Mycobacterium avium subsp. paratuberculosis in Sheep and Goats in Austria: Seroprevalence, Risk Factors and Detection from Boot Swab Samples
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Animal Sampling and Data Collection
2.1.1. Serological Screening
2.1.2. Herd-Level Examination in Selected Dairy Goat Herds
2.2. Serological Analyses
2.3. qPCR of Individual Faecal Samples
2.4. Culture and qPCR of Boot Swab and Pooled Faecal Samples
2.5. Statistical Analyses
3. Results
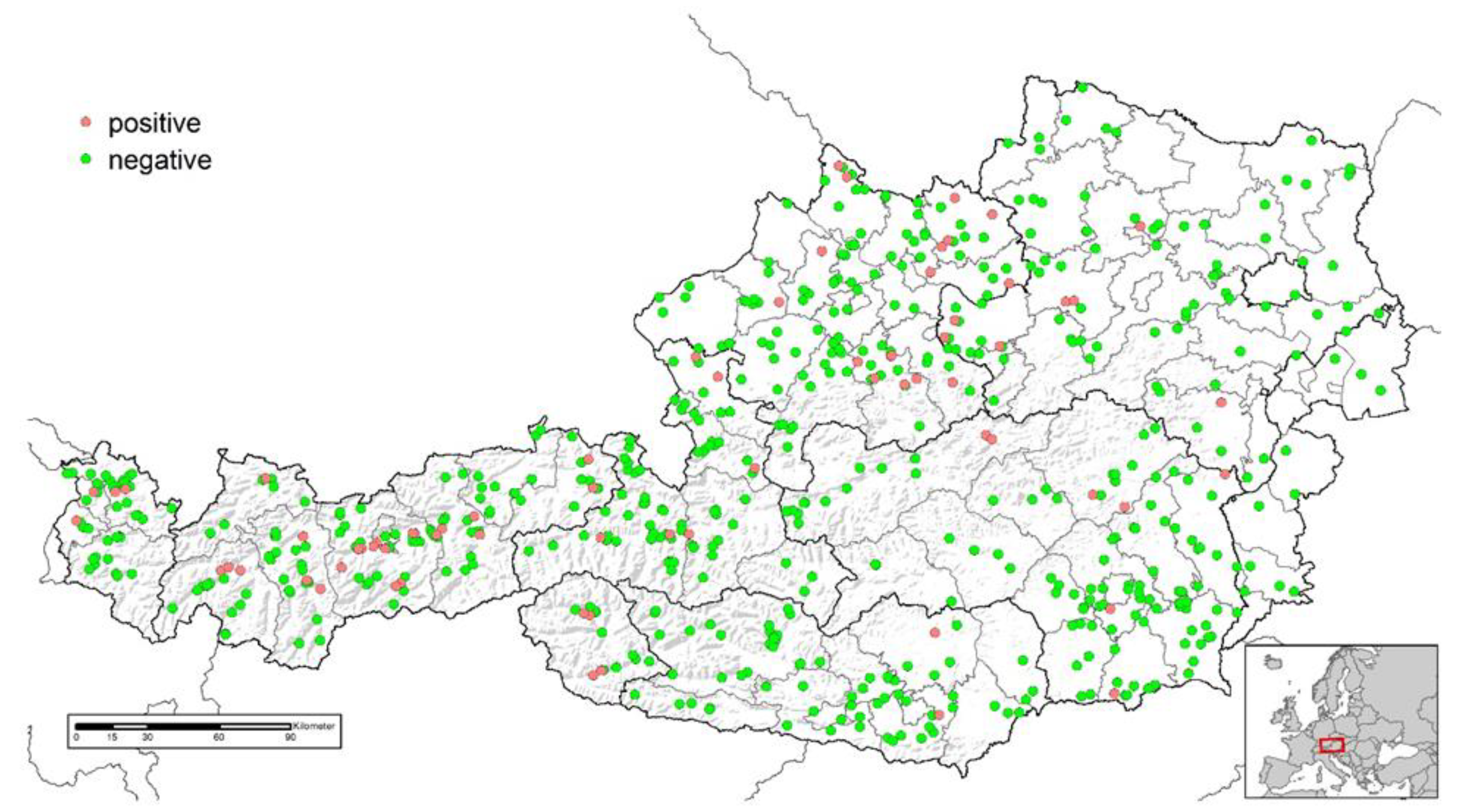
3.1. Serological Screening
| Animals (n) | Seropositive Animals (n) | Animal-Level Apparent Seroprevalence (%) | Animal-Level Calculated True Seroprevalence (%) | Herds (n) | Seropositive Herds (n) | Herd-Level Apparent Seroprevalence (%) | |
|---|---|---|---|---|---|---|---|
| Goat | 6434 | 126 | 2.0 (95% CI = 1.6–2.3) | 3.5 (95% CI = 3.1–4.0) | 638 | 71 | 11.1 (95% CI = 8.9–13.8) |
| Sheep | 15,585 | 110 | 0.7 (95% CI = 0.6–0.9) | 1.2 (95% CI = 1.0–1.4) | 1032 | 92 | 8.9 (95% CI = 7.3–10.8) |
| Total | 22,019 | 236 | 1.1 (95% CI = 0.9–1.2) | 1.9 (95% CI = 1.7–2.1) | 1670 | 163 | 9.8 (95% CI = 8.4–11.3) |
| Goat Herds | Sheep Flocks | ||||
|---|---|---|---|---|---|
| Variable a | Apparent seroprevalence in %, absolute numbers in brackets b | p-value | Apparent seroprevalence in %, absolute numbers in brackets | p-value | |
| Animal trading c | Yes No | 16.8 (25/149) 9.4 (46/489) | 0.012 * | 11.8 (48/406) 7.1 (44/616) | 0.011 * |
| Dairy herd | Yes No | 18.6 (32/172) 8.4 (39/466) | <0.001 * | 6.1 (4/66) 9.2 (88/956) | 0.388 |
| Grazing on common pastures | Yes No | 10.5 (9/86) 11.2 (62/552) | 0.833 | 10.6 (32/302) 8.3 (60/720) | 0.249 |
| Grazing on alpine pastures | Yes No | 9.9 (11/111) 11.4 (60/527) | 0.653 | 10.7 (34/319) 8.3 (58/703) | 0.213 |
| Animal Health Service membership | Yes No | 18.9 (25/132) 9.1 (46/506) | 0.001 * | 8.9 (24/270) 9.0 (68/752) | 0.940 |
| Organic farming | Yes No | 13.6 (23/169) 10.2 (48/469) | 0.232 | 8.0 (22/274) 9.4 (70/748) | 0.511 |
| Cohabitation with other animal species | |||||
| Cattle | Yes No | 8.7 (23/265) 12.9 (48/373) | 0.097 | 11.8 (34/287) 7.9 (58/735) | 0.047 * |
| Goat | Yes No | - | - | 13.0 (40/308) 7.3 (52/714) | 0.003 * |
| Sheep | Yes No | 10.1 (28/278) 11.9 (43/360) | 0.456 | - | - |
