Estimating the Effect of the Kappa Casein Genotype on Milk Coagulation Properties in Israeli Holstein Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bittante, G.; Penasa, M.; Cecchinato, A. Genetics and modeling of milk coagulation properties. J. Dairy Sci. 2012, 95, 6483–6870. [Google Scholar] [CrossRef]
- Ikonen, T.; Ahlfors, K.; Kempe, R.; Ojala, M.; Ruottinen, O. Genetic parameters for the milk coagulation properties and prevalence of noncoagulating milk in Finnish dairy cows. J. Dairy Sci. 1999, 82, 205–214. [Google Scholar] [CrossRef]
- Wedholm, A.; Larsen, L.B.; Lindmark-Månsson, H.; Karlsson, A.H.; Andrén, A. Effect of protein composition on the cheese-making properties of milk from individual dairy cows. J. Dairy Sci. 2006, 89, 3296–3305. [Google Scholar] [CrossRef]
- Cassandro, M.; Comin, A.; Ojala, M.; Dal Zotto, R.; De Marchi, L.; Gallo, L.; Carnier, P.; Bittante, G. Genetic parameters of milk coagulation properties and their relationships with milk yield and quality traits in Italian Holstein. J. Dairy Sci. 2008, 91, 371–376. [Google Scholar] [CrossRef]
- Israeli Dairy Board. 2020. Available online: https://www.israeldairy.com/category/dairy-farming/facts-and-figures/ (accessed on 1 January 2023).
- Leitner, G.; Lavon, Y.; Merin, U.; Jacoby, S.; Blum, S.E.; Krifucks, O.; Silanikove, N. Milk quality and milk transformation parameters from infected mammary glands depends on the infecting bacteria species. PLoS ONE 2019, 14, e0213817. [Google Scholar] [CrossRef]
- Guinee, T.P.; O’Kennedy, B.T.; Kelly, P.M. Effect of milk protein standardization using different methods on the composition and yields of Cheddar cheese. J. Dairy Sci. 2006, 89, 468–482. [Google Scholar] [CrossRef]
- Pretto, D.; De Marchi, M.; Penasa, M.; Cassandro, M. Effect of milk composition and coagulation traits on Grana Padano cheese yield under field conditions. J. Dairy Res. 2013, 80, 1–5. [Google Scholar] [CrossRef]
- Panthi, R.R.; Jordan, K.N.; Kelly, A.L.; Sheehan, J.D. Selection and treatment of milk for cheesemaking. In Cheese, 4th ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 23–50. [Google Scholar]
- Tyrisevä, A.M.; Vahlsten, T.; Ruottinen, O.; Ojala, M. Non-coagulation of milk in Finnish Ayrshire and Holstein-Friesian cows and effect of herds on milk coagulation ability. J. Dairy Sci. 2004, 87, 3958–3966. [Google Scholar] [CrossRef]
- Cecchinato, A.; Penasa, M.; De Marchi, M.; Gallo, L.; Bittante, G.; Carnier, P. Genetic parameters of coagulation properties, milk yield, quality, and acidity estimated using coagulating and noncoagulating milk information in Brown Swiss and Holstein-Friesian cows. J. Dairy Sci. 2011, 94, 4205–4213. [Google Scholar] [CrossRef]
