Simple Summary
This study investigates the functional adaptation and underlying molecular mechanisms of hemoglobins (Hbs) in the two species of Eremias lizards dwelling on the Qinghai-Tibet Plateau. By measuring O2 equilibrium curves of purified Hbs at different pH and temperature in the absence and presence of ATP and/or Cl−, the study found that the high-altitude populations of the two species of Eremias lizards exhibit convergent high Hb-O2 affinity compared to the respective lowland counterparts while demonstrating distinct ATP-mediated allosteric regulation. Hbs of the highland E. argus showed high ATP sensitivity and ATP-dependent strong Bohr effect compared to E. multiocellata. The underlying mechanisms of these functional variations may be attributed to the varying β2/β1 globin ratios, combined with substitutions on the β2-type globin, as suggested by Hb isoform identification and structural analysis of tetrameric Hbs. In addition, Hbs of these Eremias lizards have similarly low temperature sensitivities and relatively high Bohr effects at lower temperatures, which could minimize the impact of temperature fluctuations on Hb-O2 affinity and facilitate the release of O2 in the cold extremities at low temperatures.
Abstract
The functional adaptation and underlying molecular mechanisms of hemoglobins (Hbs) have primarily concentrated on mammals and birds, with few reports on reptiles. This study aimed to investigate the convergent and species-specific high-altitude adaptation mechanisms of Hbs in two Eremias lizards from the Qinghai-Tibet Plateau. The Hbs of high-altitude E. argus and E. multiocellata were characterized by significantly high overall and intrinsic Hb-O2 affinity compared to their low-altitude populations. Despite the similarly low Cl− sensitivities, the Hbs of high-altitude E. argus exhibited higher ATP sensitivity and ATP-dependent Bohr effects than that of E. multiocellata, which could facilitate O2 unloading in respiring tissues. Eremias lizards Hbs exhibited similarly low temperature sensitivities and relatively high Bohr effects at lower temperatures, which could help to stably deliver and release O2 to cold extremities at low temperatures. The oxygenation properties of Hbs in high-altitude populations might be attributed to varying ratios of β2/β1 globin and substitutions on the β2-type globin. Notably, the Asn12Ala in lowland E. argus could cause localized destabilization of the E-helix in the tetrameric Hb by elimination of hydrogen bonds, thereby resulting in its lowest O2 affinity. This study provides a valuable reference for the high-altitude adaptation mechanisms of hemoglobins in reptiles.
1. Introduction
High-altitude environments of the plateau provide an ideal laboratory for studying the adaptation mechanisms of animals to hypobaric hypoxia and low ambient temperature. The world’s highest-dwelling mammal is reported to be a specimen of the yellow-rumped leaf-eared mouse (Phyllotis xanthopygus rupestris), which was captured at the summit of Llullaillaco at an altitude of 6739 m [1]. The ability to regulate O2 tensions in systemic blood and respiring tissues was weaker for reptiles and amphibians because of the incomplete separation of systemic and pulmonary circulation [2]. However, the natural habitats of reptiles can extend to extremely high altitudes. The highest-altitude reptile in the world (Liolaemus aff. tacnae) was recently recorded at 5400 m elevation in Peru [3]. The red-tailed toad-head lizard (Phrynocephalus erythrurus) native to the Qinghai-Tibet Plateau (QTP) inhabits between 4500 and 5300 m in elevation [4]. The fact that high-altitude reptiles can survive and prosper at extremely high elevations indicates that they have evolved unique mechanisms to adapt to severe hypoxia and low temperatures.
Air-breathing vertebrates well adapted to high-altitude environments can adjust their respiratory and cardiovascular systems to maintain an adequate O2 supply to respiring tissues [5,6,7]. High Hb-O2 affinity could maximize O2 extraction from the lungs under hypoxia for animals that are endemic to plateaus. On the other hand, the efficiency of O2 unloading from Hb into aerobic tissues mainly depends on the sensitivity of Hb to allosteric effectors (Cl−, H+, CO2, and organic phosphates). The main organic phosphates are 2,3-diphosphoglycerate (DPG), inositol pentaphosphate (IP5), and adenosine triphosphate (ATP) in the erythrocytes of mammals, birds, and reptiles, respectively. Amphibian erythrocytes contain both DPG and ATP as allosteric effectors. Numerous studies have shown that high-altitude mammals and birds generally have a significantly higher Hb-O2 affinity compared to their low-altitude relatives or populations, such as the bar-headed goose [8,9,10,11], high-altitude passerine birds [12], high-altitude deer mice [13,14], Tibetan antelope [15], and plateau zokor (Eospalax baileyi) [16]. The high Hb-O2 affinities are the consequence of high intrinsic Hb-O2 affinity and/or reduced allosteric effector sensitivity. The underlying molecular mechanisms of high intrinsic Hb-O2 affinity are mainly attributed to the structural effects of specific amino acid substitutions. As found in the Hbs of bar-head geese and plateau zokors, substitutions located at the (αβ)2 interface could eliminate the T-state-stabilizing hydrogen connection on the interface between (α1β1/α2β2) or inside (α1/β1 and α2/β2) the two semirigid dimers, facilitating the conformational transition from the T-state to the R-state during Hb oxygenation and resulting in an increase in the intrinsic Hb-O2 affinity [10,11,16]. Amino acid substitutions located on the β chain heme pockets could fine-tune conformation of the pockets and subsequently reduce the steric hindrance of O2 binding, which could be another mechanism of the high intrinsic Hb-O2 affinity [14,16,17]. In addition, reduced allosteric effector sensitivity could also lead to high Hb-O2 affinity due to substitutions at binding sites of allosteric effectors, as exemplified by the Andean frog (Telmatobius peruvianus) and Andean llamas [18,19,20]. It has been reported that evolutionary adjustments in Hb function are also attributed to epistatic interactions between different substitutions [13]. Most of these studies focused on mammals, birds, and a few amphibians. However, little attention has been given to the high-altitude adaptation of Hb function and structure in reptiles despite their extremely high distribution, lack of physiological thermoregulation, and incomplete circulatory system.
The O2 affinity of Hb decreased with increasing temperature due to the exothermic nature of Hb oxygenation. A substantial temperature effect impairs O2 delivery to cold limbs and extremities in mammals living in cold environments [21]. Several polar and alpine mammals have been shown to possess Hbs with low temperature sensitivity [16,21,22,23]. Regional heterothermy may not exist both in ectothermic reptiles and amphibians due to a lack of physiological thermoregulatory capacity. However, our previous study revealed that Hbs of both high- and low-altitude Asiatic toads (Bufo gargarizans) exhibit low temperature sensitivity, which could help to reduce the temperature-induced fluctuations in Hb-O2 affinity [24]. Whether the same is true in alpine reptiles remains to be investigated.
Eremias argus (oviparous) and Eremias multiocellata (viviparous) are two of the eight Eremias (Family Lacertidae) species in China [4]. A molecular phylogeographical study based on 16S rRNA mitochondrial DNA revealed that E. argus clustered with E. brenchleyi on a monophyletic clade as the sister group of E. multiocellata [25]. The altitude distribution of the two Eremias lizards ranges from 0 to 3500 m in a broad dimension from the Eastern Plain to the QTP in China [4]. This provides a valuable opportunity to study the high-altitude adaptation of the two closely related lizards by comparing their high- and low-altitude populations. Recent comparative genomics analyses revealed that E. argus populations endemic to high altitudes possess many novel genomic regions under strong selective sweeps, and genes embedded in those regions are mainly associated with energy metabolism and DNA damage repair pathways [26]. However, high-altitude adaptation in the oxygen transport system has not been studied in the two Eremias lizards dwelling on the QTP.
The present study aimed to characterize the Hb isoform composition in erythrocytes, the oxygenation properties of Hbs, and the underlying genetic basis in high-altitude E. argus and E. multiocellata living on the QTP. We purified Hbs from hemolysates of high- and low-altitude E. argus and E. multiocellata, and measured intrinsic Hb-O2 affinity and their sensitivity to allosteric effectors (H+, Cl− and/or ATP) and temperature. We also analyzed Hb isoform diversity and sequenced the coding DNA sequence data for the full complement of α- and β-type globin genes from E. argus and E. multiocellata to elucidate the mechanism underlying the observed functional properties.
2. Materials and Methods
2.1. Ethics Statement
All experiments were carried out according to the principles from the China Council on Animal Care, and approved by the Ethics Committee of School of Life Sciences, Lanzhou University (protocol code EAF2021026 and date of approval: 1 April 2021). Every effort was made to minimize the numbers used and any suffering experienced by the animals in the experiment.
2.2. Sample Collection
High-altitude E. argus (n = 6, average of 3.71 g) were collected at Shazhuyu township (36°17′06″ N, 100°35′56″ E, 2860 m), Qinghai Province, China. Low-altitude E. argus (n = 6, average of 3.76 g) were collected at Xingtai city (36°58′47″ N, 114°26′28″ E, 89 m), Hebei Province, China. High-altitude E. multiocellata (n = 6, average of 3.93 g) were collected at Tianzhu Tibetan Autonomous County (37°18′16″ N, 103°10′30″ E, 2837 m), Gansu Province, China. Low-altitude E. multiocellata (n = 6, average of 4.56 g) were collected at Lanzhou City (36°18′43″ N, 103°51′44″ E, 1728 m), Gansu Province, China. All lizards are adult males and were collected in May or August 2019 before or after the breeding season. The geographical location and climate data of the four sampling sites are shown in Figure S1. The climate data for the years from 1981 to 2010 were obtained from the Chinese Climatic Data Centre (http://data.cma.cn, accessed on 5 August 2021).
