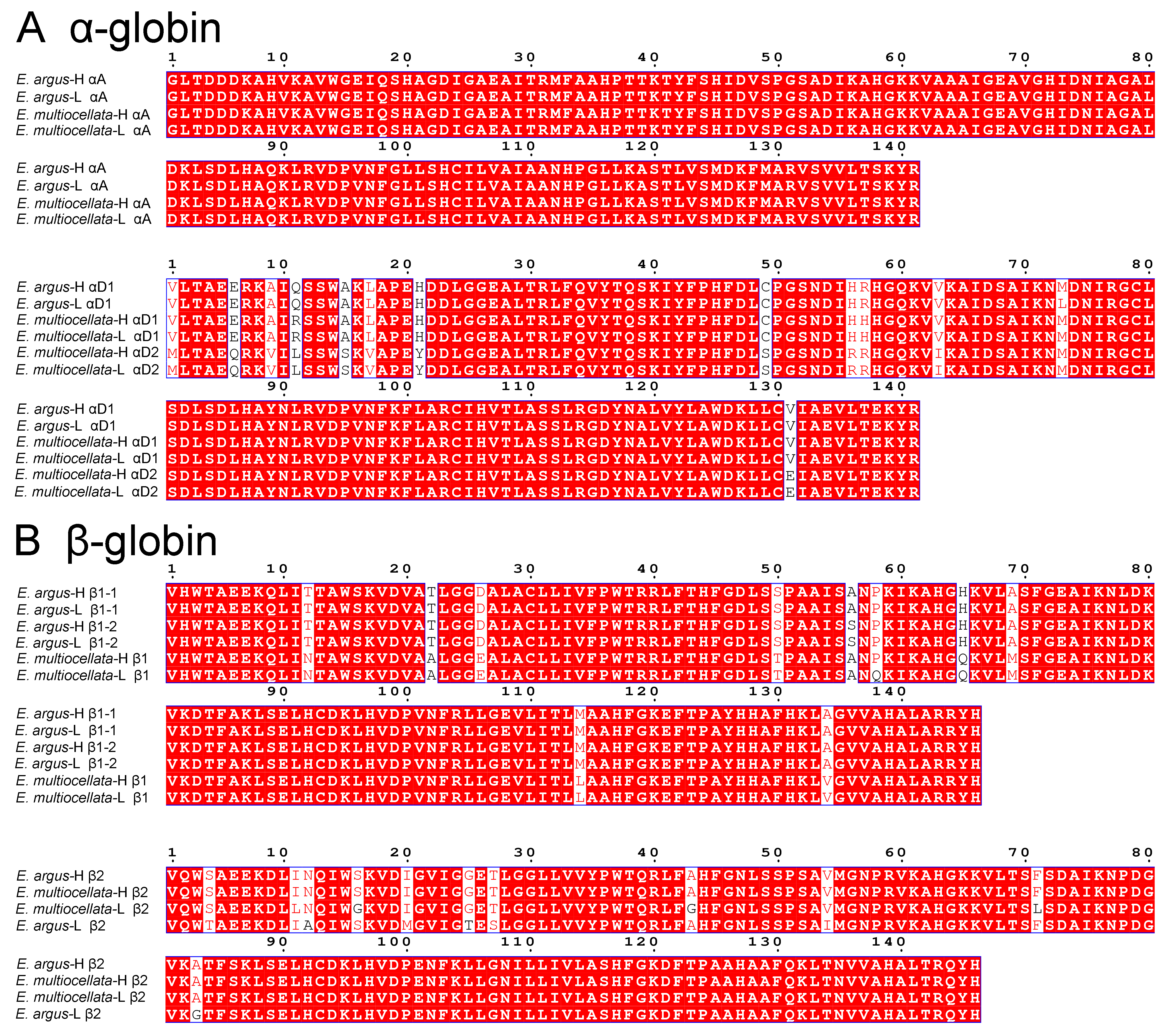
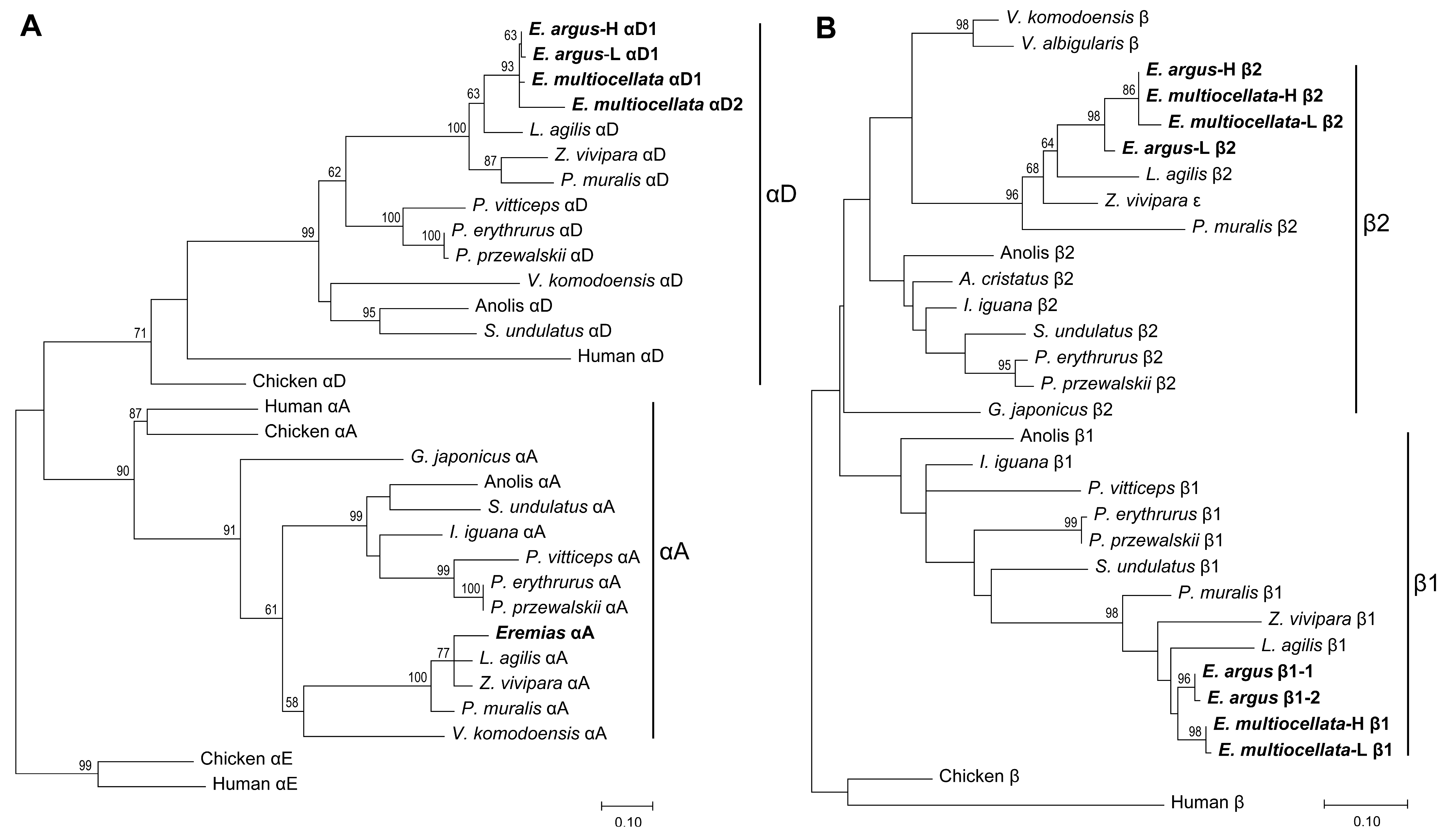
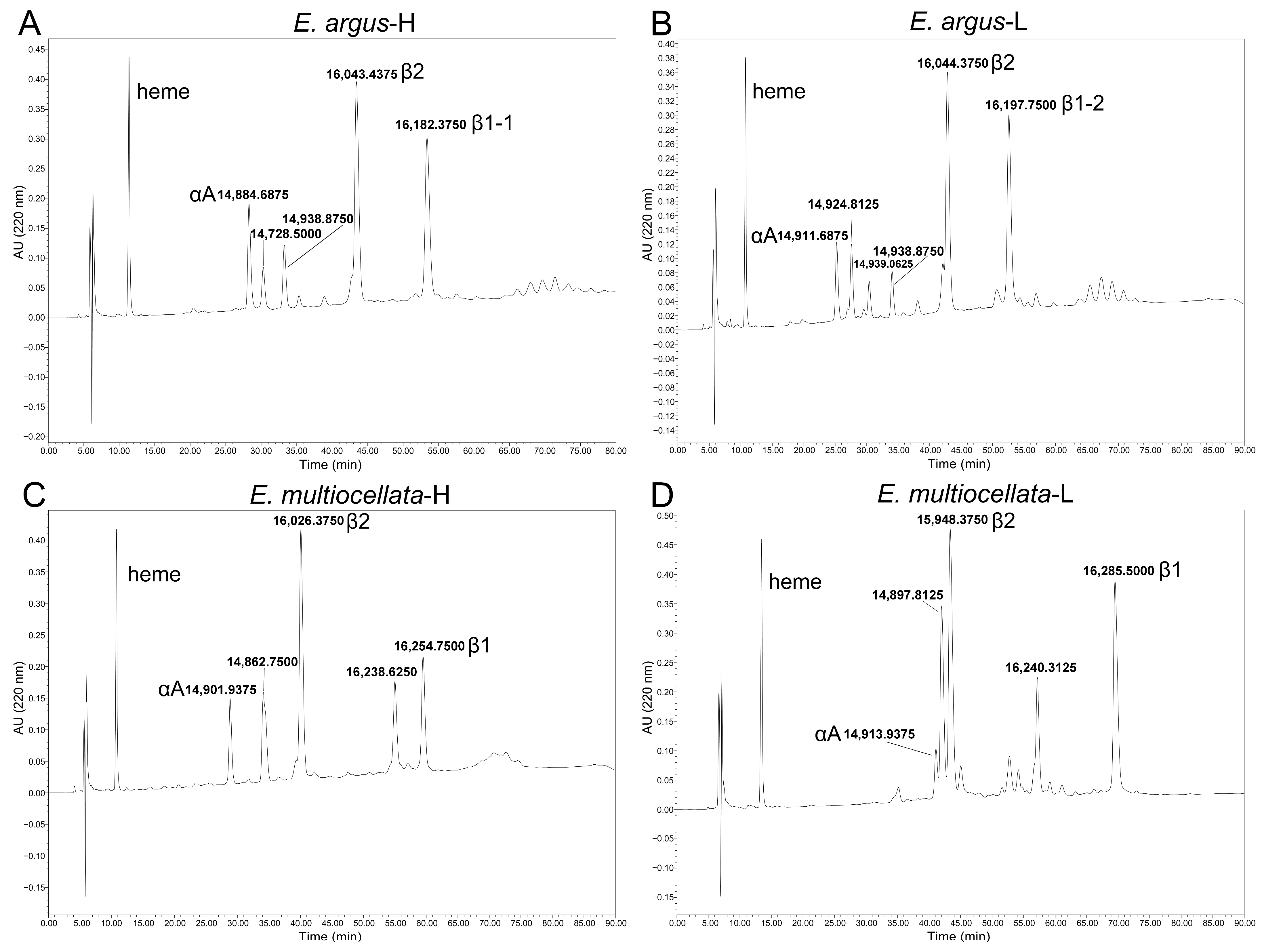
3.1. Sequence Variation, Phylogenetic Relationships, and isoHb Compositions
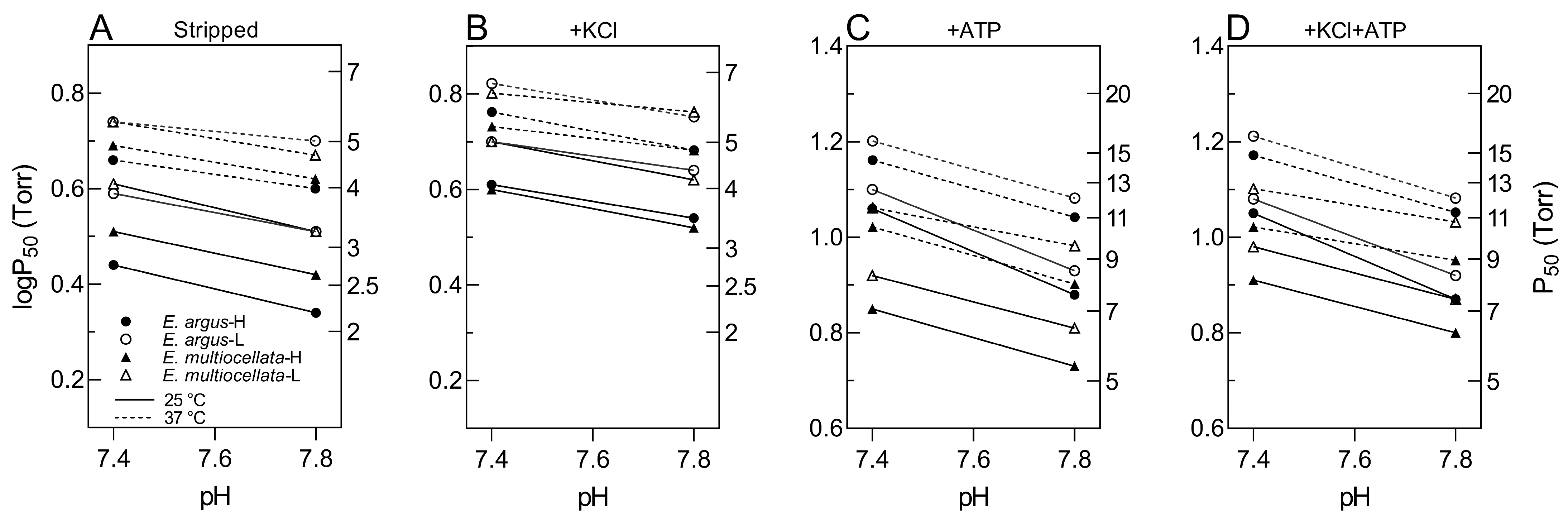
3.2. Convergent High Hb-O2 Affinity but Distinct ATP-Mediated Allosteric Regulation of Hbs in Eremias Lizards
3.3. Low Temperature Sensitivity of Hbs in Eremias Lizards
3.4. Structural Properties of Tetrameric Hbs in T-State
4. Discussion
4.1. Phylogenetic Relationship of Beta Globins in Sauria Lizards
4.2. The Physiological Significances of Oxygenation Properties of Eremias Lizards Hbs
4.3. Molecular Mechanisms Underlying the Oxygenation Properties of Eremias Hbs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QTP | Qinghai-Tibet Plateau |
| E. argus-H | high-altitude E. argus |
| E. argus-L | low-altitude E. argus |
| E. multiocellata-H | high-altitude E. multiocellata |
| E. multiocellata-L | low-altitude E. multiocellata |
| Hb | hemoglobin |
| ATP | adenosine triphosphate |
| DPG | 2,3-diphosphoglycerate |
| IP5 | inositol pentaphosphate |
| P50 | O2 tension at half-saturation |
| n50 | Hill cooperativity coefficient |
| O2 tension | |
| fractional saturation | |
| ΔH | overall change in enthalpy for oxygenation |
| Φ | Bohr factor |
| isoHb | Hb isoform |
| IEF | isoelectric focusing |
References
- Storz, J.F.; Quiroga-Carmona, M.; Opazo, J.C.; Bowen, T.; Farson, M.; Steppan, S.J.; D’Elía, G. Discovery of the world’s highest-dwelling mammal. Proc. Natl. Acad. Sci. USA 2020, 117, 18169–18171. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.W. The physiological and evolutionary significance of cardiovascular shunting patterns in reptiles. Physiology 2002, 17, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Cerdeña, J.; Farfan, J.; Quiroz, A. A high mountain lizard from Peru: The world’s highest-altitude reptile. Herpetozoa 2021, 34, 61–65. [Google Scholar] [CrossRef]
- Zhao, E.M.; Zhao, K.T.; Zhou, K.Y. Reptilia (Squamata: Lacertilia). In Fauna Sinica; Beijing Science Press: Beijing, China, 1999; Volume 2, pp. 220–243. [Google Scholar]
- Ivy, C.M.; Scott, G.R. Control of breathing and the circulation in high-altitude mammals and birds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 186, 66–74. [Google Scholar] [CrossRef] [PubMed]