- Tiezzi, F.; Pretto, D.; De Marchi, M.; Penasa, M.; Cassandro, M. Heritability and repeatability of milk coagulation properties predicted by mid-infrared spectroscopy during routine data recording, and their relationships with milk yield and quality traits. Animal 2013, 7, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, T.; Morri, S.; Tyrisevä, A.M.; Ruottinen, O.; Ojala, M. Genetic and phenotypic correlations between milk coagulation properties, milk production traits, somatic cell count, casein content, and pH of milk. J. Dairy Sci. 2004, 87, 458–467. [Google Scholar] [CrossRef]
- Amenu, B.; Cowan, T.; Deeth, H.; Moss, R. Impacts of feeding system and season on milk composition and Cheddar cheese yield in a subtropical environment. Aust. J. Exp. Agri. 2006, 46, 299–306. [Google Scholar] [CrossRef]
- Ikonen, T.; Ojala, M.; Ruottinen, O. Associations between milk protein polymorphism and first lactation milk production traits in Finnish Ayrshire cows. J. Dairy Sci. 1999, 82, 1026–1033. [Google Scholar] [CrossRef]
- Jõudu, I.; Henno, M.; Värv, S.; Viinalass, H.; Püssa, T.; Kaart, T.; Arney, D.; Kärt, O. The effect of milk proteins on milk coagulation properties in Estonian dairy breeds. Vet. Zootec. 2009, 46, 14–19. [Google Scholar]
- Ng-Kwai-Hang, K.F. Genetic polymorphism of milk proteins: Relationships with production traits, milk composition and technological properties. Can. J. Anim. Sci. 1998, 78, 131–147. [Google Scholar]
- Bobe, G.; Freeman, A.E.; Lindberg, G.L. Associations among individual proteins and fatty acids in bovine milk as determined by correlations and factor analyses. J. Dairy Res. 1999, 66, 523–526. [Google Scholar] [CrossRef]
- Hallén, E.; Andrén, A.; Lundén, A. Effect of β-casein, κ-casein and β-lactoglobulin genotypes on concentration of milk protein variants. J. Anim. Breed. Genet. 2008, 125, 119–129. [Google Scholar] [CrossRef]
- Heck, J.M.L.; Schennink, A.; van Valenberg, H.J.F.; Bovenhuis, H.; Visker, M.H.P.W.; van Arendonk, J.A.M.; van Hooijdonk, A.C.M. Effects of milk protein variants on the protein composition of bovine milk. J. Dairy Sci. 2009, 92, 1192–1202. [Google Scholar] [CrossRef]
- Walsh, C.D.; Guinee, T.P.; Reville, W.D.; Harrington, D.; Murphy, J.J.; O’Kennedy, B.T.; FitzGerald, R.J. Influence of κ-casein genetic variant on rennet gel microstructure, cheddar cheesemaking properties and casein micelle size. Int. Dairy J. 1998, 8, 707–714. [Google Scholar] [CrossRef]