Blood samples were drawn from the aortic arch directly using a heparinized glass capillary tube after the lizards were anesthetized with ether. Approximately 20–30 µL of blood sample was obtained from each lizard and then centrifuged at 4 °C (3000× g, 10 min) to obtain packed red blood cells. Liver samples were also harvested for cDNA cloning and sequencing of globins. All the samples were flash-frozen with liquid nitrogen and stored at −80 °C for subsequent experiments. Every effort was made to minimize the number of animals used and any suffering experienced by the animals during the experiments.
2.3. RT-PCR and Sequencing of α and β Globins
To investigate the genetic variation of hemoglobin in high- and low-altitude E. argus and E. multiocellata, we cloned and sequenced the adult-expressed α- and β-type globin genes from lizards. Total RNA was extracted from liver samples (40–100 mg) using RNAiso Plus reagent (Takara, Dalian, China). Then, we eliminated residual genomic DNA and amplified full-length cDNAs of adult-expressed genes using a PrimeScriptTM RT reagent kit with gDNA Eraser (Takara, Dalian, China). We designed paralog-specific primers (Table S1) using 5′ and 3′ sequences according to the annotated globin genes in the genome assemblies of the common wall lizard (Podarcis muralis) [27]. We cloned all target globin genes with the reverse-transcription (RT)-cDNA as the template using 2× Accurate Taq Master Mix (dye plus) (Accurate Biotechnology Co., Changsha, China). The target products were then connected with the pMDTM18-T vector (Takara, Dalian, China) and further transformed into DH5α-competent cells (Takara, Dalian, China). At least three positive clones per gene were sequenced (Sangon, Shanghai, China) to ensure the accuracy of the sequencing results. All new sequences have been submitted to the GenBank database under accession OL804548-OL804565.
2.4. Sequence Alignment and Phylogenetic Analysis
Nucleotide sequences of the globin genes were conceptually translated into amino acid sequences using MEGA 11 [28]. We compared our newly generated sequence data with the adult-expressed α- and β-type globin genes of 12 species of lizards from seven families of Sauria. The globin sequences of the remaining species were obtained from public databases or annotated from genome assemblies (Table S2).
It needs to be pointed out that the names of β1 and β2 in anole were inaccurate in the previous study [29]. We aligned the βII in their study to the nr database with the BLASTP of NCBI and found that βII was completely consistent with the β1 annotated in the anole genome [30]. The re-annotation of the anole genome by Lu S. (2017) also confirmed that βI and βII should be β2 and β1, respectively [31]. In addition, the β1- and β2-type globins of Lacerta agilis also had the same naming problem as anole. BLASTP results found that its β1 was most similar to β2 of P. muralis, while its β2 was most similar to β1 of Z. vivipara and P. muralis. Therefore, we renamed the β1 and β2 of the anole and L. agilis in this study based on the BLASTP results.
It has been revealed that the αE-, αD-, and αA-globin genes diverged through duplication events before the radiation of tetrapods [29,32,33]. Thus, the homologous sequences from human (Homo sapiens) and chicken (Gallus gallus) were included for alignment of amino acid sequences and reconstruction of the phylogenetic relationships of α and β globin of Eremias lizard. The amino acid sequences of α- and β-type globins were aligned using muscle implementation in MEGA 11. Subsequently, the maximum likelihood phylogenies for α-type and β-type were estimated using LG + G + I and LG + G models of amino acid substitution with five different site categories, respectively. The support rate of branch nodes was evaluated by 1000 bootstrap pseudoreplicates.
2.5. Hb Purification and Isoform Identification
The frozen red blood cells of six individuals from each population were incubated on ice for 20 min after adding a 5-fold volume of ice-cold 10 mmol/L of Hepes buffer (pH7.8, 0.5 mmol/L of EDTA). Then, hemolysates were centrifuged (9000× g, 10 min at 4 °C) to remove membranes and cellular debris, and the supernatants from the six individuals were pooled together for further purification. The ÄKTA Pure chromatography system (GE Healthcare, Chicago, IL, USA) and IexCap Q 6FF 5 mL column (Smart-lifesciences, Changzhou, China) were used to remove miscellaneous proteins and endogenous organic phosphates in supernatants. Before and after the injection of the supernatants, the anion exchange column was equilibrated with 20 mmol/L of Tris·HCl (pH8.7, 0.5 mmol/L of EDTA) and then eluted with a linear gradient of 0–400 mmol/L of NaCl at 1 mL/min flow rate to obtain mixtures of hemoglobin isoforms (Hbs). The mixtures were desalted by dialyzing against three changes of a 200-fold volume of 10 mmol/L of Hepes buffer (pH7.6, 0.5 mmol/L of EDTA) at 4 °C. The Hb isoform (isoHb) compositions were verified using isoelectric focusing (IEF) on polyacrylamide gels in the pH range of 3–10 (DYCP-37B, Liuyi Biotechnology, Beijing, China). The final purified Hbs were concentrated to ~1.3 mmol/L of heme by ultrafiltration using Amicon® Ultra-4 Centrifugal Filter Units fitted with a 10 kDa cutoff filter (Millipore, Nantong, China) and then stored at −80 °C in aliquots.
To identify whether the sequenced globins were all expressed in hemolysates, Hb subunits of native hemolysates were separated on a 15% SDS-PAGE gel, digested with trypsin and determined by liquid chromatography–tandem mass spectrometry (LC–MS/MS) using Orbitrap Fusion Lumos MS (Thermo Fisher Scientific, Waltham, MA, USA) coupled online to an EASY-nLC 1200 system in a data-dependent mode (DDA) according to the previously described method [34]. The Hb isoform compositions of erythrocytes from Eremias lizards were further measured using RP-HPLC (Bio-Bond C4 column, 5 μm, 250 × 4.6 mm, DIKMA, Beijing, China) and an ultrahigh-resolution time-of-flight mass spectrometer (MaXis 4G, Bruker-Daltonics, Billerica, MA, USA) using the methods and parameters described by Lu et al. [35].
2.6. Measuring O2 Equilibrium Curves
O2 equilibrium curves of the purified Hbs were measured using a homemade modified diffusion chamber as previously described [11,36,37,38]. Purified Hbs (0.3 mmol/L of heme) were diluted in 0.1 mol/L of Hepes buffers in the absence (stripped) and presence of Cl− (added as 0.1 mol/L of KCl) and/or ATP (7.5 mmol/L, 100-fold molar excess over tetrameric Hbs). Absorbance at 436 nm of the working Hbs solutions (≈4 µL) was continually monitored under the 100% humidified gas mixture, in which O2 tension (, Torr) was stepwise increased by mixing ultrapure N2 and air. A0 and A100 are the absorbances at zero and full O2 saturation equilibrated with ultrapure N2 and atmospheric air, respectively. Fractional saturation () under the corresponding was calculated by = (A − A0)/(A100 − A0). O2 equilibrium curves were measured in 0.1 mol/L of pH7.4 and 7.8 Hepes buffers at 25 °C and 37 °C under different allosteric conditions (stripped, Cl− and/or ATP present) to calculate the Bohr effect, enthalpy of oxygenation, and anionic cofactor sensitivities. The experimental temperature was based on the ranges of body temperatures (24.1–37.2 °C) at which adult lizards maintained 80% of the maximum sprint speed for E. multiocellata [39].
At least six technical repeats were performed for each experimental condition. The pH of the working Hbs solutions used for O2 equilibrium experiments was adjusted with NaOH to as close to 7.4 and 7.8 as possible and then precisely measured with an InLab micro pH electrode equipped with a SevenCompact pH/Ion Meter S220 and an ATC temperature probe (Mettler Toledo, Greifensee, Switzerland) after the samples were brought to the same temperature as that used in the experiments. To further assess the effect of allosteric binding of ATP on the Hb-O2 affinity of lizard Hbs, we measured O2 equilibrium curves with ATP concentrations in the range of 0–7.5 mmol/L in the absence of KCl at 0.3 mmol/L [heme] at 37 °C in 0.1 mol/L of Hepes buffer (pH7.4).
The dose–response curves of ATP on P50 were generated by fitting a hyperbolic function to the data, and the apparent affinity constant was calculated as the ATP concentration at which the increase in logP50 was half the maximum [40].
P50 is the at half-saturation, while n50 is the Hill cooperativity coefficient. We fitted the Hill’s sigmoidal equation to 5–10 saturation data (O2 saturation range ∼0.1–0.9) to estimate the values of P50 and n50 for each condition.
Nonlinear sigmoidal Hill fitting (r2 > 0.99) was performed on GraphPad Prism 8 (San Diego, CA, USA). The Bohr factor (Φ) was used to quantify the Bohr effect and was calculated as the slope of linear plots of logP50 as a function of pH in the range of pH7.4–7.8 (Φ = ΔlogP50/ΔpH). The overall change in enthalpy of oxygenation (ΔH, kJ/mol O2), which is the heat liberated upon oxygenation, was calculated by the van’t Hoff equation ΔH = 2.303R(ΔlogP50)/Δ(1/T) and used to indicate the temperature sensitivity of Hbs. R is the gas constant (8.314 J/K/mol O2), and T is the absolute temperature in Kelvin. The final ΔH values were calibrated by excluding the solution heat of O2 (ΔHsol ≈ −12.6 kJ/mol O2) [41].