- McClelland, G.B.; Scott, G.R. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu. Rev. Physiol. 2019, 81, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Scott, G.R. Life ascending: Mechanism and process in physiological adaptation to high-Altitude hypoxia. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 503–526. [Google Scholar] [CrossRef] [PubMed]
- Jendroszek, A.; Malte, H.; Overgaard, C.B.; Beedholm, K.; Natarajan, C.; Weber, R.E.; Storz, J.F.; Fago, A. Allosteric mechanisms underlying the adaptive increase in hemoglobin–oxygen affinity of the bar-headed goose. J. Exp. Biol. 2018, 221, jeb185470. [Google Scholar] [CrossRef]
- Natarajan, C.; Jendroszek, A.; Kumar, A.; Weber, R.E.; Tame, J.R.H.; Fago, A.; Storz, J.F. Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genet. 2018, 14, e1007331. [Google Scholar] [CrossRef] [PubMed]
- Jessen, T.H.; Weber, R.E.; Fermi, G.; Tame, J.; Braunitzer, G. Adaptation of bird hemoglobins to high altitudes: Demonstration of molecular mechanism by protein engineering. Proc. Natl. Acad. Sci. USA 1991, 88, 6519–6522. [Google Scholar] [CrossRef]
- Weber, R.E.; Jessen, T.H.; Malte, H.; Tame, J. Mutant hemoglobins (alpha 119-Ala and beta 55-Ser): Functions related to high-altitude respiration in geese. J. Appl. Physiol. 1993, 75, 2646–2655. [Google Scholar] [CrossRef]
- Zhu, X.; Guan, Y.; Signore, A.V.; Natarajan, C.; DuBay, S.G.; Cheng, Y.; Han, N.; Song, G.; Qu, Y.; Moriyama, H.; et al. Divergent and parallel routes of biochemical adaptation in high-altitude passerine birds from the Qinghai-Tibet Plateau. Proc. Natl. Acad. Sci. USA 2018, 115, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, C.; Inoguchi, N.; Weber, R.E.; Fago, A.; Moriyama, H.; Storz, J.F. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 2013, 340, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Runck, A.M.; Sabatino, S.J.; Kelly, J.K.; Ferrand, N.; Moriyama, H.; Weber, R.E.; Fago, A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 2009, 106, 14450–14455. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.V.; Storz, J.F. Biochemical pedomorphosis and genetic assimilation in the hypoxia adaptation of Tibetan antelope. Sci. Adv. 2020, 6, eabb5447. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Lu, S.; Niu, Z.; Zhang, T.; Zhao, Y.; Yang, X.; Zhao, Y.; Tang, X.; Chen, Q. Oxygenation properties and underlying molecular mechanisms of hemoglobins in plateau zokor (Eospalax baileyi). Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R696–R708. [Google Scholar] [CrossRef]