| Farmed game | Yes No | 33.3 (7/21) 10.4 (64/617) | 0.001 * | 0.0 (0/10) 9.1 (92/1012) | 0.318 |
| South American camelids | Yes No | 20.8 (5/24) 10.7 (66/614) | 0.123 | 10.0 (2/20) 9.0 (90/1002) | 0.875 |
3.2. Herd-Level Examination
| Herd Number | Herd Size (n) | Serum Sample Size (n) | Faecal Sample Size (n) | ELISA Positive a | PCR Positive a | Both ELISA and PCR Positive a | Boot Swab Samples (HEYM) d | Pooled Faecal Samples (HEYM) d |
|---|---|---|---|---|---|---|---|---|
| 1 | 114 | 114 | 112 c | 20.2 (23) | 10.7 (12) | 8.9 (10) | 3/3 | 1/3 |
| 2 | 126 | 126 b | 122 c | 24.0 (30) | 10.7 (13) | 10.7 (13) | 4/4 | 1/3 |
| 3 | 57 | 57 | 57 | 22.8 (13) | 10.5 (6) | 8.8 (5) | 3/3 | 2/3 |
| 4 | 600 | 75 | 73 c | 28.0 (21) | 24.7 (18) | 23.3 (17) | 2/3 | 2/3 |
| 5 | 550 | 60 | 60 | 11.7 (7) | 5.0 (3) | 1.7 (1) | 3/3 | 1/3 |
| Total | 432 | 424 | 21.8 (94) | 12.3 (52) | 10.9 (46) | 15/16 | 7/15 |
| Number of Animals | ELISA Positive | PCR Positive | |
|---|---|---|---|
| Age, years | |||
| 1 | 20.5 (88) | 18.2 (16) | 5.7 (5) |
| 2 | 19.6 (84) | 13.1 (11) | 8.3 (7) |
| 3 | 14.5 (62) | 35.5 (22) | 30.6 (19) |
| 4 | 10.0 (43) | 16.3 (7) | 18.6 (8) |
| 5 or older | 35.4 (152) | 25.0 (38) | 8.6 (13) |
| Total | 100 (429) | 21.9 (94) | 12.1 (52) |
| BCS | |||
| 1 | 0.9 (4) | 75.0 (3) | 75.0 (3) |
| 2 | 17.4 (75) | 25.3 (19) | 12.0 (9) |
| 3 | 57.4 (248) | 20.6 (51) | 11.7 (29) |
| 4 | 23.8 (103) | 20.4 (21) | 10.7 (11) |
| 5 | 0.5 (2) | 0.0 (0) | 0.0 (0) |
| Total | 100 (432) | 21.8 (94) | 12.3 (52) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.A. Paratuberculosis in sheep and goats. Vet. Microbiol. 2015, 181, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, R.; Pieragostini, E.; Rubino, G.; Petazzi, F. Impact of Mycobacterium avium subspecies paratuberculosis on profit efficiency in semi-extensive dairy sheep and goat farms of Apulia, southern Italy. Prev. Vet. Med. 2017, 136, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Toft, N. A review of prevalences of paratuberculosis in farmed animals in Europe. Prev. Vet. Med. 2009, 88, 1–14. [Google Scholar] [CrossRef]
- Barrero-Domínguez, B.; Luque, I.; Huerta, B.; Gomez-Laguna, J.; Galán-Relaño, Á.; Gómez-Gascón, L.; Sánchez, M.; Astorga, R.J. Paratuberculosis in dairy goat flocks from southern Spain: Risk factors associated with seroprevalence. Vet. Rec. 2019, 185, 600. [Google Scholar] [CrossRef]