2.7. Homology Modeling and Molecular Dynamics Simulation of Tetrameric Hb
According to our sequencing results, αA, αD, and β1 globins of high- and low-altitude populations are the same or only different at one site, and β2 globins of the two high-altitude populations are the same. The amino acid substitutions of Hb globins between high- and low-altitude populations mainly occurred on the β2-type globin for both E. argus and E. multiocellata. So, we first constructed the tetramer model (T-state) composed of β2 globin with all α-type globins (αAβ2-AB2, αD2β1-D2B1, αD2β2-D2B2) using Modeller 10.1 software [42]. The crystal structure of Turkey (Meleagiris gallopova) deoxyhemoglobin at 2.3 Å (3K8B.pdb) was selected as the template for homology modeling because of its highest percent identity (69.18%) with Eremias β2 globin. Molecular dynamics simulations were further performed on the Hb models to explore the quaternary structural properties of deoxyhemoglobin under simulated physiological conditions according to the previous method using NAMD 2.15 software [35,43]. The output dcd trajectory files of 10.5 ns molecular dynamics simulations were finally analyzed using VMD 1.9.4 software and its Plugs to explore the structural properties of different Hb models at the last 4 ns.
2.8. Statistical Analyses
Curve fitting and statistical analysis were performed using GraphPad Prism 8.4.3 (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics 26, respectively. A schematic diagram of the tetrameric Hb spatial structure was drawn via VMD. The normality and homogeneity of the P50 values were checked, and then the one-way ANOVA and independent sample t-test were used to detect significant differences (p < 0.05) between populations of the same and different species, respectively. The significant differences between the stripped state and conditions for adding KCl and/or ATP under the same temperature and pH were analyzed using the LSD (equal variances) and Games–Howell (unequal variance) method of post hoc multiple comparisons of one-way ANOVA. P50 and n50 values are presented as mean ± SE.
3. Results
3.1. Sequence Variation, Phylogenetic Relationships, and isoHb Compositions
Amino acid sequence alignment revealed that E. argus and E. multiocellata typically possess a full repertoire of αA-, αD-, β1-, and β2-type globins similar to most phylogenetically related lizards (Figure 1). The phylogenetic relationships based on the amino acid sequences showed that the αA- and αD-type globins of lizards are orthologous and sister to the αE-globin clade of additional amniote outgroup taxa (Figure 2). The phylogenetic analysis of β-type globins indicated that the β1- and β2-type globins of Sauria lizards were products of a duplication event that occurred before the divergence of Sauria lizards, as the β1 globins of all lizards are sister to the β2 globin of all lizards (Figure 2). The αA-, αD-, β1-, and β2-type globins of Lacertidae lizards (Eremias, L. Agilis, Z. vivipara, and P. muralis) are clustered on a clade, and all globins of Eremias are sister to those of L. Agilis (Figure 2). Surprisingly, high- and low-altitude E. multiocellata additionally expressed a distinct αD-type globin (αD2), which grouped in the same clade with αD1 (Figure 2). The second position of the N-terminus in E. multiocellata αD2-globin is Met compared with the αD of other species. As shown in Figure 1, the amino acid sequences of the αA globin are the same in E. argus and E. multiocellata; one substitution was observed in the αD1 globin between high- and low-altitude E. argus (Met73Leu) and in the β1 globin between high- and low-altitude E. multiocellata (Gln58Pro). The β1 globin of E. argus is heterozygous at site 56 (Ala/Ser). The amino acid sequences of the β2 globin are the same in the high-altitude E. argus and E. multiocellata (Figure 1), and four substitutions occurred on the β2 globin of the low-altitude E. multiocellata (Leu11Ile, Gly16Ser, Gly43Ala, Leu71Phe); seven substitutions occurred on the β2 globin of the low-altitude E. argus (Thr4Ser, Ala12Asn, Met20Ile, Thr25Gly, Ser27Thr, Ile54Val, Gly83Ala).
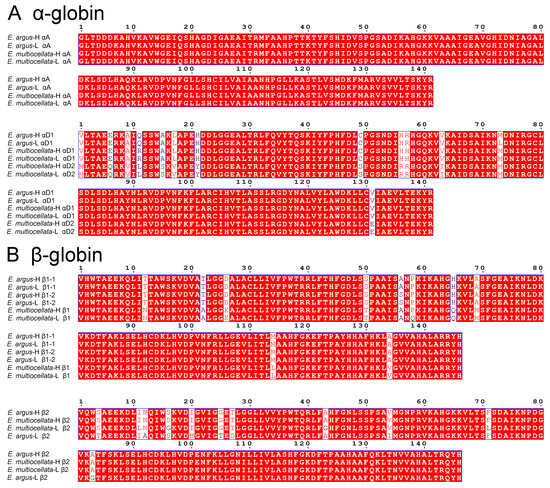
Figure 1.
Alignment of amino acid sequences for the adult-expressed α- (A) and β-type globin genes (B) from high- and low-altitude E. argus and E. multiocellata performed on the ESPript 3.0 online tool.
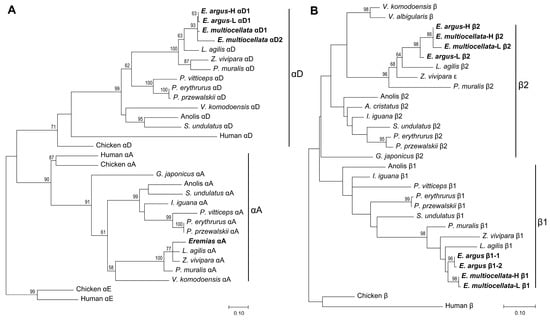
Figure 2.
Maximum likelihood phylogenies of α- (A) and β-type globins (B) of Sauria lizards constructed by amino acid sequences, and the full set of adult-expressed α- and β-type globins from E. argus and E. multiocellata are highlighted with bold font. Bootstrap percentages are shown on relevant nodes to indicate the support values.
There are three major Hb isoforms with isoelectric points approximately equal to pH8.0, pH7.8, and 7.5 in the purified Hbs mixture of both E. argus and E. multiocellata (Figure S2). The results from the Orbitrap Fusion Lumos MS confirmed that all the sequenced α- and β-type globins were expressed in the hemolysates of the four populations of Eremias lizards. The results from RP-HPLC and ultrahigh-resolution time-of-flight mass spectrometer showed approximately equal amounts but quite different shapes of globin peaks in the two species of Eremias lizards (Figure 3). However, only αA, β1, and β2 globins were verified by similar molecular weights (MWs) to the theoretical values in these populations (Table S3 and Figure 3). Conversely, αD globin was not detected in any of the populations, as no peaks with MWs between 15,972.32 Da and 15,999.37 Da were detected.
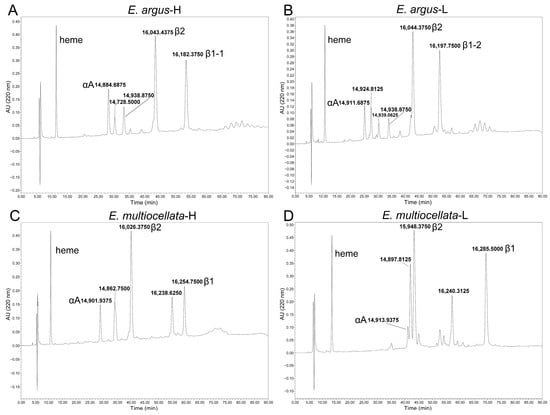
Figure 3.
RP-HPLC chromatograms for erythrocyte hemolysates of Eremias lizards. Five and six globin chain peaks of high-altitude (A) and low-altitude E. argus (B) were eluted from the C4 RP-HPLC column, respectively. Five globin chain peaks were eluted for both high-altitude (C) and low-altitude E. multiocellata (D). The corresponding molecular weight and the inferred globin based on the molecular weight are shown beside the peaks.
αA globins were the first to be eluted, β1 globins were the last to be eluted in all studied populations, and β2 globins were the penultimate globins eluted in two populations of E. argus, while the antepenultimate globins were eluted in two populations of E. multiocellata (Figure 3). The ratio of β2/β1 globin was the highest in high-altitude E. multiocellata (~2.25), the same in two populations of E. argus (~1.38), and the lowest in low-altitude E. multiocellata (~1.23) based on the analysis of the HPLC peak using ImageJ 1.54g software. However, there are additional peaks whose MWs did not match our sequenced globins. Two peaks with MW values of 14,728.5000 Da and 14,938.8750 Da, and three peaks with MW values of 14,924.8425 Da, 14,939.0625 Da, and 14,938.8750 Da, were identified in high-altitude and low-altitude E. argus, respectively. Two distinct peaks, with similar interpopulation elution times and MW values, were identified in high-altitude (MW = 14,862.7500 Da, 16,238.6250 Da) and low-altitude (MW = 14,897.8125 Da, 16,240.3125 Da) E. multiocellata.