- Tufts, D.M.; Natarajan, C.; Revsbech, I.G.; Projecto-Garcia, J.; Hoffmann, F.G.; Weber, R.E.; Fago, A.; Moriyama, H.; Storz, J.F. Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol. Biol. Evol. 2014, 32, 287–298. [Google Scholar] [CrossRef]
- Weber, R.E.; Ostojic, H.; Fago, A.; Dewilde, S.; Hauwaert, M.-L.V.; Moens, L.; Monge, C. Novel mechanism for high-altitude adaptation in hemoglobin of the Andean frog Telmatobius peruvianus. Am. J. Physiol. Integr. Comp. Physiol. 2002, 283, R1052–R1060. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, T.; März, J.; Jürgens, K.D.; Braunitzer, G. Interaction of allosteric effectors with alpha-globin chains and high altitude respiration of mammals. The primary structure of two tylopoda hemoglobins with high oxygen affinity: Vicuna (Lama vicugna) and alpaca (Lama pacos). Biol. Chem. Hoppe-Seyler. 1986, 367, 153. [Google Scholar] [CrossRef]
- Piccinini, M.; Kleinschmidt, T.; Jürgens, K.D.; Braunitzer, G. Primary structure and oxygen-binding properties of the hemoglobin from guanaco (Lama guanacoë, Tylopoda). Biol. Chem. Hoppe-Seyler. 1990, 371, 641. [Google Scholar] [CrossRef]
- Weber, R.E.; Campbell, K.L. Temperature dependence of haemoglobin–oxygen affinity in heterothermic vertebrates: Mechanisms and biological significance. Acta Physiol. 2011, 202, 549–562. [Google Scholar] [CrossRef]
- Brix, O.; Bårdgard, A.; Mathisen, S.; Tyler, N.; Nuutinen, M.; Condo, S.G.; Giardina, B. Oxygen transport in the blood of arctic mammals: Adaptation to local heterothermia. J. Comp. Physiol. B 1990, 159, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Storz, J.F.; Fago, A. Bohr effect and temperature sensitivity of hemoglobins from highland and lowland deer mice. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 195, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Zhao, Y.; Niu, Z.; Cao, W.; Zhang, T.; He, J.; Wang, J.; Tang, X.; Chen, Q. Comparison of hematological traits and oxygenation properties of hemoglobins from highland and lowland Asiatic toad (Bufo gargarizans). J. Comp. Physiol. B 2021, 191, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Sun, S.; Jin, Y.; Yan, Y.; Liu, N. Molecular phylogeography of the Chinese lacertids of the genus Eremias (Lacertidae) based on 16S rRNA mitochondrial DNA sequences. Amphib.-Reptil. 2007, 28, 33–41. [Google Scholar] [CrossRef]
