On-Farm Methane Mitigation and Animal Health Assessment of a Commercially Available Tannin Supplement in Organic Dairy Heifers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Sample Collection
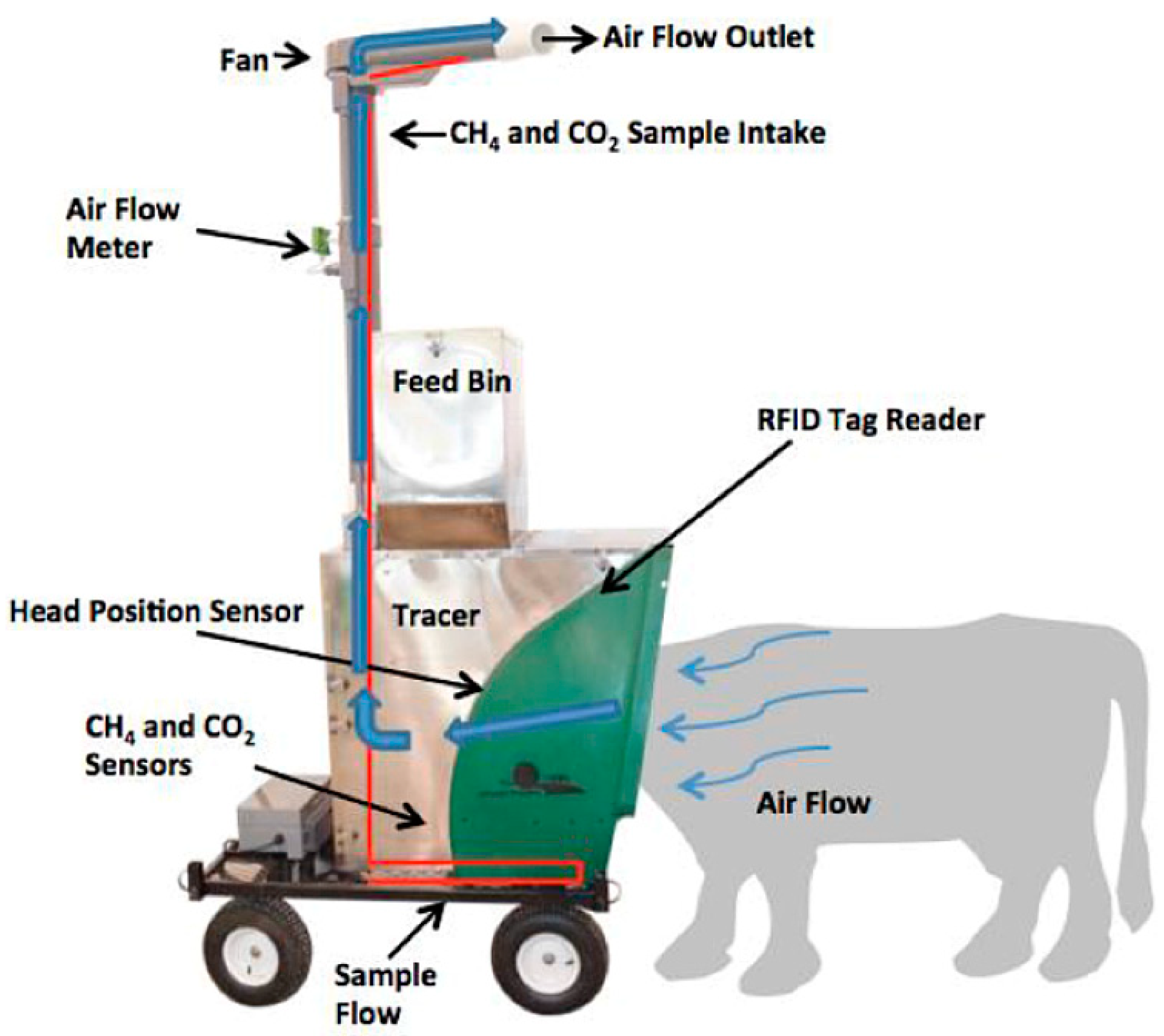
2.2.1. Enteric CH4 Emissions
2.2.2. Animal Feed Intake
2.2.3. Feed Samples
2.2.4. Animal Body Weights
2.2.5. Blood Samples
2.3. Statistical Analysis
3. Results
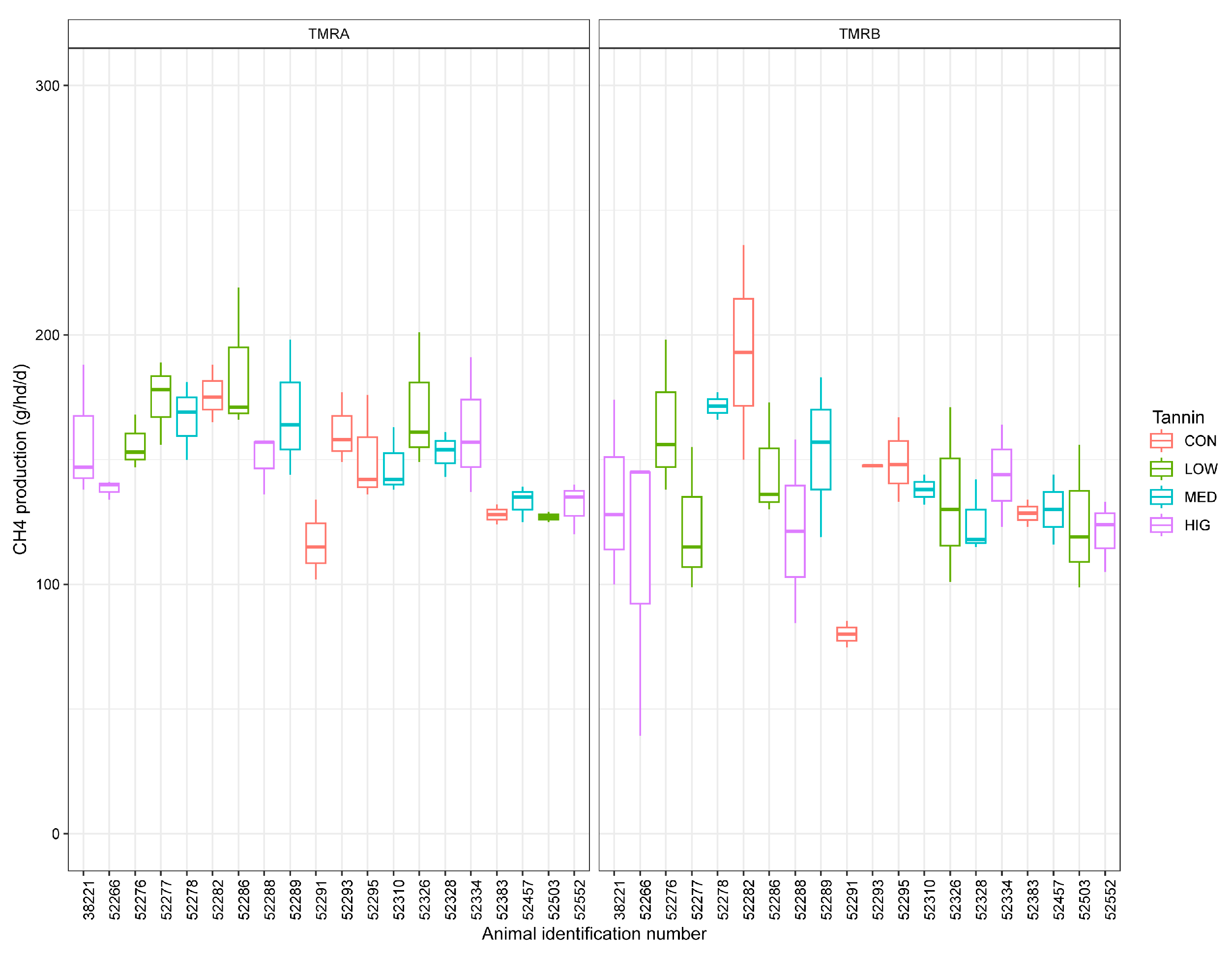
3.1. Methane Emissions Parameters
3.2. Animal Performance Parameters
3.3. Blood Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2019 (No. EPA-430-R-21-005); United States Environmental Protection Agency: Washington, DC, USA, 2021. [Google Scholar]
- Rotz, A.; Stout, R.; Leytem, A.; Feyereisen, G.; Waldrip, H.; Thoma, G.; Holly, M.; Bjorneberg, D.; Baker, J.; Vadas, P.; et al. Environmental assessment of United States dairy farms. J. Clean. Prod. 2021, 315, 128153. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional Management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21. [Google Scholar] [CrossRef]
- Place, S.E.; Mitloehner, F.M. Invited review: Contemporary environmental issues: A review of the dairy industry’s role in climate change and air quality and the potential of mitigation through improved production efficiency. J. Dairy. Sci. 2010, 93, 3407–3416. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Oh, J.; Firkins, J.L.; Dijkstra, J.; Kebreab, E.; Waghorn, G.; Makkar, H.P.; Adesogan, A.T.; Yang, W.; Lee, C.; et al. Special topics—Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J. Anim. Sci. 2013, 91, 5045–5069. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty Years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Field, J.A.; Kortekaas, S.; Lettinga, G. The tannin theory of methanogenic toxicity. Biol. Wastes 1989, 29, 241–262. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Technol. 2005, 123–124, 403–419. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Physiol. Anim. Nutr. 2011, 96, 365–375. [Google Scholar] [CrossRef]