3.2. Convergent High Hb-O2 Affinity but Distinct ATP-Mediated Allosteric Regulation of Hbs in Eremias Lizards
For both E. argus and E. multiocellata, the O2 equilibrium curves were left-shifted, and the P50 values were significantly lower in the Hbs of the high-altitude population than in those of the low-altitude population under all experiment conditions (F1,11 = 32.76–1173.98 for comparisons among populations of the same species, p < 0.01, Figure 4 and Figure 5, Tables S4 and S5). The P50 values for the Hbs of the four populations significantly increased in the presence of KCl and/or ATP compared with those in the stripped state (F1,11 = 51.75–47,310.77, p < 0.05, Tables S4 and S5). In the stripped state, P50 values of high-altitude E. argus (2860 m) were the lowest. However, the interspecies differences in the P50 values of the two high-altitude populations in the stripped state and the presence of KCl were small, as were those of the two low-altitude populations (Figure 4). In the presence of ATP and ATP + Cl−, P50 values of E. multiocellata-H (2837 m), E. multiocellata-L (1728 m), and E. argus-L (89 m) were greatly decreased with the increase in elevation; however, high-altitude E. argus (2860 m) exhibited significantly higher P50 values compared to high- and low-altitude E. multiocellata (Figure 5). This is attributed to the anion allosteric effector sensitivity, which was expressed as the logP50 difference between adding Cl− and/or ATP and the stripped state (Table 1). The Hbs of high-altitude E. argus possessed the highest ATP and ATP + Cl− sensitivities, while these sensitivities were relatively low for high- and low-altitude E. multiocellata (Table 1). Furthermore, the ATP and ATP + Cl− sensitivities of Hbs increased with decreasing temperature and pH in E. argus but not in E. multiocellata. Hbs of the four populations exhibit dramatically lower Cl− sensitivities than ATP and ATP + Cl− sensitivities. O2 binding was cooperative under all conditions (n50 = 1.89–3.08; Tables S4 and S5), reflecting a normal allosteric T–R shift upon oxygenation.
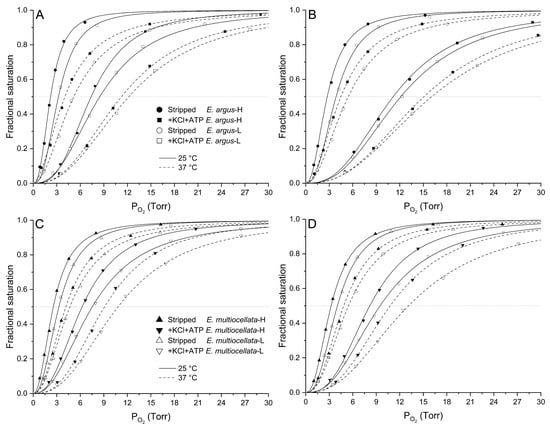
Figure 4.
O2 equilibrium curves of high- and low-altitude E. argus Hbs ((A): pH7.8, (B): pH7.4) and high- and low-altitude E. multiocellata Hbs ((C): pH7.8, (D): pH7.4) at 25 °C (continued lines) and 37 °C (dotted lines) in the absence (stripped) and presence of Cl− (added as 0.1 mol/L of KCl) and ATP (100-fold molar excess over tetrameric Hbs). In E. argus, the stripped status and presence of Cl− and ATP were denoted by circles and squares, respectively. In E. multiocellata, the stripped status and presence of Cl− and ATP were indicated by triangles and upside-down triangles. The high- and low-altitude populations are represented by solid and open symbols, respectively. n = 6 lizards for each population, and 6 technical repeats were performed for each experimental condition.
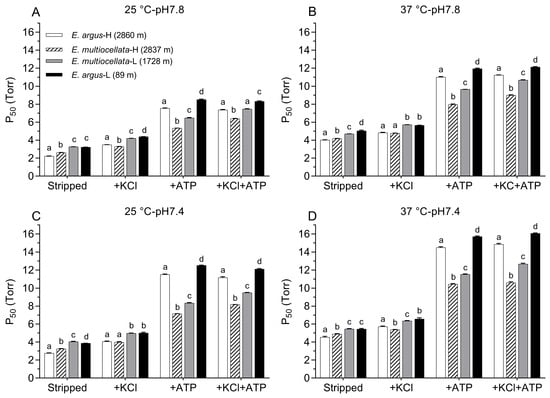
Figure 5.
P50 values (mean ± SE) of Hbs from high- and low-altitude populations of E. argus and E. multiocellata measured at 25 °C pH7.8 (A), 37 °C pH7.8 (B), 25 °C pH7.4 (C), and 37 °C pH7.4 (D) in the absence (stripped) and presence of Cl− (added as 0.1 mol l− of KCl) and/or ATP (100-fold molar excess over tetrameric Hbs). Different letters above the P50 values of the four populations indicate significant differences between each pair of populations under the same allosteric condition according to one-way ANOVA using the LSD method of post hoc multiple comparisons (p < 0.05). n = 6 lizards for each population, and 6 technical repeats were performed for each experimental condition.

Table 1.
Anionic cofactor sensitivities of high- and low-altitude E. argus and E. multiocellata Hbs under various experimental conditions.
Dose–response curves (Figure S3) showed that the maximum ATP-induced logP50 value appeared at 2.0 mmol/L of ATP (approximately 27-fold molar excess over tetrameric Hbs), which indicated that the ATP concentration (7.5 mmol/L, 100-fold molar excess over tetrameric Hbs) used in our experiment was sufficient to fully reflect its effects on the Hb-O2 affinity. E. argus Hbs had a higher affinity for ATP than E. multiocellata Hbs, with estimated apparent binding constants of 0.19, 0.18, 0.31, and 0.27 mmol/L for E. argus-H, E. argus-L, E. multiocellata-H, and E. multiocellata-L, respectively. This result was consistent with the difference in ATP sensitives (ΔlogP50(ATP-stripped)) between the two species at 37 °C (Table 1).
The O2-binding curves shifted to the right when the pH decreased from 7.8 to 7.4 (Figure 4). The Bohr effect was indicated by the magnitude of Bohr factors (Φ = ∆logP50/∆pH), which are equal to the slopes of the linear plots in Figure 6. The Bohr factors of Hbs in high-altitude and low-altitude populations were similar under each allosteric condition for both species (Table 2). In addition, the Bohr factors at 25 °C were greater than those at 37 °C for the four populations in the stripped state and the presence of ATP (Figure 6). For the interspecific comparison, the Bohr factors were similar in E. argus and E. multiocellata Hbs in the absence of ATP (stripped state and only KCl added). In the presence of ATP, Bohr factors of E. argus Hbs markedly increased, but not for E. multiocellata Hbs, resulting in a stronger Bohr effect of E. argus Hbs than that of E. multiocellata Hbs in the presence of ATP (except for E. multiocellata-H when ATP was solely added, Table 2).
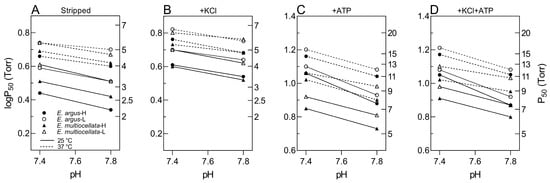
Figure 6.
Bohr plots (logP50 vs. pH) of high- and low-altitude E. argus and E. multiocellata in the absence ((A): stripped) and presence of KCl (B), ATP (C), and +KCl+ATP (D) at 25 °C (continuous lines) and 37 °C (dotted lines). E. argus and E. multiocellata were denoted by circles and triangles, respectively. The high- and low-altitude populations are represented by solid and open symbols, respectively. The slope of these linear plots is equal to the corresponding Bohr factor (Φ, Table 2), which was used to indicate the magnitude of the Bohr effect. n = 6 lizards for each population and 6 technical repeats were performed for each experimental condition.

Table 2.
Bohr factors for high- and low-altitude E. argus and E. multiocellata Hbs at 25 °C and 37 °C in the absence (stripped) and presence of Cl− (0.1 mol/L of KCl) and/or ATP (100-fold molar excess over tetrameric Hbs).
3.3. Low Temperature Sensitivity of Hbs in Eremias Lizards
The O2-binding curves shifted to the right when the temperature increased from 25 to 37 °C (Figure 4). As shown in Table 3, the temperature sensitivity of purified Hbs was quantified using the calibrated overall change in enthalpy (ΔH, kJ/mol O2). The ΔH at pH7.8 was higher than that at pH7.4 for the Hbs of the four populations under each allosteric condition except for E. argus in the presence of Cl−. For the stripped state, the ΔH of high-altitude population Hbs was higher than that of low-altitude population Hbs for both E. argus and E. multiocellata. The presence of Cl−, ATP, and ATP + Cl− decreased the ΔH except for low-altitude E. multiocellata Hbs in the presence of ATP. Under the simulated physiological conditions in which both ATP and Cl− were added, ΔH values were consistently low at pH7.4 (−4.12 to 5.97 kJ/mol O2) and pH7.8 (−9.41 to −14.52 kJ/mol O2).

Table 3.
Temperature effects (reflected by the overall change in enthalpy for oxygenation ΔH) on the O2 affinities of high- and low-altitude E. argus and E. multiocellata Hbs at pH7.8 and pH7.4 in the absence (stripped) and presence of Cl− (added as 0.1 mol/L of KCl) and/or ATP (100-fold molar excess over tetrameric Hbs).
3.4. Structural Properties of Tetrameric Hbs in T-State
The average backbone RMSD of AB2 (αAβ2) and DB2 (αD1β2 and αD2β2) Hb models of the four populations reached equilibrium in the last 5 ns of the simulations (Figure S4). The analysis of hydrogen bond interactions showed that side-chain -NH2 of 12Asn on the β subunit can form hydrogen bonds with 75Ile and/or 76Lys on the adjacent helix both in the AB2 and DB2 models of high-altitude E. argus and E. multiocellata, and low-altitude E. multiocellata. However, these hydrogen bonds were lost when 12β2 was Ala in the lowest E. argus (Figure 7). As for the four different amino acid substitutions of low-altitude E. multiocellata β2 globin in the AB2 and DB2 models, they only form hydrogen bonds with neighboring amino acids like the high-altitude E. multiocellata.