- Li, W.; Du, J.; Yang, L.; Liang, Q.; Yang, M.; Zhou, X.; Du, W. Chromosome-level genome assembly and population genomics of Mongolian racerunner (Eremias argus) provide insights into high-altitude adaptation in lizards. BMC Biol. 2023, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Pinho, C.; Pérez i de Lanuza, G.; Afonso, S.; Brejcha, J.; Rubin, C.-J.; Wallerman, O.; Pereira, P.; Sabatino, S.J.; Bellati, A.; et al. Regulatory changes in pterin and carotenoid genes underlie balanced color polymorphisms in the wall lizard. Proc. Natl. Acad. Sci. USA 2019, 116, 5633. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Hoffmann, F.G.; Opazo, J.C.; Sanger, T.J.; Moriyama, H. Developmental regulation of hemoglobin synthesis in the green anole lizard Anolis carolinensis. J. Exp. Biol. 2011, 214, 575–581. [Google Scholar] [CrossRef]
- Alföldi, J.; Di Palma, F.; Grabherr, M.; Williams, C.; Kong, L.; Mauceli, E.; Russell, P.; Lowe, C.B.; Glor, R.E.; Jaffe, J.D.; et al. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 2011, 477, 587–591. [Google Scholar] [CrossRef]
- Lu, S. The Adaptive Mechanism of Globin Family to High Altitude Hypoxia in Phrynocephalus Lizards. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Hoffmann, F.G.; Storz, J.F. The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol. Biol. Evol. 2007, 24, 1982–1990. [Google Scholar] [CrossRef]
- Hoffmann, F.G.; Storz, J.F.; Gorr, T.A.; Opazo, J.C. Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol. Biol. Evol. 2010, 27, 1126–1138. [Google Scholar] [CrossRef]
- Zhang, S.-P.; Feng, H.-Z.; Wang, Q.; Quan, S.-W.; Yu, X.-Q.; Tao, X.; Wang, Y.; Guo, D.-D.; Peng, L.; Feng, H.-Y.; et al. Proteomic analysis reveals the mechanism of different environmental stress-induced tolerance of Pseudomonas aeruginosa to monochloramine disinfection. J. Hazard. Mater. 2021, 417, 126082. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xin, Y.; Tang, X.; Yue, F.; Wang, H.; Bai, Y.; Niu, Y.; Chen, Q. Differences in hematological traits between high- and low-altitude lizards (Genus Phrynocephalus). PLoS ONE 2015, 10, e0125751. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J. Appl. Physiol. 1992, 72, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E. Cationic control of O2 affinity in lugworm erythrocruorin. Nature 1981, 292, 386–387. [Google Scholar] [CrossRef]
