Glucose Regulates Glucose Transport and Metabolism via mTOR Signaling Pathway in Bovine Placental Trophoblast Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. BPTCs Growth Curve
2.3. Immunofluorescence Identification of BPTCs
2.4. Experimental Design
2.5. Cell Morphology and Viability Assay
2.6. Flow Cytometric Analyze Cell Cycle
2.7. ATP Content Determination
2.8. RNA Extraction and Quantitative Real-Time PCR
2.9. Western Blotting
3. Statistical Analysis
4. Results
4.1. Identification of the BPTCs
4.2. Effect of Glucose on Morphology, Viability and ATP Content of BPTCs
4.3. Effect of Glucose on Cell Cycle of BPTCs
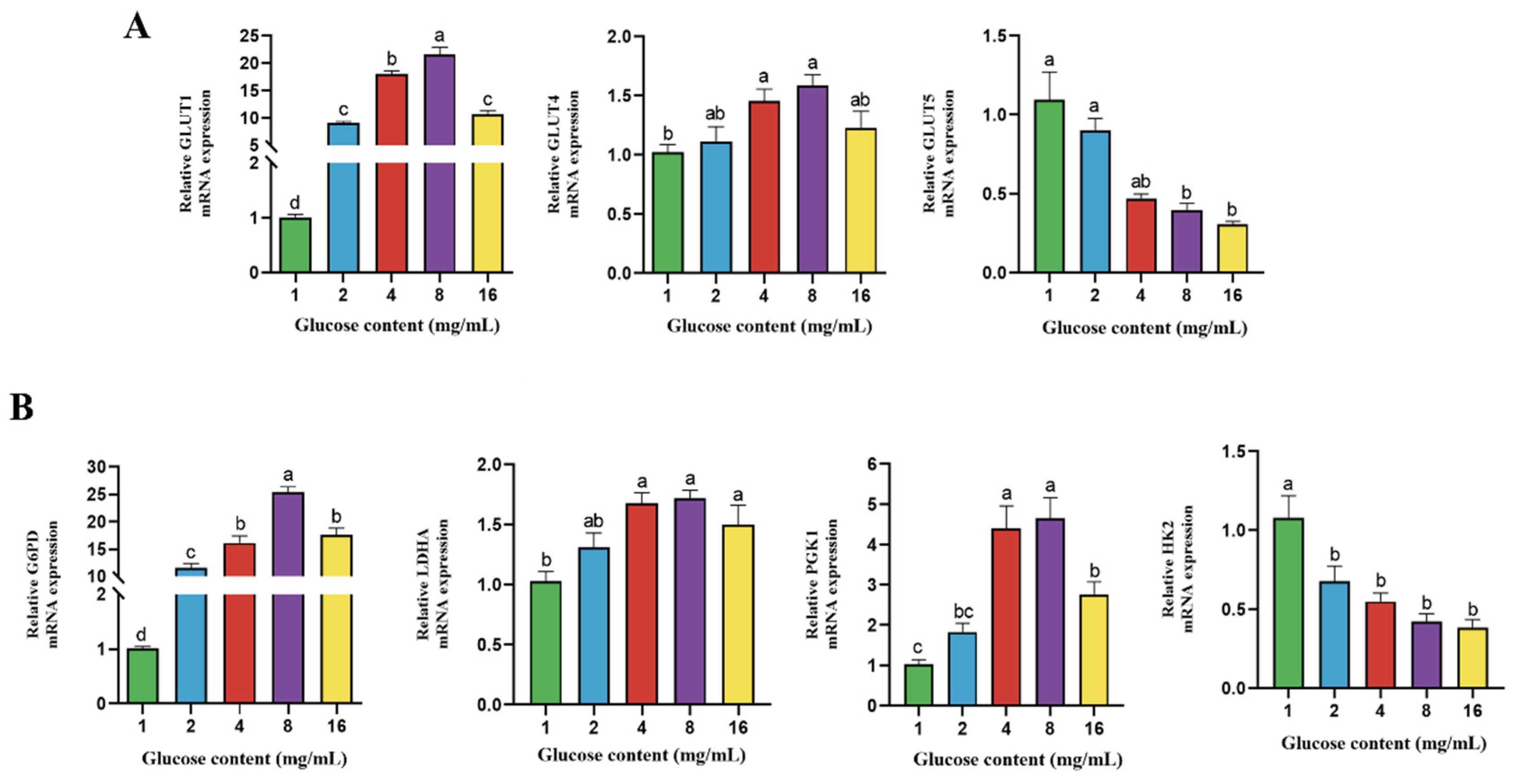
4.4. Effect of Glucose on Gene Expression of Transporter and Enzymes Related to Glucose Metabolism in BPTCs
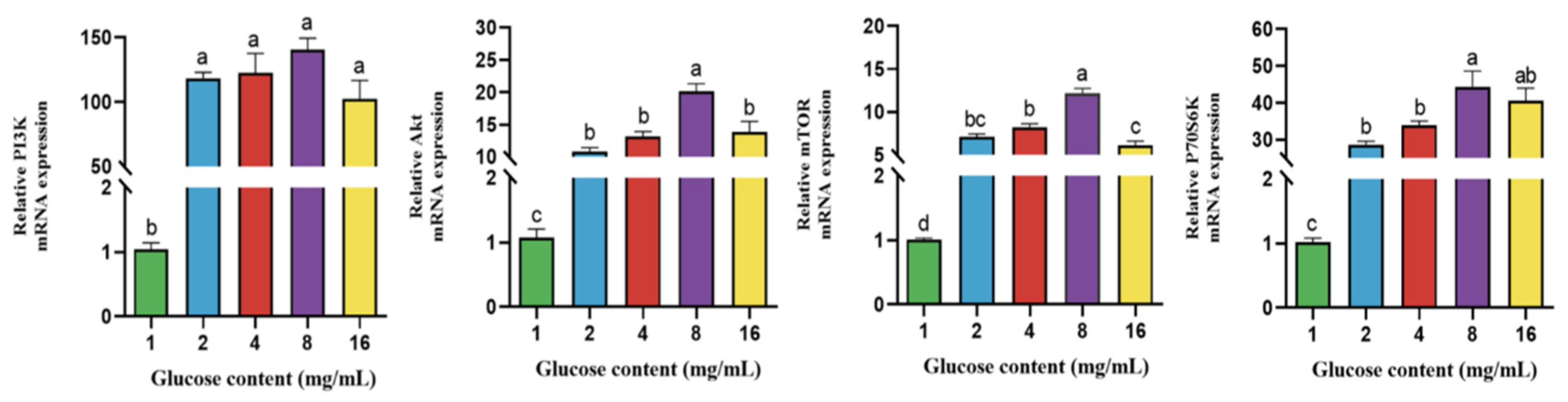
4.5. Effect of Glucose on Gene Expression of PI3K-Akt/mTOR Signaling Pathway in BPTCs
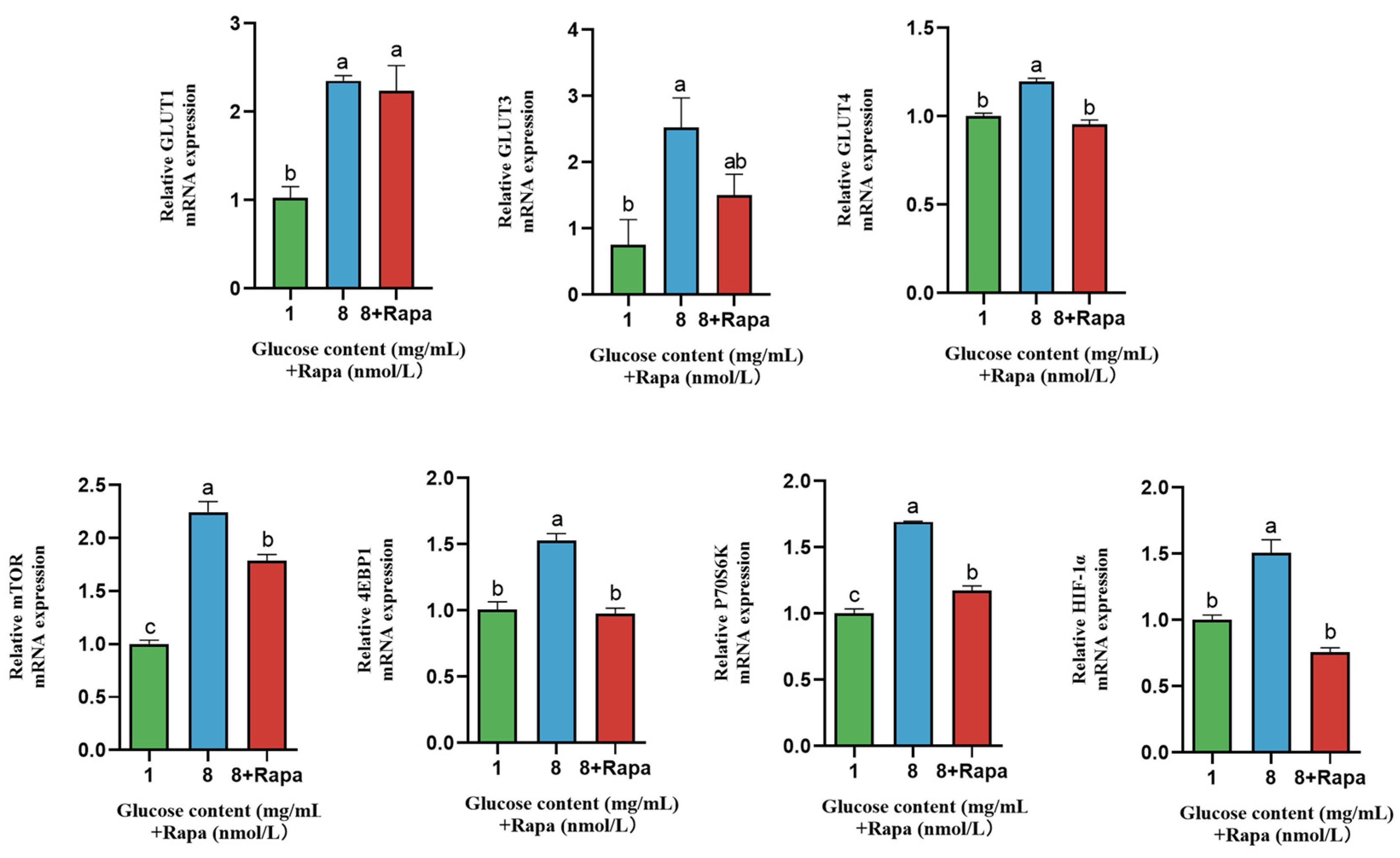
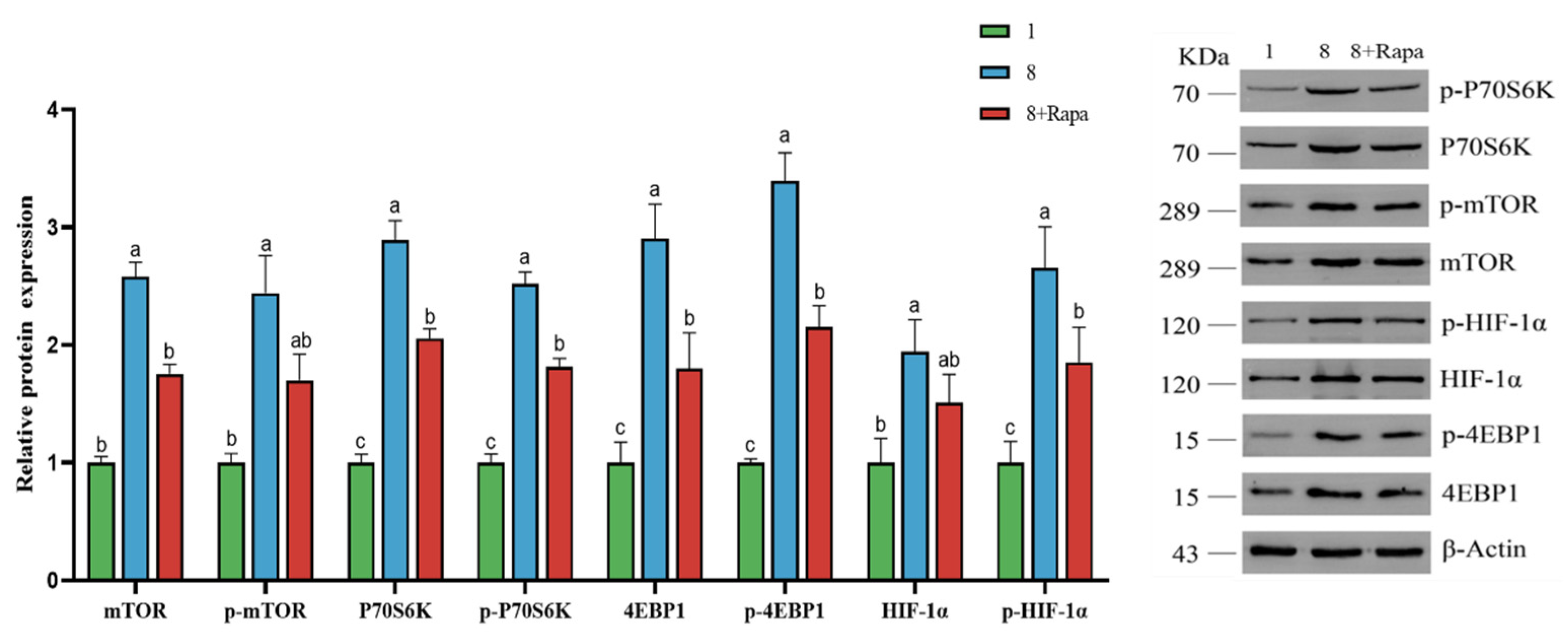
4.6. Effect of Rapamycin (Rapa) Decreased the Stimulation of Glucose Transporters in BPTCs
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozcan, S. Two different signals regulate repression and induction of gene expression by glucose. J. Biol. Chem. 2002, 277, 46993–46997. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K.; Overton, T.R.; Douglas, G.N. Adaptations of glucose and long-chain fatty acid metabolism in liver of dairy cows during the periparturient period. J. Dairy Sci. 2001, 84, E100–E112. [Google Scholar] [CrossRef]
- Horst, E.; Kvidera, S.; Baumgard, L. Invited review: The influence of immune activation on transition cow health and performance-A critical evaluation of traditional dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W. Regulation of placental metabolism by glucose supply. Reprod. Fertil. Dev. 1995, 7, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.H.; Murphy, H.A.; Chapman, H.; George, E.M. Syncytialization alters the extracellular matrix and barrier function of placental trophoblasts. Am. J. Physiol.-Cell Physiol. 2021, 321, C694–C703. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F. The ruminant placental trophoblast binucleate cell: An evolutionary breakthrough. Biol. Reprod. 2022, 107, 705–716. [Google Scholar] [CrossRef]