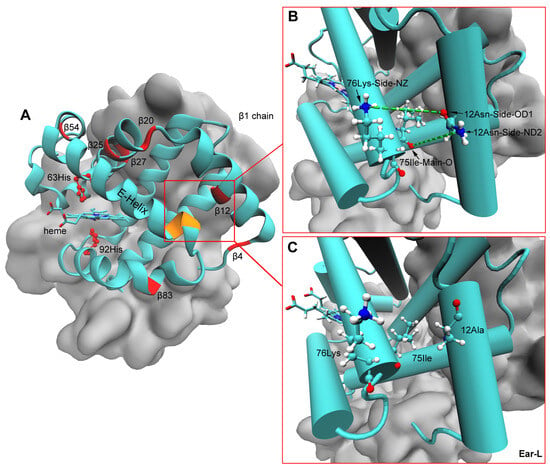
Figure 7.
Spatial locations of heme, distal 63 His, and proximal 92 His on the β subunit of Hb models (A). The distal 63 His and proximal 92 His (represented by ball-and-stick) and the seven different amino acid residues in the β2 globin of the low-altitude E. argus compared with those of the other three Eremias lizards were marked in red, and the 75Ile and 76Lys were marked in yellow in (A). Hydrogen bonds (indicated by the green dotted line) formed between 12β2 and 75Ile and/or 76Lys (represented by ball-and-stick) on the adjacent E-helix in all Hb models composed of β2 globin with all α globins in high-altitude E. argus and high- and low-altitude E. multiocellata Hbs (B). However, these two hydrogen bonds were lost in the Hbs of low-altitude E. argus (Ear-L) due to the replacement of Asn with Ala at β12 (C). The β1 chain is represented by cyan, and the β2 and two α chains are represented by silver in the QuickSurf drawing method of VMD.
4. Discussion
4.1. Phylogenetic Relationship of Beta Globins in Sauria Lizards
The phylogenetic relationships of the α globin genes of Sauria lizards and outgroups (Figure 2A) estimated using our globin sequencing results (Figure 1) were consistent with previous studies. The squamates have retained αD and αA and lost the αE globin genes [32,33,44]. With respect to the β-globin, previous studies have suggested that each of the major groups of Sauropsids (birds, crocodilians, testudines, and squamates) evolved distinct β-globin repertoires via repeated rounds of lineage-specific gene duplication [33,44]. However, this phenomenon has not been clearly verified in Sauria lizards. In addition to the high similarity of β1- and β2-globin, several limitations may account for the unresolved evolutionary pattern of the β-globin gene in lizards [44]. Firstly, the lizards used in the previous analysis only come from only a few families of Sauria. Secondly, the β-globins used in the previous analysis were inaccurately annotated and did not contain the completed repertoires of β globin (lack β1 or β2). As described in the Supplementary Materials (Table S2), the name of the β1 and β2 of anole and L. agilis were inaccurate, P. erythrurus and P. przewalskii only expressed β1 and β2 globins, which was corrected in the followed study [31]. In this study, we collected more complete repertoires of β-globin from lizards and corrected some inaccurate annotations. Our estimated phylogenetic relationships showed that the adult-expressed β2 globins of lizards were nested within a clade containing β1 globins, and the ancestral β globins of the two lizard β-type globins were sister to those of chickens and humans (Figure 2B). This result suggested that the distinct β-globin repertoires of Sauria lizards may also have derived from lineage-specific duplication, similar to other lineages of Sauropsids [33,44].
4.2. The Physiological Significances of Oxygenation Properties of Eremias Lizards Hbs
One of the primary findings of this study is that the Hbs of high-altitude E. argus and E. multiocellata both evolved genetically based high Hb-O2 affinity compared with their low-altitude populations. Given that the ATP and/or Cl− sensitivities of Hbs were similar between high- and low-populations, the overall high Hb-O2 affinities of the high-altitude population for the two species were mainly attributed to their significantly elevated intrinsic Hb-O2 affinities. Significantly increased Hb-O2 affinity has been found in other high-altitude ectothermic reptiles and amphibians, such as the high-altitude red-tailed toad-head lizard (P. erythrurus) and Asiatic toad (B. gargarizans) from the QTP, and Telmatobius frogs and Bufo spinulosus flavolineatus from the Andes [18,24,35,45,46]. The evolved high Hb-O2 affinity could be the primary strategy for high-altitude reptiles and amphibians to ensure pulmonary O2 uptake under hypoxia in the incomplete systemic circulation.
The distinct ATP-mediated allosteric regulation of Hbs in the two closely related Eremias lizards may be associated with their different habitat preference [47]. E. multiocellata prefers open habitats that may increase O2 demand to evade predators. The high Hb-O2 affinity of high-altitude E. multiocellata under all allosteric conditions could ensure an adequate O2 supply. E. argus prefers close habitats that may minimize the impact of predators. The high ATP sensitivity and strong Bohr effect in the presence of ATP of high-altitude E. argus could then facilitate O2 unloading in systemic capillaries and therefore compensate for the side effect of its high Hb-O2 affinity on O2 unloading in tissues. The inherent adaptive increase in Hb-O2 affinity in high-altitude E. multiocellata may be compensated by other mechanisms, such as an increase in the tissue O2 diffusion capacity via increased muscle capillarization and plastic changes in Hb concentration and hematocrit (unpublished data).
In the stripped state and the presence of Cl−, the Bohr effects were generally similar in the four populations’ Hbs (−0.15 to −0.25 at 25 °C, −0.08 to −0.18 at 37 °C). The presence of ATP could enhance the Bohr effects of high- and low-altitude E. argus Hbs (−0.41 to −0.45 at 25 °C, −0.30 at 37 °C), indicating that ATP could promote the binding of H+ to E. argus Hbs. However, this ATP enhancement was not found in E. multiocellata (Table 2). Similarly, in snake Hb, the alkaline Bohr effect is greatly enhanced in the presence of ATP [48,49,50], and this effect could result from ATP-induced polymerization of subunits [51]. In addition, the Bohr effects at 25 °C were greater than those at 37 °C for Hbs of all Eremias in the presence of ATP (Table 2, Figure 6). Given that the body temperature of ectotherm is determined by the ambient temperature, a higher Bohr effect at lower temperatures could facilitate O2 unloading to the metabolic tissue at low temperature. This is especially important for high-altitude E. argus and E. multiocellata, for which the mean ground and air temperatures during their active period (April to October) range from 2.3 to 18.6 °C (Figure S1C,D). A thermoregulation study in the cold-climate Phrynocephalus guinanensis showed that the heating and cooling rates of limbs and tails were relatively faster than those of torsos [52]. This phenomenon may also exist in Eremias lizards, which have a body size similar to that of Phrynocephalus lizards. Thus, the relatively high Bohr effect of Eremias lizard’s Hbs at lower temperatures could promote the release of O2 in the limbs and tails when the ambient temperature drops rapidly, thereby enhancing their low temperature tolerance. Higher Bohr effects at lower temperatures were also found in high-altitude Asiatic toads from the QTP (Φ = −0.50 and −0.38 at 10 and 20 °C in the presence of ATP and KCl, respectively) [24]. However, this phenomenon is not exclusive to ectotherms and can also be found in human and horse Hb [53].
The temperature sensitivity of Hbs in Eremias lizards decreased with decreasing pH under almost all allosteric conditions (Table 3), indicating the endothermic binding of H+ to Hbs. The overall temperature sensitivities (on average −9.68 kJ/mol O2, Table 3) of Hbs in the two Eremias species were noticeably lower than those of Hbs in other species, such as temperature-sensitive human HbA (−50.7 kJ/mol O2), temperature-insensitive bovine Hb (−22.9 kJ/mol O2), deer mouse Hbs (on average −10.5 kJ/mol O2), and Asiatic toad Hbs (on average −16.57 kJ/mol O2) [23,24,54]. As mentioned above, the cooling rates of limbs and tails were relatively faster than those of torsos in cold-climate lizards. Therefore, when the ambient temperature drops rapidly, the low temperature sensitivity of Hbs in Eremias lizards could minimize the hindering effect of temperature-induced high Hb-O2 affinity on the transport and release of O2 to the limbs and tail. In addition, the body temperature of Eremias lizards is mainly determined by the ambient temperature, and the mean air and ground temperatures fluctuate greatly during different months throughout the year at the sampling sites (Figure S1C,D), and the temperature difference between day and night is also great in the plateau environment. Thus, the low temperature sensitivity of Eremias lizard Hbs, especially under relatively low pH conditions, could minimize the temperature- and pH-induced fluctuations in Hb-O2 affinity during the transport of O2 to cold limbs and metabolic tissue and finally safeguard the tissue O2 supply [21].
Low Cl− sensitivity (∆logP50 = 0.04–0.20) was found in the Hbs of high- and low-altitude populations in both species compared with the ATP sensitivity (∆logP50 = 0.30–0.62) (Table 1). Disregarding any other variations, this property will ensure O2 loading under hypoxia without the need for decreasing erythrocytic organic phosphate levels and the related allosteric regulatory capacity [18]. The drastically suppressed Cl− sensitivity has been revealed in other reptiles and amphibians, such as Phrynocephalus lizards from China (unpublished data from the author), the aquatic Andean frog T. peruvianus, Asiatic toad (B. gargarizans), and black-spotted frog (Pelophylax nigromaculatus) [18,24,55,56]. We speculate that the anion-related allosteric regulation of hemoglobins in some reptiles and amphibians is primarily mediated by organic phosphates rather than chloride ions. More investigations into the oxygenation properties of Hbs in reptiles and amphibians are necessary to verify this hypothesis.