- Díaz Carrasco, J.M.; Cabral, C.; Redondo, L.M.; Pin Viso, N.D.; Colombatto, D.; Farber, M.D.; Fernández Miyakawa, M.E. Impact of chestnut and quebracho tannins on rumen microbiota of Bovines. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef]
- Makkar, H.P.; Becker, K.; Abel, H.; Szegletti, C. Degradation of condensed tannins by rumen microbes exposed to quebracho tannins (QT) in Rumen Simulation Technique (RUSITEC) and effects of qt on fermentative processes in the RUSITEC. J. Sci. Food Agric. 1995, 69, 495–500. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic Archaea and protozoal populations. J. Dairy. Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Carulla, J.E.; Kreuzer, M.; Machmüller, A.; Hess, H.D. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust. J. Agric. Res. 2005, 56, 961. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: A Review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Makkar, H.P. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- United States Code of Federal Regulations, 7 CFR § 205, December 21, 2000. 21 December.
- Hristov, A.N.; Oh, J.; Giallongo, F.; Frederick, T.; Weeks, H.; Zimmerman, P.R.; Harper, M.T.; Hristova, R.A.; Zimmerman, R.S.; Branco, A.F. The use of an automated system (GreenFeed) to monitor enteric methane and carbon dioxide emissions from ruminant animals. J. Vis. Exp. 2015, 103, e52904. [Google Scholar] [CrossRef]
- Gunter, S.A.; Bradford, J.A. Technical note: Effect of bait delivery interval in an automated head-chamber system on respiration gas estimates when cattle are grazing rangeland. Prof. Anim. Sci. 2017, 33, 490–497. [Google Scholar] [CrossRef]
- Gunter, S.A.; Duke, S.E.; Beck, M.R. Measuring the respiratory gas exchange of grazing cattle using the GreenFeed emissions monitoring system. J. Anim. Sci. 2017, 95 (Suppl. 4), 359. [Google Scholar] [CrossRef]
- Huhtanen, P.; Ramin, M.; Hristov, A.N. Enteric methane emission can be reliably measured by the GreenFeed monitoring unit. Livest. Sci. 2019, 222, 31–40. [Google Scholar] [CrossRef]
- Velazco, J.I.; Mayer, D.G.; Zimmerman, S.; Hegarty, R.S. Use of short-term breath measures to estimate daily methane production by cattle. Animal 2016, 10, 25–33. [Google Scholar] [CrossRef]