- Paul, M.; Chakraborty, S.; Islam, S.; Ain, R. Trans-differentiation of trophoblast stem cells: Implications in placental biology. Life Sci. Alliance 2023, 6, e202201583. [Google Scholar] [CrossRef]
- Wooding, F.; Fowden, A.; Bell, A.; Ehrhardt, R.; Limesand, S.; Hay, W. Localisation of glucose transport in the ruminant placenta: Implications for sequential use of transporter isoforms. Placenta 2005, 26, 626–640. [Google Scholar] [CrossRef]
- Joshi, N.P.; Mane, A.R.; Sahay, A.S.; Sundrani, D.P.; Joshi, S.R.; Yajnik, C.S. Role of placental glucose transporters in determining fetal growth. Reprod. Sci. 2022, 29, 2744–2759. [Google Scholar] [CrossRef]
- Lüscher, B.P.; Marini, C.; Joerger-Messerli, M.S.; Huang, X.; Hediger, M.A.; Albrecht, C.; Baumann, M.U.; Surbek, D.V. Placental glucose transporter (GLUT)-1 is down-regulated in preeclampsia. Placenta 2017, 55, 94–99. [Google Scholar] [CrossRef]
- Kramer, A.C.; Steinhauser, C.B.; Gao, H.; Seo, H.; McLendon, B.A.; Burghardt, R.C.; Wu, G.; Bazer, F.W.; Johnson, G.A. Steroids regulate SLC2A1 and SLC2A3 to deliver glucose into trophectoderm for metabolism via glycolysis. Endocrinology 2020, 161, bqaa098. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Chao, A.-S.; Chang, S.-D.; Cheng, P.-J. Placental glucose transporter 1 and 3 gene expression in Monochorionic twin pregnancies with selective fetal growth restriction. BMC Pregnancy Childbirth 2021, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Stanirowski, P.J.; Szukiewicz, D.; Pyzlak, M.; Abdalla, N.; Sawicki, W.; Cendrowski, K. Impact of pre-gestational and gestational diabetes mellitus on the expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 in human term placenta. Endocrine 2017, 55, 799–808. [Google Scholar] [CrossRef]