- Damsgaard, C.; Storz, J.F.; Hoffmann, F.G.; Fago, A. Hemoglobin isoform differentiation and allosteric regulation of oxygen binding in the turtle, Trachemys scripta. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R961–R967. [Google Scholar] [CrossRef]
- Tang, X.L.; Yue, F.; He, J.Z.; Wang, N.B.; Ma, M.; Mo, J.R.; Chen, Q. Ontogenetic and sexual differences of thermal biology and locomotor performance in a lacertid lizard, Eremias multiocellata. Zoology 2013, 116, 331. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, O.; Mould, R.; Brittain, T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem. J. 1995, 306, 367–370. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands; North-Holland Publishing Company: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781. [Google Scholar] [CrossRef]
- Hoffmann, F.G.; Vandewege, M.W.; Storz, J.F.; Opazo, J.C. Gene turnover and diversification of the α- and β-globin gene families in Sauropsid vertebrates. Genome Biol. Evol. 2018, 10, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, V.H.; Haines, H.B.; Engbretson, G. Aquatic life at high altitude: Respiratory adaptations in the Lake Titicaca frog, Telmatobius culeus. Respir. Physiol. 1976, 27, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, H.; Monge-C, C.; Cifuentes, V. Hemoglobin affinity for oxygen in three subspecies of toads (Bufo sp.) living at different altitudes. Biol. Res. 2000, 33, 5–10. [Google Scholar] [CrossRef]
- Zeng, Z.-G.; Bi, J.H.; Li, S.-R.; Wang, Y.; Robbins, T.R.; Chen, S.-Y.; Du, W.-G. Habitat alteration influences a desert steppe lizard community: Implications of species-specific preferences and performance. Herpetol. Monogr. 2016, 30, 34–48. [Google Scholar] [CrossRef]
- Bonilla, G.O.; Oyama, S.; Nagatomo, C.L.; Matsuura, M.S.A.; Focesi, A. Interactions of adenosine triphosphate with snake hemoglobins. Studies in Liophis miliaris, Boa constrictor and Bothrops alternatus. Comp. Biochem. Physiol. Part B Comp. Biochem. 1994, 109, 701–707. [Google Scholar] [CrossRef]
- Lombardi, F.R.; Anazetti, M.C.; Santos, G.C.; Olivieri, J.R.; de Azevedo, W.F., Jr.; Bonilla-Rodriguez, G.O. Rattlesnake hemoglobins: Functional properties and tetrameric stability. Protein Pept. Lett. 2006, 13, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Anazetti, M.; Santos, G.; Polizelli, P.; Olivieri, J.; De Azevedo Jr, W.; Bonilla-Rodriguez, G. Oxygen binding properties and tetrameric stability of hemoglobins from the snakes Crotalus durissus terrificus and Liophis miliaris. In Frontiers in Protein and Peptide Sciences; Bentham Science: Beijing, China, 2014; pp. 3–30. [Google Scholar]