- Begg, D.J.; Purdie, A.C.; de Silva, K.; Dhand, N.K.; Plain, K.M.; Whittington, R.J. Variation in susceptibility of different breeds of sheep to Mycobacterium avium subspecies paratuberculosis following experimental inoculation. Vet. Res. 2017, 48, 36. [Google Scholar] [CrossRef]
- ESG Sergeant. Ovine Johne’s disease in Australia—The first 20 years. Aust. Vet. J. 2001, 79, 484–491. [Google Scholar] [CrossRef]
- Stau, A.; Seelig, B.; Walter, D.; Schroeder, C.; Ganter, M. Seroprevalence of Mycobacterium avium subsp. paratuberculosis in small ruminants in Germany. Small Rumin. Res. 2012, 105, 361–365. [Google Scholar] [CrossRef]
- Morales-Pablos, M.I.; Mejía-Sánchez, P.; Díaz-Aparicio, E.; Palomares-Resendiz, E.G.; Gutiérrez-Hernández, J.L.; Reyna-Granados, J.R.; Luna-Nevárez, P.; Munguía-Xóchihua, J.A.; Segura-Correa, J.C.; Leyva-Corona, J.C. Risk factors associated with the seroprevalence of paratuberculosis in sheep flocks in the hot-arid region of Sonora, México. Trop. Anim. Health Prod. 2020, 52, 1357–1363. [Google Scholar] [CrossRef]
- Angelidou, E.; Kostoulas, P.; Leontides, L. Flock-level factors associated with the risk of Mycobacterium avium subsp. paratuberculosis (MAP) infection in Greek dairy goat flocks. Prev. Vet. Med. 2014, 117, 233–241. [Google Scholar] [CrossRef]
- Freitas, T.D.; de Azevedo, S.S.; Silva, M.L.C.R.; Júnior, F.G.; Santos, C.d.S.A.B.; Clementino, I.J.; Amaral, F.R.-C.; Alves, C.J. Epidemiological characterization and risk factors associated with Mycobacterium avium subsp. paratuberculosis infection in dairy goats in the Brazilian semiarid region. Semin. Ciências Agrárias 2015, 36, 267–275. [Google Scholar] [CrossRef]
- OIE. Chapter 3.1.15. Paratuberculosis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2021. [Google Scholar]
- Donat, K.; Hahn, N.; Eisenberg, T.; Schlez, K.; KÖHler, H.; Wolter, W.; Rohde, M.; Pützschel, R.; RöSler, U.; Failing, K.; et al. Within-herd prevalence thresholds for the detection of Mycobacterium avium subspecies paratuberculosis-positive dairy herds using boot swabs and liquid manure samples. Epidemiol. Infect. 2016, 144, 413–424. [Google Scholar] [CrossRef]