- Kang, K.; Zeng, L.; Ma, J.; Shi, L.; Hu, R.; Zou, H.; Peng, Q.; Wang, L.; Xue, B.; Wang, Z. High energy diet of beef cows during gestation promoted growth performance of calves by improving placental nutrients transport. Front. Vet. Sci. 2022, 9, 1053730. [Google Scholar] [CrossRef]
- Lucy, M.; Green, J.; Meyer, J.; Williams, A.; Newsom, E.; Keisler, D. Short communication: Glucose and fructose concentrations and expression of glucose transporters in 4- to 6-week pregnancies collected from Holstein cows that were either lactating or not lactating. J. Dairy Sci. 2012, 95, 5095–5101. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Sawai, K.; Hirayama, M.; Hirai, T.; Kageyama, S.; Onoe, S.; Minamihashi, A.; Moriyasu, S. Prepartum maternal plasma glucose concentrations and placental glucose transporter mRNA expression in cows carrying somatic cell clone fetuses. J. Reprod. Dev. 2011, 57, 57–61. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, W. Role of mTOR in glucose and lipid metabolism. Int. J. Mol. Sci. 2018, 19, 2043. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X. Research progress of mTOR inhibitors. Eur. J. Med. Chem. 2020, 208, 112820. [Google Scholar] [CrossRef]
- Yang, X.; Yang, C.; Farberman, A.; Rideout, T.; De Lange, C.; France, J.; Fan, M. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. J. Anim. Sci. 2008, 86, E36–E50. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef]
- Roos, S.; Lagerlöf, O.; Wennergren, M.; Powell, T.; Jansson, T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am. J. Physiol.-Cell Physiol. 2009, 297, C723–C731. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, T.M.; Holme, A.M.; Holm, M.B.; Roland, M.C.; Haugen, G.; Powell, T.L.; Jansson, T.; Henriksen, T. Uteroplacental glucose uptake and fetal glucose consumption: A quantitative study in human pregnancies. J. Clin. Endocrinol. Metab. 2019, 104, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mi, A.; Liu, Y.; Wang, D.; Ran, L.; Wu, Y. The Maternal Impact of AdGHRKO Mice on Glucose Metabolism. Diabetes 2018, 67 (Suppl. S1), 17-OR. [Google Scholar] [CrossRef]