- Ron, M.; Yoffe, O.; Ezra, E.; Medrano, J.F.; Weller, J.I. Determination of effects of milk protein genotype on production traits of Israeli Holsteins. J. Dairy Sci. 1994, 77, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M. Breeding for Kappa Casein to Increase Cheese Yield; The Bullvine: Hamilton, ON, USA, 2017; Available online: https://www.thebullvine.com/genetics/breeding-for-kappa-casein-to-increase-cheese-yield/ (accessed on 1 January 2023).
- Zepeda-Batista, J.; Alarcón-Zúñiga, B.; Ruíz-Flores, A.; Núñez-Domínguez, R.; Ramírez-Valverde, R. Polymorphism of three milk protein genes in Mexican Jersey cattle. Electron. J. Biotechnol. 2015, 18, 1–4. [Google Scholar] [CrossRef]
- Bartonova, P.; Vrtkova, I.; Kaplanova, K.; Urban, T. Association between CSN3 and BCO2 gene polymorphisms and milk performance traits in the Czech Fleckvieh cattle breed. Gen. Mol. Res. 2012, 27, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Morkūnienė, K.; Baltrėnaitė, L.; Puišytė, A.; Bižienė, R.; Pečiulaitienė, N.; Makštutienė, N.; Mišeikienė, R.; Miceikienė, I.; Kerzienė, S. Associations of kappa casein polymorphism with milk yield and milk protein genomic values in cows reared in Lithuania. Vet. Zootec. 2016, 74, 27–32. [Google Scholar]
- Leitner, G.; Merin, U.; Silanikove, N. Effects of glandular bacterial infection and stage of lactation on milk quality: Comparison among cows, goats and sheep. Int. Dairy J. 2011, 21, 279–285. [Google Scholar] [CrossRef]
- Lavon, Y.; Ezra, E.; Leitner, G.; Wolfenson, D. Association of conception rate with pattern and level of somatic cell count elevation relative to time of insemination in dairy cows. J. Dairy Sci. 2011, 94, 4538–45445. [Google Scholar] [CrossRef]
- Weller, J.I.; Gershoni, M.; Ezra, E. Breeding dairy cattle for fertility and production in the age of genomics. Vet. Sci. 2022, 9, 434–447. [Google Scholar] [CrossRef]
- Hristov, P.; Neov, B.; Sbirkova, H.; Teofanova, D.; Radoslavov, G.; Shivachev, B. Genetic polymorphism of kappa casein and casein micelle size in the Bulgarian Rhodopean cattle breed. Biotechnol. Anim. Husb. 2014, 30, 561–570. [Google Scholar] [CrossRef]
- Aleandri, R.; Buttazzoni, L.G.; Schneider, J.C.; Caroli, A.; Davoli, R. The effects of milk protein polymorphisms on milk components and cheese-producing ability. J. Dairy Sci. 1990, 73, 241–255. [Google Scholar] [CrossRef]
- Devold, T.G.; Brovold, M.J.; Langsrud, T.; Vegarud, G.E. Size of native and heated casein micelles, content of protein and minerals in milk from Norwegian Red Cattle—Effect of milk protein polymorphism and different feeding regimes. Int. Dairy J. 2000, 10, 313–323. [Google Scholar] [CrossRef]