- Arthur, P.F.; Barchia, I.M.; Weber, C.; Bird-Gardiner, T.; Donoghue, K.A.; Herd, R.M.; Hegarty, R.S. Optimizing test procedures for estimating daily methane and carbon dioxide emissions in cattle using short-term breath measures. J. Anim. Sci. 2017, 95, 645–656. [Google Scholar] [CrossRef]
- Thompson, L.R.; Beck, M.R.; Gunter, S.A.; Williams, G.D.; Place, S.E.; Reuter, R.R. An energy and monensin supplement reduces methane emission intensity of stocker cattle grazing winter wheat. Appl. Anim. Behav. Sci. 2019, 35, 433–440. [Google Scholar] [CrossRef]
- Armstrong, D.; Browne, R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the Clinical Chemistry Laboratory. Adv. Exp. Med. Biol. 1994, 366, 43–58. [Google Scholar] [CrossRef]
- Beck, M.R.; Gregorini, P. How dietary diversity enhances hedonic and eudaimonic well-being in grazing ruminants. Front. Vet. Sci. 2020, 7, 191. [Google Scholar] [CrossRef]
- Malmstrom, B.G.; Andreasson, L.-E.; Reinhammar, B. Copper-containing oxidases and superoxide dismutase. Enzymes 1975, 12, 507–579. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A.; McGinn, S.M.; Martinez, T.F.; McAllister, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Ebert, P.J.; Bailey, E.A.; Shreck, A.L.; Jennings, J.S.; Cole, N.A. Effect of condensed tannin extract supplementation on growth performance, nitrogen balance, gas emissions, and energetic losses of beef steers. J. Anim. Sci. 2017, 95, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effects of quebracho tannin extract on intake, digestibility, rumen fermentation, and methane production in crossbred heifers fed low-quality tropical grass. Trop. Anim. Health Prod. 2017, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Almaraz, M.; Rudnick, J.; Parker, L.E.; Ostoja, S.M.; Khalsa, S.D. Farmer adoption of climate-smart practices is driven by farm characteristics, information sources, and practice benefits and challenges. Sustainability 2023, 15, 8083. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-smart agriculture for food security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Ramana, D. Climate Change Impacts and Innovative Adoption Options for Smart Animal-Agriculture. In Impact of Climate Change on Livestock Health and Production; CRC Press: Boca Raton, FL, USA, 2023; pp. 77–81. [Google Scholar]