- Duque Quintero, M.; Olivera, M.; Rosero Noguera, R. Metabolismo energético en vacas durante la lactancia temprana y el efecto de la suplementación con grasa protegida. Rev. Colomb. Cienc. Pecu. 2011, 24, 74–82. [Google Scholar]
- Su, Y.; Li, Q.; Zhang, Q.; Li, Z.; Yao, X.; Guo, Y.; Xiao, L.; Wang, X.; Ni, H. Exosomes derived from placental trophoblast cells regulate endometrial epithelial receptivity in dairy cows during pregnancy. J. Reprod. Dev. 2022, 68, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cui, H.; Cui, Y.; Bai, C.; Gong, T.; Peng, X. Cell-cycle blockage associated with increased apoptotic cells in the thymus of chickens fed on diets high in fluorine. Human Exp. Toxicol. 2011, 30, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, M.A.; Bursac, Z.; Eby, W.M.; Singh, K.P. Mathematical modeling of stem cell proliferation. Med. Biol. Eng. Comput. 2011, 49, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Cui, H.; Peng, X.; Chen, Z.; He, M.; Tang, L. Developmental changes in cell proliferation and apoptosis in the normal duck thymus. Anat. Histol. Embryol. 2011, 40, 457–465. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- He, B.; Bai, J.; Wu, Z. Glucosamine enhances proliferation, barrier, and anti-oxidative functions in porcine trophectoderm cells. Food Funct. 2022, 13, 4551–4561. [Google Scholar] [CrossRef]
- Arponen, M.; Jalava, N.; Widjaja, N.; Ivaska, K.K. Glucose transporters GLUT1, GLUT3, and GLUT4 have different effects on osteoblast proliferation and metabolism. Front. Physiol. 2022, 13, 2511. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V. Abstract IA10: Metabolic regulation of cell proliferation. In Proceedings of the Abstracts: AACR Special Conference on Tumor Immunology and Immunotherapy, Miami Beach, FL, USA, 27–30 November 2018. [Google Scholar]