- Storz, J.F.; Natarajan, C.; Moriyama, H.; Hoffmann, F.G.; Wang, T.; Fago, A.; Malte, H.; Overgaard, J.; Weber, R.E. Oxygenation properties and isoform diversity of snake hemoglobins. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R1178–R1191. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tong, H.; Zhang, K. The Impact of phenotypic characteristics on thermoregulation in a cold-climate Agamid lizard, Phrynocephalus guinanensis. Asian Herpetol. Res. 2016, 7, 210–219. [Google Scholar]
- Antonini, E.; Wyman, J.; Brunori, M.; Fronticelli, C.; Bucci, E.; Rossi-Fanelli, A. Studies on the relations between molecular and functional properties of hemoglobin: V. The influence of temperature on the Bohr effect in human and in horse hemoglobin. J. Biol. Chem. 1965, 240, 1096–1103. [Google Scholar] [CrossRef]
- Weber, R.E.; Fago, A.; Campbell, K.L. Enthalpic partitioning of the reduced temperature sensitivity of O2 binding in bovine hemoglobin. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 176, 20–25. [Google Scholar] [CrossRef]
- Weber, R.E. Enthalpic consequences of reduced chloride binding in Andean frog (Telmatobius peruvianus) hemoglobin. J. Comp. Physiol. B 2014, 184, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Pu, P.; Niu, Z.; Zhang, T.; Wu, J.; Tang, X.; Chen, Q. A novel mechanism for high-altitude adaptation in hemoglobin of black-spotted frog (Pelophylax nigromaculatus). Front. Ecol. Evol. 2023, 11, 1103406. [Google Scholar] [CrossRef]
- Opazo, J.C.; Hoffmann, F.G.; Natarajan, C.; Witt, C.C.; Berenbrink, M.; Storz, J.F. Gene turnover in the avian globin gene families and evolutionary changes in hemoglobin isoform expression. Mol. Biol. Evol. 2015, 32, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Runck, A.M.; Moriyama, H.; Weber, R.E.; Fago, A. Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 2010, 213, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Cheviron, Z.A.; Natarajan, C.; Projecto-Garcia, J.; Eddy, D.K.; Jones, J.; Carling, M.D.; Witt, C.C.; Moriyama, H.; Weber, R.E.; Fago, A. Integrating evolutionary and functional tests of adaptive hypotheses: A case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Mol. Biol. Evol. 2014, 31, 2948–2962. [Google Scholar] [CrossRef] [PubMed]