- Liu, H.W.; Zhou, D.W.; Li, K. Effect of chestnut tannins on performance and antioxidative status of transition dairy cows. J. Dairy Sci. 2013, 96, 5901–5907. [Google Scholar] [CrossRef]
| Diet Composition 1 | ||
|---|---|---|
| Ingredients | TMRA | TMRB |
| Alfalfa Hay, % | 4.8 | 36.8 |
| Oat Hay, % | 9.1 | 9.8 |
| Straw, % | 4.4 | 0.0 |
| Haylage, % | 15.2 | 23.0 |
| Corn Silage, % | 23.9 | 17.8 |
| Mixed Grains, % | 30.6 | 7.4 |
| Ground Corn, % | 8.3 | 4.7 |
| Minerals, % | 3.8 | 0.56 |
| Feed Nutritive Analysis 1 | |||||
|---|---|---|---|---|---|
| Item 2 | TMRA | TMRB | AP | SF | TAN |
| DM, % | 45.8 | 45.0 | 92.0 | 93.0 | 91.6 |
| CP, % | 19.0 | 18.6 | 20.0 | 21.1 | 1.8 |
| TDN, % | 72 | 70 | 61 | 81 | 90 |
| GE, cal/g | 4571 | 4603 | 4668 | 4841 | 5341 |
| % ADF 3 | 20.9 | 27.7 | 30.8 | 7.6 | 0.2 |
| % NDF 4 | 29.7 | 36.7 | 35 | 17.6 | 0.3 |
| Control | Tannin | SE | p-Value 1 | Treatment 2 | SE | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter 4 | CON | LOW | MED | HIG | Linear | Quadratic | TMR | |||||
| n | 5 | 15 | 5 | 5 | 5 | 5 | ||||||
| CH4 Production, g CH4/hd/d | 146 | 145 | 5.2 | 0.88 | 146 | 145 | 149 | 142 | 5.2 | 0.56 | 0.52 | 0.002 |
| Ym,%GE intake | 6.7 | 6.8 | 0.01 | 0.92 | 6.7 | 7.7 | 6.8 | 5.7 | 0.01 | 0.13 | 0.21 | 0.01 |
| MY, g CH4/kg DMI | 23.4 | 23.6 | 2.2 | 0.91 | 23.4 | 27.1 | 23.9 | 19.9 | 2.2 | 0.13 | 0.21 | 0.01 |
| EI, g CH4/kg ADG | 109.9 | 141.8 | 30.3 | 0.30 | 109.9 | 148.2 | 126.9 | 150.1 | 26.8 | 0.38 | 0.79 | 0.004 |
| Control | Tannin | SE | p-Value 1 | Treatment 2 | SE | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter 4 | CON | LOW | MED | HIG | Linear | Quadratic | TMR | |||||
| n | 5 | 15 | 5 | 5 | 5 | 5 | ||||||
| ADG, kg BW gain/d | 0.50 | 0.52 | 0.23 | 0.92 | 0.50 | 0.57 | 0.50 | 0.50 | 0.20 | 0.91 | 0.90 | <0.001 |
| FBW, kg | 250 | 252 | 1.13 | 0.11 | 250 | 254 | 251 | 251 | 1.13 | 0.70 | 0.07 | - |
| DMI, kg DMI/d | 6.2 | 6.7 | 0.72 | 0.61 | 6.2 | 6.4 | 6.6 | 7.0 | 0.72 | 0.42 | 0.96 | 0.06 |