- Knipp, G.T.; Audus, K.L.; Soares, M.J. Nutrient transport across the placenta. Adv. Drug Deliv. Rev. 1999, 38, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Cao, G.; Chen, D.; Liu, J.; Yu, B.; Liu, M.; Li, Y.-X.; Cao, B.; Sadovsky, Y.; Wang, Y.-L. Placental trophoblast syncytialization potentiates macropinocytosis via mTOR signaling to adapt to reduced amino acid supply. Proc. Natl. Acad. Sci. USA 2021, 118, e2017092118. [Google Scholar] [CrossRef]
- Hung, T.-H.; Wu, C.-P.; Chen, S.-F. Differential changes in Akt and AMPK phosphorylation regulating mTOR activity in the placentas of pregnancies complicated by fetal growth restriction and gestational diabetes mellitus with large-for-gestational age infants. Front. Med. 2021, 2529, 788969. [Google Scholar] [CrossRef] [PubMed]
- Le Bouteiller, P.; Bensussan, A. Up-and-down immunity of pregnancy in humans. F1000Research 2017, 6, 1216. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Karahoda, R. Trophoblast: The central unit of fetal growth, protection and programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Han, C.; He, F.; Song, Q.; Kang, B.; Liu, H.; Li, L.; Xu, H.; Zeng, X. Inhibition of PI 3K-Akt-mTOR signal pathway dismissed the stimulation of glucose on goose liver cell growth. J. Anim. Physiol. Anim. Nutr. 2017, 101, e133–e143. [Google Scholar] [CrossRef]
- Klip, A.; Tsakiridis, T.; Marette, A.; Ortiz, P.A. Regulation of expression of glucose transporters by glucose: A review of studies in vivo and in cell cultures. FASEB J. 1994, 8, 43–53. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, H.; Wang, H.; Zhou, M.; Liu, J. Effect of glucose availability on glucose transport in bovine mammary epithelial cells. Animal 2012, 6, 488–493. [Google Scholar] [CrossRef]
- Zöllkau, J.; Swiderski, L.; Schmidt, A.; Weschenfelder, F.; Groten, T.; Hoyer, D.; Schneider, U. The Relationship between Gestational Diabetes Metabolic Control and Fetal Autonomic Regulation, Movement and Birth Weight. J. Clin. Med. 2021, 10, 3378. [Google Scholar] [CrossRef]