- Sodoma, E.; Mitterhuemer, S.; Altmann, M.; Mader, C.; Kössler, J.; Ortner, P.; Vill, M.; Duenser, M. Results and experiences of laboratory diagnostic analysis within the voluntary paratuberculosis control program in Tyrol. Berl. Und Munch. Tierarztl. Wochenschr. 2021, 134. [Google Scholar] [CrossRef]
- Sodoma, E.; Altmann, M.; Mitterhuemer, S.; Moebius, P.; Duenser, M. First comprehensive study on molecular diversity of Austrian Mycobacterium avium subspecies paratuberculosis isolates from domestic and wild ruminants. Berl. Und Munch. Tierarztl. Wochenschr. 2018, 131, 2–11. [Google Scholar]
- Dreier, S.; Khol, J.L.; Stein, B.; Fuchs, K.; Gütler, S.; Baumgartner, W. Serological, Bacteriological and Molecularbiological Survey of Paratuberculosis (Johne’s Disease) in Austrian Cattle. J. Vet. Med. Ser. B 2006, 53, 477–481. [Google Scholar] [CrossRef]
- Baumgartner, W.; Damoser, J.; Khol, J. Comparison of two studies concerning the prevalence of bovine paratuberculosis (Johne’s Disease) in Austrian cattle in the years 1995-97 and 2002/03. Wien. Tierarztl. Mon. 2005, 92, 274–277. [Google Scholar]
- Khol, J.L.; Eisenberg, S.; Noll, I.; Zschöck, M.; Eisenberg, T.; Donat, K. Two-stage control of paratuberculosis: Herd-status surveillance as the basis for operational measures to reduce the prevalence. Experiences from Lower Saxony, Hesse, Thuringia and Tyrol [Article in German]. Tierarztl Prax Ausg G Grosstiere Nutztiere 2019, 47, 171–183. [Google Scholar] [CrossRef]
- Khol, J.L.; Damoser, J.; Dünser, M.; Baumgartner, W. Paratuberculosis, a notifiable disease in Austria—Current status, compulsory measures and first experiences. Prev. Vet. Med. 2007, 82, 302–307. [Google Scholar] [CrossRef]
- Statistics Austria. Sheep and Goat Livestock. 1 December 2020. Available online: https://www.statistik.at/web_en/statistics/Economy/agriculture_and_forestry/livestock_animal_production/livestock/index.html (accessed on 21 December 2021).
- AgrarMarktAustria. Sheep and Goat Grazing on Alpine Pastures 2020. Available online: https://www.ama.at/ (accessed on 9 February 2022).
- Regulation(EU)2020/689; Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 Supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as Regards Rules for Surveillance, Eradication Programmes, and Disease-Free Status for Certain Listed and Emerging Diseases. European Parliament: Strasbourg, France, 2019.