| TMR, kg TMR DMI/d | 4.9 | 5.3 | 0.72 | 0.66 | 4.9 | 5.0 | 5.2 | 5.6 | 0.72 | 0.45 | 0.93 | 0.07 |
| SF, kg SF DMI/d | 0.93 | 0.93 | 0.00 | - | 0.93 | 0.93 | 0.93 | 0.93 | 0.00 | - | - | - |
| GF, kg GF DMI/d | 0.34 | 0.40 | 0.03 | 0.07 | 0.34 | 0.44 | 0.38 | 0.40 | 0.03 | 0.46 | 0.27 | 0.02 |
| G:F, ADG/DMI | 0.10 | 0.09 | 0.03 | 0.66 | 0.10 | 0.11 | 0.08 | 0.08 | 0.03 | 0.46 | 0.94 | <0.001 |
| Control | Tannin | SE | p-Value 1 | Treatment 2 | SE | p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter 4 | CON | LOW | MED | HIG | Linear | Quadratic | TMR | |||||
| n | 5 | 15 | 5 | 5 | 5 | 5 | ||||||
| MDA, μM | 0.51 | 0.63 | 0.22 | 0.55 | 0.51 | 0.41 | 0.75 | 0.75 | 0.19 | 0.23 | 0.87 | 0.34 |
| SOD, units/mL | 58.18 | 51.59 | 4.01 | 0.11 | 58.18 | 51.07 | 60.85 | 42.84 | 3.53 | 0.01 | 0.11 | 0.02 |
| GSH, μM | 7.13 | 10.49 | 1.07 | 0.003 | 7.13 | 8.80 | 11.7 | 10.9 | 0.94 | 0.003 | 0.04 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schilling-Hazlett, A.; Raynor, E.J.; Thompson, L.; Velez, J.; Place, S.; Stackhouse-Lawson, K. On-Farm Methane Mitigation and Animal Health Assessment of a Commercially Available Tannin Supplement in Organic Dairy Heifers. Animals 2024, 14, 9. https://doi.org/10.3390/ani14010009
Schilling-Hazlett A, Raynor EJ, Thompson L, Velez J, Place S, Stackhouse-Lawson K. On-Farm Methane Mitigation and Animal Health Assessment of a Commercially Available Tannin Supplement in Organic Dairy Heifers. Animals. 2024; 14(1):9. https://doi.org/10.3390/ani14010009
Chicago/Turabian StyleSchilling-Hazlett, Ashley, Edward J. Raynor, Logan Thompson, Juan Velez, Sara Place, and Kim Stackhouse-Lawson. 2024. "On-Farm Methane Mitigation and Animal Health Assessment of a Commercially Available Tannin Supplement in Organic Dairy Heifers" Animals 14, no. 1: 9. https://doi.org/10.3390/ani14010009
APA StyleSchilling-Hazlett, A., Raynor, E. J., Thompson, L., Velez, J., Place, S., & Stackhouse-Lawson, K. (2024). On-Farm Methane Mitigation and Animal Health Assessment of a Commercially Available Tannin Supplement in Organic Dairy Heifers. Animals, 14(1), 9. https://doi.org/10.3390/ani14010009