- Holman, G.D. Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 1155–1175. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Iwata, S. Structure and Molecular Mechanism of the Mammalian Fructose Transporter GLUT5. Nihon Kessho Gakkaishi 2016, 58, 133–138. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Peng, W.; Qi, H.-B. Glucose Metabolism-Derived Nicotinamide Adenine Dinucleotide Phosphate in Late-Onset Preeclampsia Placenta Tissue and Its Correlation with Oxidative Stress. Sichuan Da Xue Xue Bao Yi Xue Ban = J. Sichuan Univ. Med. Sci. Ed. 2022, 53, 1028–1032. [Google Scholar]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Sanden, M.; Frøyland, L.; Hemre, G.-I. Modulation of glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase and malic enzyme activity by glucose and alanine in Atlantic salmon, Salmo salar L. hepatocytes. Aquaculture 2003, 221, 469–480. [Google Scholar] [CrossRef]
- Kumar, P.; Rajput, S.; Verma, A.; De, S.; Datta, T.K. Expression pattern of glucose metabolism genes in relation to development rate of buffalo (Bubalus bubalis) oocytes and in vitro–produced embryos. Theriogenology 2013, 80, 914–922. [Google Scholar] [CrossRef]
- Minemura, T.; Fukuhara, A.; Otsuki, M.; Shimomura, I. Lactate dehydrogenase regulates basal glucose uptake in adipocytes. Biochem. Biophys. Res. Commun. 2022, 607, 20–27. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef]



| Gene Name | Forward (5′→3′) | Reverse (5′→3′) | Accession No. |
|---|---|---|---|
| mTOR | AAACCCAGGTGTGATCAATAATGTC | CATCAACCCATTTCCTCATTTCA | XM_002694043.6 |
| PI3K | CCGGTTCCGCCAGTGTT | CCCATGCCGGCGTAAAA | NM_001206047.2 |
| Akt | CCAGGTATTTTGATGAGGAGTTC | GTCTTGGTCAGGTGGCGTAA | NM_173986.2 |
| P70S6K | TTTGCCTCCCTACCTCACG | GCCAGCAGTTCTTCCCAGTT | NM_205816.1 |
| G6PD | CGCCTCAACAGCCACATA | CAGGTCCCTCCCAAACG | NM_001244135.2 |
| PGK1 | CATCCTGGGCGGAGCTAAAGTTG | GGTCCATTCCACACGATCTGCTTAG | NM_001034299.1 |
| LDHA | TTGGTCCAGCGTAACGTGAACATC | ACTCCACTCCATACAGGCACACTAG | NM_174099.2 |
| HK2 | GGAGATTGCTAAGCGTTTTCG | AAGCCGTAGGGTGAGTGGTG | XM_015473383.2 |
| GLUTI | TGGGCTTCTCAAAACTGGG | GGATGCCGACGACGATG | NM_174602.2 |