4.3. Molecular Mechanisms Underlying the Oxygenation Properties of Eremias Hbs
The molecular mechanisms underlying the high Hb-O2 affinity are not only related to evolutionary changes in globin sequences but are also influenced by Hb isoform diversity [57,58]. αD-type globin was detected in all hemolysates of Eremias lizards by Orbitrap Fusion Lumos MS but not by the time-of-flight mass spectrometry using products separated by RP-HPLC. These inconsistent results suggest that αD-type globin accounts for a small proportion of the hemolysate of Eremias lizards, while αA-type hemoglobin is the predominant isoform. Our results align with the Hb isoform differentiation observed in turtles and birds. In most turtles and birds, HbA composed of αA-type globin is the major isoform, and HbD composed of αD-type globin is the minor isoform [38,57,59,60]. However, the opposite pattern of isoHb differentiation was found in the green anole lizard (Anolis carolinensis) and South American rattlesnake (Crotalus durissus) [29,51]. In these species, HbD is the major isoform, and its O2 affinity is lower than that of HbA. Thus, Hb isoform diversity might be species-specific in squamate reptiles. In addition, a consistently higher O2 affinity of HbD than that of HbA in all examined turtles and birds was not verified in Eremias lizards because the individual Hb isoforms were not separated in this study, which should be resolved in further studies [61,62]. For globin sequence variation, the amino acid sequences of the αA globin were identical in the highland and lowland E. argus and E. multiocellata.
The oxygenation properties of Hbs in the studied Eremias lizards might largely be attributed to differences in β-globin. The relative abundance of β2 globin to β1 globin was the highest in high-altitude E. multiocellata (~2.25), followed by the two populations of E. argus (~1.38), and the lowest in low-altitude E. multiocellata (~1.23). For interpopulation comparison in E. argus, high O2 affinity of Hbs in the high-altitude population could be attributed to the seven substitutions in the β2 globin (Figure 1), as the β2/β1 ratio and amino acid sequence of the β1 globin were identical in the high- and low-altitude populations (Figure 1 and Figure 3). For interpopulation comparison in E. multiocellata, high O2 affinity of Hbs in the high-altitude population could be attributed to four substitutions on the β2 globin, the high relative abundance of the β2 globin, and one substitution on the β1 globin (Gln → Pro) (Figure 1 and Figure 3). In the comparison between the high-altitude populations of E. argus and E. multiocellata, the amino acid sequences of their β2 globins were identical, and the main differences were the β2/β1 globin ratio and eight species-specific amino acid variations in the β1 globin, which may result in the higher ATP sensitivities and ATP-dependent high Bohr effect in the Hbs of highland E. argus relative to those of E. multiocellata. In addition, because of their distinct ATP-dependent oxygenation properties, the O2 affinity of highland E. argus was higher than that of highland E. multiocellata in the absence of ATP but lower than that of highland E. multiocellata in the presence of ATP. Furthermore, additional peaks whose MWs cannot match our sequenced results may also be attributable to the differences in the oxygenation properties of the highland populations of the two species. These peaks may be unsequenced globins or products of the deamination or other forms of posttranslational modification of the sequenced globins. Studies on the isoform diversity of snake hemoglobins also showed that products of the β2 globin gene form the main isoHbs in the South American rattlesnake and leaf-nosed viper (Eristicophis macmahonii) snakes [51,63].
The lowest intrinsic Hb-O2 affinity of low-altitude E. argus Hbs may also be related to the localized destabilization of the E-helix secondary structure in Hb models composed of β2 globin with all α globins (Figure 7). The substitution of Asn to Ala at the 12 position on β2 globin in the lowland E. argus caused the elimination of hydrogen bonds formed between 12β2 and 75Ile and/or 76Lys on the adjacent E-helix compared with the other three Eremias lizards (Figure 7B,C). A similar structural mechanism was previously found in high-altitude American pika (Ochotona princeps), where the β62 Ala → Thr substitution could increase the rigidity of the E-helix, consequently leading to an increased oxygen affinity [17].
It is still not clear which amino acid residues are responsible for the different ATP sensitivities of the two closely related species of Eremias lizards since β1Val, β82Lys, and β143Arg are conserved in Eremias Hbs, except that the positively charged β2His is replaced by negatively charged β2Glu. Studies on fish Hbs have shown that substitutions of Glu or His at the position 2 in the β chain do not affect DPG sensitivity [64,65]. We speculate that the high ATP-binding affinities of the high-altitude E. argus Hbs could be attributed to the combined effects of different amino acid substitutions of the αD and β2 globins, coupled with isoform diversity in the β globins.
Cl− binds to an α-chain site (between α1Val and α131Ser) and a β-chain site (between β1Val and β82Lys) in human HbA [66]. However, the polar 131Ser is replaced by nonpolar Val and electronegative Glu in αD1 and αD2 of Eremias Hbs (Figure 4A), respectively. In addition, the N-terminal residue 1Val of αD2-globin in E. multiocellata was replaced by Met (Figure 1A). These substitutions may result in the elimination of Cl− binding sites in Hbs formed by αD1- and αD2-type globins. Similar mechanisms were found in the Andean frog (T. peruvianus) and Indian python (Python molurus) Hb [18,51]. However, it is uncertain whether the loss of Cl− binding sites at the αD2 globin could cause the dramatically low Cl− sensitivities of Eremias lizard Hbs, as the αD globins were not isolated by HPLC in this study.
5. Conclusions
In this study, we investigated the oxygenation properties and underlying mechanisms of Hbs in two closely related species of Eremias lizards from the QTP compared with their low-altitude counterparts. Phylogenetic analysis suggested that the evolved distinct β-globin repertoires of Sauria lizards may also be derived from lineage-specific duplications. The significantly high overall and intrinsic Hb-O2 affinity of the highland E. argus and E. multiocellata Hbs can ensure efficient pulmonary O2 uptake under hypoxic conditions compared to lowland populations. The Hbs of high-altitude E. argus exhibit higher ATP sensitivities and stronger ATP-dependent Bohr effects than that of E. multiocellata, and the mechanism may be related to the differential abundance of β2 and β1 globins, as well as specific amino acid substitutions in the β2-type globin. These properties could compensate for the high Hb-O2 affinity and thus facilitate O2 unloading in respiring tissues for the high-altitude E. argus. Furthermore, Hbs of Eremias lizards Hbs exhibited low temperature sensitivities, which decreased with decreasing pH, and higher Bohr effects were observed at lower temperatures. Taken together, these characteristics could minimize the temperature- and pH-induced fluctuations in the Hb-O2 affinity and facilitate the transport and release of O2 to cold limbs and metabolic tissue at low temperatures. Our results could provide a valuable reference for the study of adaptation mechanisms of Hbs in other high-altitude reptiles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14101440/s1, Table S1. PCR primers are used to amplify adult-expressed α- and β-type globin genes of high- and low-altitude E. argus and E. multiocellata; Table S2. (a) List of α-globin amino acid sequences included in the phylogenetic analyses, (b) List of β-globin amino acid sequences included in the phylogenetic analyses; Table S3. The theoretical molecular weight (MW) of α- and β-type globins sequenced in high- and low-altitude E. argus and E. multiocellata; Table S4. O2 affinities (indexed as P50 values) and Hill’s cooperativity coefficients (indexed as n50 values) for high- and low-altitude E. argus and Hbs measured in pH 7.8, 7.4 Hepes buffers at 25 °C and 37 °C in the absence (stripped) and presence of Cl− (added as 0.1 mol/L KCl) and/or ATP (100-fold molar excess over tetrameric Hbs); Table S5. O2 affinities (indexed as P50 values) and Hill’s cooperativity coefficients (indexed as n50 values) for high- and low-altitude E. multiocellata Hbs measured in pH 7.8, 7.4 Hepes buffers at 25 °C and 37 °C in the absence (stripped) and presence of Cl− (added as 0.1 mol/L KCl) and/or ATP (100-fold molar excess over tetrameric Hbs); Figure S1. The geographical location (A) and climate data (B–D) from 1981 to 2010 of four sample sites of Eremias argus (Shazhuyu and Xingtai) and Eremias multiocellata (Tianzhu and Lanzhou). B, atmospheric pressure; C, mean ground temperature; D, mean air temperature; Figure S2. Isoelectric focusing (pH 3–10) of the pooled purified isoform Hb mixture from high-altitude (Ear-H) and low-altitude (Ear-L) E. argus, and high-altitude (Emu-H) and low-altitude (Emu-L) E. multiocellata. Samples in lanes Ear-H-2, Ear-L-2, Emu-H-2 and Emu-L-2 are dilutions of samples in lanes Ear-H-1, Ear-L-1, Emu-H-1 and Emu-L-1, respectively; Figure S3. Dose-response curves showing effects of ATP on P50 of high- (solid) and low-(open) altitude E. argus (circle) and E. multiocellata (triangle) Hbs measured at 37 °C pH7.4 in the absence of Cl−. n = 6 lizards for each population and 6 technical repeats were performed for each experiment condition; Figure S4. Evolution of root mean square deviation (RMSD) of the atoms in the backbone of the Hbs over time in AB2 Hb models (A) molecular dynamics simulations and DB2 Hb models (B) molecular dynamics simulations. The average RMSD over the last 4 ns for AB2 and DB2 models were also shown in A and B. Ear-H and Ear-L indicate the high- and low-altitude E. argus, Emu-H, and Emu-L indicate the high- and low-altitude E. multiocellata. References [29,31,67] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Q.C. and P.P.; methodology, P.P., Z.N. and M.M.; software, P.P.; validation, P.P. and X.T.; formal analysis, P.P.; investigation, P.P.; resources, X.T.; data curation, Z.N.; writing—original draft preparation, P.P.; writing—review and editing, P.P. and Q.C.; visualization, P.P.; supervision, Q.C.; project administration, Q.C.; funding acquisition, Q.C. and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (32270502 to Qiang Chen), the Gansu Province Science and Technology Foundation for Youths (23JRRA1700 to Peng Pu), and the Young Scholars Science Foundation of Lanzhou Jiaotong University (2023018 to Peng Pu).