- Grispo, M.T.; Natarajan, C.; Projecto-Garcia, J.; Moriyama, H.; Weber, R.E.; Storz, J.F. Gene Duplication and the Evolution of Hemoglobin Isoform Differentiation in Birds. J. Biol. Chem. 2012, 287, 37647–37658. [Google Scholar] [CrossRef] [PubMed]
- Hiebl, I.; Weber, R.E.; Schneeganss, D.; Braunitzer, G. High-altitude respiration of Falconiformes. The primary structures and functional properties of the major and minor hemoglobin components of the adult White-headed vulture (Trigonoceps occipitalis, Aegypiinae). Biol. Chem. 1989, 370, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hiebl, I.; Weber, R.E.; Schneeganss, D.; KÖSTERS, J.; Braunitzer, G. High-Altitude Respiration of Birds. Structural Adaptations in the Major and Minor Hemoglobin Components of adult Rüppell’s Griffon (Gyps rueppellii, Aegypiinae): A New Molecular Pattern for Hypoxic Tolerance. Biol. Chem. 1988, 369, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Waheed, H.; Doman, S.K.; Ahmed, A. The partial amino acid sequence of leaf-nosed vper (Eristicophis macmahonii) snake hemoglobin. J. Anim. Plant Sci. 2018, 28, 1622–1628. [Google Scholar]
- Clementi, M.; De Rosa, M.; Bertonati, C.; Capo, C.; Cataldi, E.; Petruzzelli, R.; Giardina, B. Functional and structural properties of the hemoglobin components from Italian sturgeon (Acipenser naccarii). Fish Physiol. Biochem. 2001, 24, 191–200. [Google Scholar] [CrossRef]
- Barra, D.; Bossa, F.; Brunori, M. Structure of binding sites for heterotropic effectors in fish haemoglobins. Nature 1981, 293, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Riggs, A.F. The Bohr Effect. Annu. Rev. Physiol. 1988, 50, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.; Li, Q.; Lian, J.; O’Meally, D.; Deakin, J.; Wang, Z.; Zhang, P.; Fujita, M.; Patel, H.R.; Holleley, C.E.; et al. High-coverage sequencing and annotated assembly of the genome of the Australian dragon lizard Pogona vitticeps. GigaScience 2015, 4, 45. [Google Scholar] [CrossRef] [PubMed]



| Cofactor Sensitivities | E. argus-H | E. argus-L | E. multiocellata-H | E. multiocellata-L | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | ||||||||
| pH | 7.80 | 7.40 | 7.80 | 7.40 | 7.80 | 7.40 | 7.80 | 7.40 | 7.80 | 7.40 | 7.80 | 7.40 | 7.80 | 7.40 | 7.80 | 7.40 |
| ∆logP50 | ||||||||||||||||
| KCl-stripped | 0.20 | 0.17 | 0.08 | 0.10 | 0.13 | 0.11 | 0.05 | 0.08 | 0.10 | 0.09 | 0.06 | 0.04 | 0.11 | 0.09 | 0.09 | 0.07 |
| ATP-stripped | 0.53 | 0.62 | 0.44 | 0.50 | 0.42 | 0.51 | 0.37 | 0.46 | 0.31 | 0.34 | 0.28 | 0.33 | 0.30 | 0.31 | 0.31 | 0.32 |