| GLUT3 | CGGCAACCCATCATTATCTC | CTGGACACCCGCATCTTT | NM_174603.3 |
| GLUT4 | AGGAGGAGAAGCGGAAGC | AATGGCGATGACGAGGG | NM_174604.1 |
| GLUT5 | CGTGGTGGAACTAATGGGG | CAAGCGGTGAAACAGACAGAG | NM_001101042.2 |
| β-actin | TCCCTGGAGAAGAGCTACGA | TCCCTGGAGAAGAGCTACGA | NM_173979.3 |
| Antibody | Dilution Ratio | Source | Manufacturer |
|---|---|---|---|
| mTOR | 1:1000 | Rabbit | Affinity |
| p-mTOR | 1:1000 | Rabbit | Affinity |
| P70S6K | 1:1000 | Rabbit | ABclonal |
| p-P70S6K | 1:1000 | Rabbit | ABclonal |
| 4EBP1 | 1:500 | Rabbit | ABclonal |
| p-4EBP1 | 1:1000 | Rabbit | ABclonal |
| HIF-1α | 1:1000 | Rabbit | ABclonal |
| p-HIF-1α | 1:1000 | Rabbit | ABclonal |
| β-actin | 1:2000 | Rabbit | Affinity |
| Goat Anti-rabbit IgG | 1:5000 | Goat | Affinity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Kang, K.; Wang, Z.; Wang, J.; Xiao, J.; Peng, Q.; Hu, R.; Zhou, J.; Zhang, X.; Yue, Z.; et al. Glucose Regulates Glucose Transport and Metabolism via mTOR Signaling Pathway in Bovine Placental Trophoblast Cells. Animals 2024, 14, 40. https://doi.org/10.3390/ani14010040
Shi L, Kang K, Wang Z, Wang J, Xiao J, Peng Q, Hu R, Zhou J, Zhang X, Yue Z, et al. Glucose Regulates Glucose Transport and Metabolism via mTOR Signaling Pathway in Bovine Placental Trophoblast Cells. Animals. 2024; 14(1):40. https://doi.org/10.3390/ani14010040
Chicago/Turabian StyleShi, Liyuan, Kun Kang, Zhisheng Wang, Junmei Wang, Jianxin Xiao, Quanhui Peng, Rui Hu, Jia Zhou, Xiaohong Zhang, Ziqi Yue, and et al. 2024. "Glucose Regulates Glucose Transport and Metabolism via mTOR Signaling Pathway in Bovine Placental Trophoblast Cells" Animals 14, no. 1: 40. https://doi.org/10.3390/ani14010040
APA StyleShi, L., Kang, K., Wang, Z., Wang, J., Xiao, J., Peng, Q., Hu, R., Zhou, J., Zhang, X., Yue, Z., Zou, H., Xue, B., & Wang, L. (2024). Glucose Regulates Glucose Transport and Metabolism via mTOR Signaling Pathway in Bovine Placental Trophoblast Cells. Animals, 14(1), 40. https://doi.org/10.3390/ani14010040