Institutional Review Board Statement
The study was approved by the Ethics Committee of School of Life Sciences, Lanzhou University (protocol code EAF2021026 and date of approval: 1 April 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank the Core Facility of School of Life Sciences, Lanzhou University, for technical assistance. This work is supported by the Supercomputing Center of Lanzhou University. We thank Tao Zhang and Jinzhou Wang for their assistance in the capture of lizards. We thank Fei Meng and Lu Xi for their help with data acquisition, and Professor Shouliang Dong and Feiyun Gao of Lanzhou University, China, for their help with the HPLC and mass spectrometry experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| QTP | Qinghai-Tibet Plateau |
| E. argus-H | high-altitude E. argus |
| E. argus-L | low-altitude E. argus |
| E. multiocellata-H | high-altitude E. multiocellata |
| E. multiocellata-L | low-altitude E. multiocellata |
| Hb | hemoglobin |
| ATP | adenosine triphosphate |
| DPG | 2,3-diphosphoglycerate |
| IP5 | inositol pentaphosphate |
| P50 | O2 tension at half-saturation |
| n50 | Hill cooperativity coefficient |
| O2 tension | |
| fractional saturation | |
| ΔH | overall change in enthalpy for oxygenation |
| Φ | Bohr factor |
| isoHb | Hb isoform |
| IEF | isoelectric focusing |
References
- Storz, J.F.; Quiroga-Carmona, M.; Opazo, J.C.; Bowen, T.; Farson, M.; Steppan, S.J.; D’Elía, G. Discovery of the world’s highest-dwelling mammal. Proc. Natl. Acad. Sci. USA 2020, 117, 18169–18171. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.W. The physiological and evolutionary significance of cardiovascular shunting patterns in reptiles. Physiology 2002, 17, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Cerdeña, J.; Farfan, J.; Quiroz, A. A high mountain lizard from Peru: The world’s highest-altitude reptile. Herpetozoa 2021, 34, 61–65. [Google Scholar] [CrossRef]
- Zhao, E.M.; Zhao, K.T.; Zhou, K.Y. Reptilia (Squamata: Lacertilia). In Fauna Sinica; Beijing Science Press: Beijing, China, 1999; Volume 2, pp. 220–243. [Google Scholar]
- Ivy, C.M.; Scott, G.R. Control of breathing and the circulation in high-altitude mammals and birds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 186, 66–74. [Google Scholar] [CrossRef] [PubMed]
- McClelland, G.B.; Scott, G.R. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annu. Rev. Physiol. 2019, 81, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Scott, G.R. Life ascending: Mechanism and process in physiological adaptation to high-Altitude hypoxia. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 503–526. [Google Scholar] [CrossRef] [PubMed]
- Jendroszek, A.; Malte, H.; Overgaard, C.B.; Beedholm, K.; Natarajan, C.; Weber, R.E.; Storz, J.F.; Fago, A. Allosteric mechanisms underlying the adaptive increase in hemoglobin–oxygen affinity of the bar-headed goose. J. Exp. Biol. 2018, 221, jeb185470. [Google Scholar] [CrossRef]
- Natarajan, C.; Jendroszek, A.; Kumar, A.; Weber, R.E.; Tame, J.R.H.; Fago, A.; Storz, J.F. Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genet. 2018, 14, e1007331. [Google Scholar] [CrossRef] [PubMed]
- Jessen, T.H.; Weber, R.E.; Fermi, G.; Tame, J.; Braunitzer, G. Adaptation of bird hemoglobins to high altitudes: Demonstration of molecular mechanism by protein engineering. Proc. Natl. Acad. Sci. USA 1991, 88, 6519–6522. [Google Scholar] [CrossRef]
- Weber, R.E.; Jessen, T.H.; Malte, H.; Tame, J. Mutant hemoglobins (alpha 119-Ala and beta 55-Ser): Functions related to high-altitude respiration in geese. J. Appl. Physiol. 1993, 75, 2646–2655. [Google Scholar] [CrossRef]
- Zhu, X.; Guan, Y.; Signore, A.V.; Natarajan, C.; DuBay, S.G.; Cheng, Y.; Han, N.; Song, G.; Qu, Y.; Moriyama, H.; et al. Divergent and parallel routes of biochemical adaptation in high-altitude passerine birds from the Qinghai-Tibet Plateau. Proc. Natl. Acad. Sci. USA 2018, 115, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, C.; Inoguchi, N.; Weber, R.E.; Fago, A.; Moriyama, H.; Storz, J.F. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 2013, 340, 1324–1327. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Runck, A.M.; Sabatino, S.J.; Kelly, J.K.; Ferrand, N.; Moriyama, H.; Weber, R.E.; Fago, A. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 2009, 106, 14450–14455. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.V.; Storz, J.F. Biochemical pedomorphosis and genetic assimilation in the hypoxia adaptation of Tibetan antelope. Sci. Adv. 2020, 6, eabb5447. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Lu, S.; Niu, Z.; Zhang, T.; Zhao, Y.; Yang, X.; Zhao, Y.; Tang, X.; Chen, Q. Oxygenation properties and underlying molecular mechanisms of hemoglobins in plateau zokor (Eospalax baileyi). Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R696–R708. [Google Scholar] [CrossRef]
- Tufts, D.M.; Natarajan, C.; Revsbech, I.G.; Projecto-Garcia, J.; Hoffmann, F.G.; Weber, R.E.; Fago, A.; Moriyama, H.; Storz, J.F. Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol. Biol. Evol. 2014, 32, 287–298. [Google Scholar] [CrossRef]
- Weber, R.E.; Ostojic, H.; Fago, A.; Dewilde, S.; Hauwaert, M.-L.V.; Moens, L.; Monge, C. Novel mechanism for high-altitude adaptation in hemoglobin of the Andean frog Telmatobius peruvianus. Am. J. Physiol. Integr. Comp. Physiol. 2002, 283, R1052–R1060. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, T.; März, J.; Jürgens, K.D.; Braunitzer, G. Interaction of allosteric effectors with alpha-globin chains and high altitude respiration of mammals. The primary structure of two tylopoda hemoglobins with high oxygen affinity: Vicuna (Lama vicugna) and alpaca (Lama pacos). Biol. Chem. Hoppe-Seyler. 1986, 367, 153. [Google Scholar] [CrossRef]
- Piccinini, M.; Kleinschmidt, T.; Jürgens, K.D.; Braunitzer, G. Primary structure and oxygen-binding properties of the hemoglobin from guanaco (Lama guanacoë, Tylopoda). Biol. Chem. Hoppe-Seyler. 1990, 371, 641. [Google Scholar] [CrossRef]
- Weber, R.E.; Campbell, K.L. Temperature dependence of haemoglobin–oxygen affinity in heterothermic vertebrates: Mechanisms and biological significance. Acta Physiol. 2011, 202, 549–562. [Google Scholar] [CrossRef]
- Brix, O.; Bårdgard, A.; Mathisen, S.; Tyler, N.; Nuutinen, M.; Condo, S.G.; Giardina, B. Oxygen transport in the blood of arctic mammals: Adaptation to local heterothermia. J. Comp. Physiol. B 1990, 159, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Storz, J.F.; Fago, A. Bohr effect and temperature sensitivity of hemoglobins from highland and lowland deer mice. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 195, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Zhao, Y.; Niu, Z.; Cao, W.; Zhang, T.; He, J.; Wang, J.; Tang, X.; Chen, Q. Comparison of hematological traits and oxygenation properties of hemoglobins from highland and lowland Asiatic toad (Bufo gargarizans). J. Comp. Physiol. B 2021, 191, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Sun, S.; Jin, Y.; Yan, Y.; Liu, N. Molecular phylogeography of the Chinese lacertids of the genus Eremias (Lacertidae) based on 16S rRNA mitochondrial DNA sequences. Amphib.-Reptil. 2007, 28, 33–41. [Google Scholar] [CrossRef]
- Li, W.; Du, J.; Yang, L.; Liang, Q.; Yang, M.; Zhou, X.; Du, W. Chromosome-level genome assembly and population genomics of Mongolian racerunner (Eremias argus) provide insights into high-altitude adaptation in lizards. BMC Biol. 2023, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Pinho, C.; Pérez i de Lanuza, G.; Afonso, S.; Brejcha, J.; Rubin, C.-J.; Wallerman, O.; Pereira, P.; Sabatino, S.J.; Bellati, A.; et al. Regulatory changes in pterin and carotenoid genes underlie balanced color polymorphisms in the wall lizard. Proc. Natl. Acad. Sci. USA 2019, 116, 5633. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Hoffmann, F.G.; Opazo, J.C.; Sanger, T.J.; Moriyama, H. Developmental regulation of hemoglobin synthesis in the green anole lizard Anolis carolinensis. J. Exp. Biol. 2011, 214, 575–581. [Google Scholar] [CrossRef][Green Version]
- Alföldi, J.; Di Palma, F.; Grabherr, M.; Williams, C.; Kong, L.; Mauceli, E.; Russell, P.; Lowe, C.B.; Glor, R.E.; Jaffe, J.D.; et al. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature 2011, 477, 587–591. [Google Scholar] [CrossRef]