- Lodes, A.; Buchberger, J.; Krause, J.; Aumann, J.; Klostermeyer, H. The influence of genetic variants of milk proteins on the compositional and technological properties of milk. 3. Content of protein, casein, whey protein, and casein number. Milchwissenschaft 1997, 52, 3–8. [Google Scholar]
- Mao, I.L.; Buttazzoni, L.G.; Aleandri, R. Effects of polymorphic milk protein genes on milk yield and composition traits in Holstein cattle. Acta Agric. Scand. Sect. A Anim. Sci. 1992, 42, 1–7. [Google Scholar] [CrossRef]
- Comin, A.; Cassandro, M.; Chessa, S.; Ojala, M.; Zotto, R.D.; Marchi, M.D.; Carnier, P.; Gallo, L.; Pagnacco, G.; Bittante, G. Effects of composite β- and κ-casein genotypes on milk coagulation, quality, and yield traits in Italian Holstein cows. J. Dairy Sci. 2008, 91, 4022–4027. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.B.; Holland, J.W.; Poulsen, N.A.; Larsen, L.B. Milk protein genetic variants and isoforms identified in bovine milk representing extremes in coagulation properties. J. Dairy Sci. 2012, 95, 2891–2903. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Szymanowska, M.; Zwierzchowski, L.; Leroux, C. The impact of genetic polymorphisms on the protein composition of ruminant milks. Reprod. Nutr. Dev. 2002, 42, 433–459. [Google Scholar] [CrossRef]
- Lundén, A.; Nilsson, M.; Janson, L. Marked effect of β-lactoglobulin polymorphism on the ratio of casein to total protein in milk. J. Dairy Sci. 1997, 80, 2996–3005. [Google Scholar] [CrossRef]
- Hallén, E.; Allmere, T.; Näslund, J.; Andrén, A.; Lundén, A. Effect of genetic polymorphism of milk proteins on rheology of chymosin-induced milk gels. Int. Dairy J. 2007, 17, 791–799. [Google Scholar] [CrossRef]

| Frequency (%) | n | Allele | Frequency (%) | n | Genotype 1 |
|---|---|---|---|---|---|
| 57.11 | 6706 | A | 32.47 | 1908 | AA |
| 40.28 | 4743 | B | 46.33 | 2723 | AB |
| 2.61 | 305 | E | 2.84 | 167 | AE |
| 16.05 | 943 | BB | |||
| 2.28 | 134 | BE | |||
| 0.03 | 2 | EE | |||
| 100 | 11,754 | 100 | 5877 | Total |
| Frequency (%) | N | Allele | Frequency (%) | n 2 | Genotype 1 |
|---|---|---|---|---|---|
| 43.31 | 311 | A | 17.83 | 64 | AA |
| 44.43 | 319 | B | 39.55 | 142 | AB |
| 12.26 | 88 | E | 11.42 | 41 | AE |
| 18.11 | 65 | BB | |||
| 13.09 | 47 | BE | |||
| 0 | 0 | EE | |||
| 100 | 718 | 100 | 359 | Total |
| Genotype 1 | n 2 | Milk (kg) | Fat 3 (%) | Protein (%) | SCS | DIM |
|---|---|---|---|---|---|---|
| AA | 64 | 42.6 | 3.18 | 3.20 | 1.8 | 135 |
| AB | 142 | 40.3 | 3.54 | 3.30 | 2.1 | 147 |
| AE | 41 | 39.7 | 3.41 | 3.30 | 2.2 | 161 |
| BE | 47 | 39.6 | 3.49 | 3.36 | 2.4 | 172 |
| BB | 65 | 43.5 | 3.43 | 3.27 | 1.8 | 140 |