| (KCl + ATP)-stripped | 0.52 | 0.60 | 0.45 | 0.51 | 0.41 | 0.49 | 0.38 | 0.47 | 0.39 | 0.40 | 0.33 | 0.34 | 0.36 | 0.37 | 0.36 | 0.37 |
| E. argus-H | E. argus-L | E. multiocellata-H | E. multiocellata-L | |||||
|---|---|---|---|---|---|---|---|---|
| Temperature | 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C | 25 °C | 37 °C |
| Bohr factor (Φ) | ||||||||
| Stripped | −0.25 | −0.13 | −0.20 | −0.08 | −0.23 | −0.17 | −0.23 | −0.16 |
| +KCl | −0.17 | −0.18 | −0.15 | −0.17 | −0.21 | −0.13 | −0.18 | −0.12 |
| +ATP | −0.46 | −0.30 | −0.42 | −0.30 | −0.31 | −0.29 | −0.28 | −0.19 |
| +KCl+ATP | −0.45 | −0.30 | −0.41 | −0.30 | −0.27 | −0.18 | −0.26 | −0.19 |
| E. argus-H | E. argus-L | E. multiocellata-H | E. multiocellata-L | |||||
|---|---|---|---|---|---|---|---|---|
| pH | 7.8 | 7.4 | 7.8 | 7.4 | 7.8 | 7.4 | 7.8 | 7.4 |
| ΔH (kJ/mol O2) | ||||||||
| Stripped | −25.74 | −19.02 | −16.06 | −9.21 | −17.27 | −13.63 | −10.64 | −6.53 |
| +KCl | −8.22 | −9.18 | −3.58 | −4.79 | −11.47 | −6.67 | −6.97 | −2.96 |
| +ATP | −11.64 | −2.14 | −9.13 | −1.96 | −12.97 | −11.65 | −13.02 | −8.11 |
| +KCl+ATP | −14.52 | −5.66 | −11.70 | −5.57 | −9.41 | −4.12 | −10.33 | −5.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, P.; Niu, Z.; Ma, M.; Tang, X.; Chen, Q. Convergent High O2 Affinity but Distinct ATP-Mediated Allosteric Regulation of Hemoglobins in Oviparous and Viviparous Eremias Lizards from the Qinghai-Tibet Plateau. Animals 2024, 14, 1440. https://doi.org/10.3390/ani14101440
Pu P, Niu Z, Ma M, Tang X, Chen Q. Convergent High O2 Affinity but Distinct ATP-Mediated Allosteric Regulation of Hemoglobins in Oviparous and Viviparous Eremias Lizards from the Qinghai-Tibet Plateau. Animals. 2024; 14(10):1440. https://doi.org/10.3390/ani14101440
Chicago/Turabian StylePu, Peng, Zhiyi Niu, Ming Ma, Xiaolong Tang, and Qiang Chen. 2024. "Convergent High O2 Affinity but Distinct ATP-Mediated Allosteric Regulation of Hemoglobins in Oviparous and Viviparous Eremias Lizards from the Qinghai-Tibet Plateau" Animals 14, no. 10: 1440. https://doi.org/10.3390/ani14101440
APA StylePu, P., Niu, Z., Ma, M., Tang, X., & Chen, Q. (2024). Convergent High O2 Affinity but Distinct ATP-Mediated Allosteric Regulation of Hemoglobins in Oviparous and Viviparous Eremias Lizards from the Qinghai-Tibet Plateau. Animals, 14(10), 1440. https://doi.org/10.3390/ani14101440