- Lu, S. The Adaptive Mechanism of Globin Family to High Altitude Hypoxia in Phrynocephalus Lizards. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Hoffmann, F.G.; Storz, J.F. The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol. Biol. Evol. 2007, 24, 1982–1990. [Google Scholar] [CrossRef]
- Hoffmann, F.G.; Storz, J.F.; Gorr, T.A.; Opazo, J.C. Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol. Biol. Evol. 2010, 27, 1126–1138. [Google Scholar] [CrossRef]
- Zhang, S.-P.; Feng, H.-Z.; Wang, Q.; Quan, S.-W.; Yu, X.-Q.; Tao, X.; Wang, Y.; Guo, D.-D.; Peng, L.; Feng, H.-Y.; et al. Proteomic analysis reveals the mechanism of different environmental stress-induced tolerance of Pseudomonas aeruginosa to monochloramine disinfection. J. Hazard. Mater. 2021, 417, 126082. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xin, Y.; Tang, X.; Yue, F.; Wang, H.; Bai, Y.; Niu, Y.; Chen, Q. Differences in hematological traits between high- and low-altitude lizards (Genus Phrynocephalus). PLoS ONE 2015, 10, e0125751. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E. Use of ionic and zwitterionic (Tris/BisTris and HEPES) buffers in studies on hemoglobin function. J. Appl. Physiol. 1992, 72, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E. Cationic control of O2 affinity in lugworm erythrocruorin. Nature 1981, 292, 386–387. [Google Scholar] [CrossRef]
- Damsgaard, C.; Storz, J.F.; Hoffmann, F.G.; Fago, A. Hemoglobin isoform differentiation and allosteric regulation of oxygen binding in the turtle, Trachemys scripta. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R961–R967. [Google Scholar] [CrossRef][Green Version]
- Tang, X.L.; Yue, F.; He, J.Z.; Wang, N.B.; Ma, M.; Mo, J.R.; Chen, Q. Ontogenetic and sexual differences of thermal biology and locomotor performance in a lacertid lizard, Eremias multiocellata. Zoology 2013, 116, 331. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, O.; Mould, R.; Brittain, T. Allosteric modulation of oxygen binding to the three human embryonic haemoglobins. Biochem. J. 1995, 306, 367–370. [Google Scholar] [CrossRef]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reactions with Ligands; North-Holland Publishing Company: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781. [Google Scholar] [CrossRef]
- Hoffmann, F.G.; Vandewege, M.W.; Storz, J.F.; Opazo, J.C. Gene turnover and diversification of the α- and β-globin gene families in Sauropsid vertebrates. Genome Biol. Evol. 2018, 10, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, V.H.; Haines, H.B.; Engbretson, G. Aquatic life at high altitude: Respiratory adaptations in the Lake Titicaca frog, Telmatobius culeus. Respir. Physiol. 1976, 27, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, H.; Monge-C, C.; Cifuentes, V. Hemoglobin affinity for oxygen in three subspecies of toads (Bufo sp.) living at different altitudes. Biol. Res. 2000, 33, 5–10. [Google Scholar] [CrossRef]
- Zeng, Z.-G.; Bi, J.H.; Li, S.-R.; Wang, Y.; Robbins, T.R.; Chen, S.-Y.; Du, W.-G. Habitat alteration influences a desert steppe lizard community: Implications of species-specific preferences and performance. Herpetol. Monogr. 2016, 30, 34–48. [Google Scholar] [CrossRef]
- Bonilla, G.O.; Oyama, S.; Nagatomo, C.L.; Matsuura, M.S.A.; Focesi, A. Interactions of adenosine triphosphate with snake hemoglobins. Studies in Liophis miliaris, Boa constrictor and Bothrops alternatus. Comp. Biochem. Physiol. Part B Comp. Biochem. 1994, 109, 701–707. [Google Scholar] [CrossRef]
- Lombardi, F.R.; Anazetti, M.C.; Santos, G.C.; Olivieri, J.R.; de Azevedo, W.F., Jr.; Bonilla-Rodriguez, G.O. Rattlesnake hemoglobins: Functional properties and tetrameric stability. Protein Pept. Lett. 2006, 13, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Anazetti, M.; Santos, G.; Polizelli, P.; Olivieri, J.; De Azevedo Jr, W.; Bonilla-Rodriguez, G. Oxygen binding properties and tetrameric stability of hemoglobins from the snakes Crotalus durissus terrificus and Liophis miliaris. In Frontiers in Protein and Peptide Sciences; Bentham Science: Beijing, China, 2014; pp. 3–30. [Google Scholar]
- Storz, J.F.; Natarajan, C.; Moriyama, H.; Hoffmann, F.G.; Wang, T.; Fago, A.; Malte, H.; Overgaard, J.; Weber, R.E. Oxygenation properties and isoform diversity of snake hemoglobins. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R1178–R1191. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tong, H.; Zhang, K. The Impact of phenotypic characteristics on thermoregulation in a cold-climate Agamid lizard, Phrynocephalus guinanensis. Asian Herpetol. Res. 2016, 7, 210–219. [Google Scholar]
- Antonini, E.; Wyman, J.; Brunori, M.; Fronticelli, C.; Bucci, E.; Rossi-Fanelli, A. Studies on the relations between molecular and functional properties of hemoglobin: V. The influence of temperature on the Bohr effect in human and in horse hemoglobin. J. Biol. Chem. 1965, 240, 1096–1103. [Google Scholar] [CrossRef]
- Weber, R.E.; Fago, A.; Campbell, K.L. Enthalpic partitioning of the reduced temperature sensitivity of O2 binding in bovine hemoglobin. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 176, 20–25. [Google Scholar] [CrossRef]
- Weber, R.E. Enthalpic consequences of reduced chloride binding in Andean frog (Telmatobius peruvianus) hemoglobin. J. Comp. Physiol. B 2014, 184, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Pu, P.; Niu, Z.; Zhang, T.; Wu, J.; Tang, X.; Chen, Q. A novel mechanism for high-altitude adaptation in hemoglobin of black-spotted frog (Pelophylax nigromaculatus). Front. Ecol. Evol. 2023, 11, 1103406. [Google Scholar] [CrossRef]
- Opazo, J.C.; Hoffmann, F.G.; Natarajan, C.; Witt, C.C.; Berenbrink, M.; Storz, J.F. Gene turnover in the avian globin gene families and evolutionary changes in hemoglobin isoform expression. Mol. Biol. Evol. 2015, 32, 871–887. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Runck, A.M.; Moriyama, H.; Weber, R.E.; Fago, A. Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 2010, 213, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Cheviron, Z.A.; Natarajan, C.; Projecto-Garcia, J.; Eddy, D.K.; Jones, J.; Carling, M.D.; Witt, C.C.; Moriyama, H.; Weber, R.E.; Fago, A. Integrating evolutionary and functional tests of adaptive hypotheses: A case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Mol. Biol. Evol. 2014, 31, 2948–2962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grispo, M.T.; Natarajan, C.; Projecto-Garcia, J.; Moriyama, H.; Weber, R.E.; Storz, J.F. Gene Duplication and the Evolution of Hemoglobin Isoform Differentiation in Birds. J. Biol. Chem. 2012, 287, 37647–37658. [Google Scholar] [CrossRef] [PubMed]
- Hiebl, I.; Weber, R.E.; Schneeganss, D.; Braunitzer, G. High-altitude respiration of Falconiformes. The primary structures and functional properties of the major and minor hemoglobin components of the adult White-headed vulture (Trigonoceps occipitalis, Aegypiinae). Biol. Chem. 1989, 370, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hiebl, I.; Weber, R.E.; Schneeganss, D.; KÖSTERS, J.; Braunitzer, G. High-Altitude Respiration of Birds. Structural Adaptations in the Major and Minor Hemoglobin Components of adult Rüppell’s Griffon (Gyps rueppellii, Aegypiinae): A New Molecular Pattern for Hypoxic Tolerance. Biol. Chem. 1988, 369, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Waheed, H.; Doman, S.K.; Ahmed, A. The partial amino acid sequence of leaf-nosed vper (Eristicophis macmahonii) snake hemoglobin. J. Anim. Plant Sci. 2018, 28, 1622–1628. [Google Scholar]
- Clementi, M.; De Rosa, M.; Bertonati, C.; Capo, C.; Cataldi, E.; Petruzzelli, R.; Giardina, B. Functional and structural properties of the hemoglobin components from Italian sturgeon (Acipenser naccarii). Fish Physiol. Biochem. 2001, 24, 191–200. [Google Scholar] [CrossRef]
- Barra, D.; Bossa, F.; Brunori, M. Structure of binding sites for heterotropic effectors in fish haemoglobins. Nature 1981, 293, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Riggs, A.F. The Bohr Effect. Annu. Rev. Physiol. 1988, 50, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.; Li, Q.; Lian, J.; O’Meally, D.; Deakin, J.; Wang, Z.; Zhang, P.; Fujita, M.; Patel, H.R.; Holleley, C.E.; et al. High-coverage sequencing and annotated assembly of the genome of the Australian dragon lizard Pogona vitticeps. GigaScience 2015, 4, 45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).