| Total | 359 | 41.4 | 3.43 | 3.28 | 2.0 | 149 |
| Factor | Level | RCT | SE | Pr > |t| | CF-60 (V) | SE | Pr > |t| |
|---|---|---|---|---|---|---|---|
| Genotype | 0.3409 | <0.0001 | |||||
| AA | 1.36 | 0.848 | −2.14 | 0.253 | |||
| AB | −0.01 | 0.711 | −0.85 | 0.212 | |||
| AE | 0.24 | 0.959 | −2.44 | 0.286 | |||
| BE | 0.94 | 0.908 | −1.27 | 0.271 | |||
| BB | 0.00 | - | 0.00 | - | |||
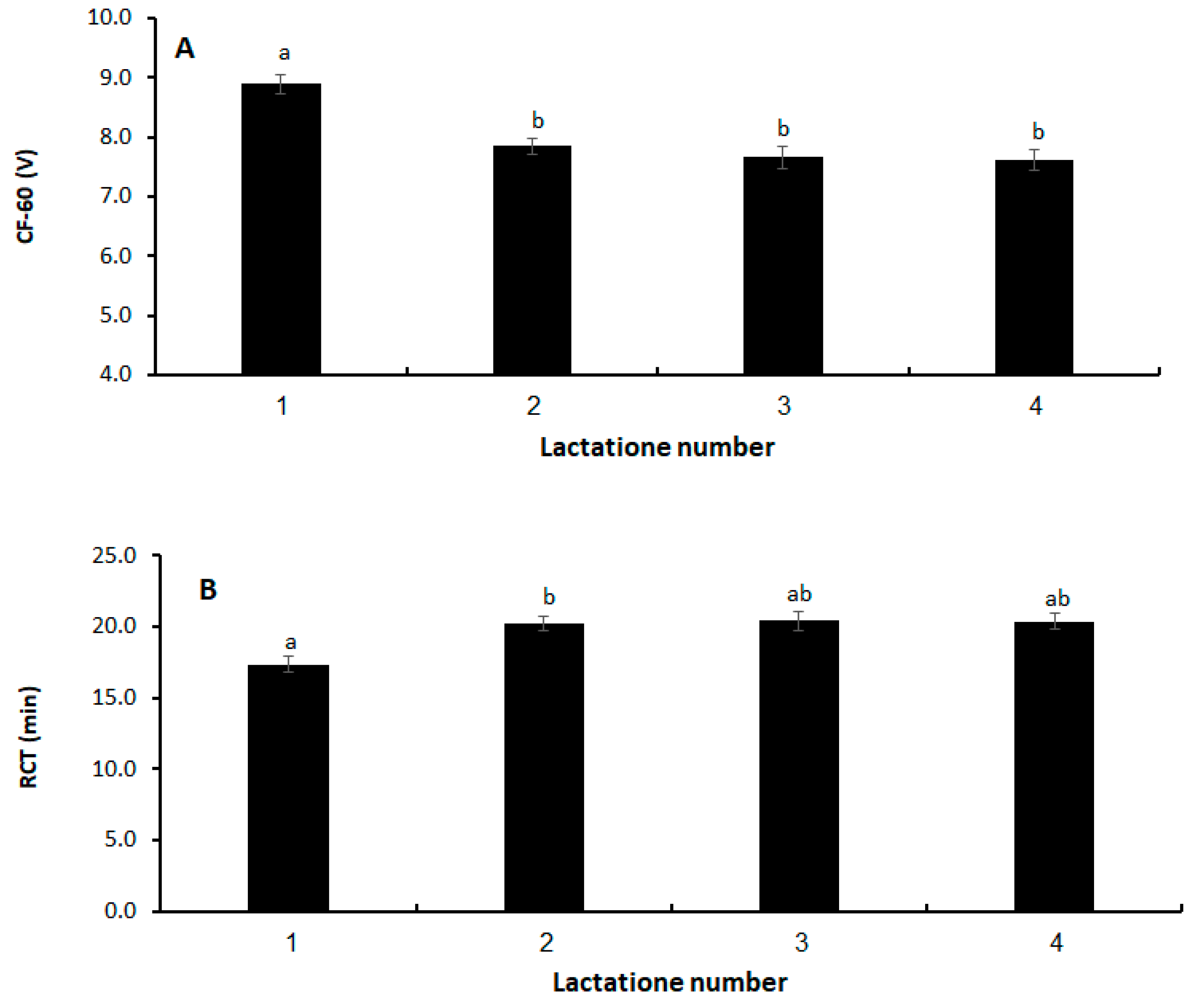
| Lactation number | 0.0006 | <0.0001 | |||||
| 1 | −2.73 | 0.829 | 1.20 | 0.247 | |||
| 2 | 0.23 | 0.720 | 0.21 | 0.214 | |||
| 3 | −0.02 | 0.810 | 0.05 | 0.241 | |||
| 4 | 0.00 | - | 0.00 | - | |||
| SCS | 0.36 | 0.168 | 0.0349 | −0.03 | 0.050 | ||
| Milk (kg) | −0.11 | 0.040 | 0.0064 | 0.05 | 0.012 | <0.0001 | |
| Fat (%) | −1.03 | 0.413 | 0.0132 | 0.77 | 0.123 | <0.0001 | |
| Protein (%) | 1.67 | 0.979 | NS | 2.69 | 0.292 | <0.0001 | |
| DIM | 0.01 | 0.004 | 0.0006 | 0.0028 | 0.001 | 0.0237 |
| 10 Cows with the Highest CF-60 Scores | 10 Cows with the Lowest CF-60 Scores | ||||||
|---|---|---|---|---|---|---|---|
| Visual Index | RCT | CF-60 | Genotype | Visual Index | RCT | CF-60 | Genotype |
| 3.7 | 15.8 | 14.6 | BB | 2.8 | 14.3 | 4.2 | AE |
| 3.7 | 19.5 | 14.4 | BE | 1.0 | 21.9 | 4.2 | AA |
| 3.7 | 18.5 | 14.1 | AA | 2.5 | 19.1 | 4.1 | AE |
| 3.3 | 16.1 | 13.4 | AB | 3.4 | 17.3 | 4.0 | AE |
| 3.8 | 22.8 | 13.2 | BB | 2.6 | 18.6 | 3.8 | AE |
| 3.8 | 16.4 | 13.0 | BB | 0.0 | 42.4 | 3.4 | AB |
| 3.7 | 21.1 | 12.8 | AB | 0.0 | 42.1 | 3.4 | AE |
| 3.9 | 14.9 | 12.8 | BB | 3.5 | 12.7 | 3.3 | AE |
| 3.6 | 20.8 | 12.3 | BB | 1.9 | 13.7 | 2.8 | AA |
| 3.6 | 18.9 | 12.3 | BB | 0.2 | 31.6 | 0.3 | AA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavon, Y.; Weller, J.I.; Zeron, Y.; Ezra, E. Estimating the Effect of the Kappa Casein Genotype on Milk Coagulation Properties in Israeli Holstein Cows. Animals 2024, 14, 54. https://doi.org/10.3390/ani14010054
Lavon Y, Weller JI, Zeron Y, Ezra E. Estimating the Effect of the Kappa Casein Genotype on Milk Coagulation Properties in Israeli Holstein Cows. Animals. 2024; 14(1):54. https://doi.org/10.3390/ani14010054
Chicago/Turabian StyleLavon, Yaniv, Joel I. Weller, Yoel Zeron, and Ephraim Ezra. 2024. "Estimating the Effect of the Kappa Casein Genotype on Milk Coagulation Properties in Israeli Holstein Cows" Animals 14, no. 1: 54. https://doi.org/10.3390/ani14010054
APA StyleLavon, Y., Weller, J. I., Zeron, Y., & Ezra, E. (2024). Estimating the Effect of the Kappa Casein Genotype on Milk Coagulation Properties in Israeli Holstein Cows. Animals, 14(1), 54. https://doi.org/10.3390/ani14010054