- Baumgartner, W.; Wittek, T. Propaedeutics of Domestic Animals; Enke: Stuttgart, Germany, 2017; Volume 9. [Google Scholar]
- Eisenberg, T.; Wolter, W.; Lenz, M.; Schlez, K.; Zschöck, M. Boot swabs to collect environmental samples from common locations in dairy herds for Mycobacterium avium ssp. paratuberculosis (MAP) detection. J. Dairy Res. 2013, 80, 485–489. [Google Scholar] [CrossRef]
- Hervieu, J.; Morand-Fehr, P.; Schmidely, P.; Fedele, V.; Delfa, R. Measures anatomiques permettant d’expliquer les variations des notes sternales, lombaires et caudales utilisées pour estimer l’état corporel des chèvres laitières. Options Méditerranéennes 1991, 13, 43–56. [Google Scholar]
- Englund, S.; Ballagi-Pordány, A.; Bölske, G.; Johansson, K.-E. Single PCR and nested PCR with a mimic molecule for detection of Mycobacterium avium subsp. paratuberculosis. Diagn. Microbiol. Infect. Dis. 1999, 33, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Khol, J.; Stein, B.; Dreier, S.; Baumgartner, W. Paratuberculosis (JOHNE’s disease) in small ruminants in Austria. Slov. Vet. Res. 2006, 43, 129–130. [Google Scholar]
- Gumber, S.; Eamens, G.; Whittington, R.J. Evaluation of a Pourquier ELISA kit in relation to agar gel immunodiffusion (AGID) test for assessment of the humoral immune response in sheep and goats with and without Mycobacterium paratuberculosis infection. Vet. Microbiol. 2006, 115, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Köhler, H.; Soschinka, A.; Meyer, M.; Kather, A.; Reinhold, P.; Liebler-Tenorio, E. Characterization of a caprine model for the subclinical initial phase of Mycobacterium avium subsp. paratuberculosis infection. BMC Vet. Res. 2015, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Toft, N. Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-γ assay and faecal culture techniques. Vet. Microbiol. 2008, 129, 217–235. [Google Scholar] [CrossRef]
- Greiner, M.; Gardner, I.A. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev. Vet. Med. 2000, 45, 3–22. [Google Scholar] [CrossRef]
- Verdugo, C.; Jones, G.; Johnson, W.; Wilson, P.; Stringer, L.; Heuer, C. Estimation of flock/herd-level true Mycobacterium avium subspecies paratuberculosis prevalence on sheep, beef cattle and deer farms in New Zealand using a novel Bayesian model. Prev. Vet. Med. 2014, 117, 447–455. [Google Scholar] [CrossRef]
- Kao, R.R.; Haydon, D.T.; Lycett, S.J.; Murcia, P.R. Supersize me: How whole-genome sequencing and big data are transforming epidemiology. Trends Microbiol. 2014, 22, 282–291. [Google Scholar] [CrossRef]
- Holstad, G.; Sigurðardóttir, Ó.G.; Storset, A.K.; Tharaldsen, J.; Nyberg, O.; Schönheit, J.; Djønne, B. Description of the Infection Status in a Norwegian Cattle Herd Naturally Infected by Mycobacterium avium subsp. paratuberculosis. Acta Vet. Scand. 2005, 46, 45. [Google Scholar] [CrossRef]
- Fridriksdottir, V.; Gunnarsson, E.; Sigurdarson, S.; Gudmundsdottir, K.B. Paratuberculosis in Iceland: Epidemiology and control measures, past and present. Vet. Microbiol. 2000, 77, 263–267. [Google Scholar] [CrossRef]
- Muskens, J.; Bakker, D.; Boer, J.d.; Keulen, L.v. Paratuberculosis in sheep: Its possible role in the epidemiology of paratuberculosis in cattle. Vet. Microbiol. 2001, 78, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, C.; Pleydell, E.; Price-Carter, M.; Prattley, D.; Collins, D.; de Lisle, G.; Vogue, H.; Wilson, P.; Heuer, C. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis isolated from sheep, cattle and deer on New Zealand pastoral farms. Prev. Vet. Med. 2014, 117, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Djønne, B.; Pavlik, I.; Svastova, P.; Bartos, M.; Holstad, G. IS900 Restriction Fragment Length Polymorphism (RFLP) Analysis of Mycobacterium avium subsp. paratuberculosisIsolates from Goats and Cattle in Norway. Acta Vet. Scand. 2005, 46, 13. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, I.; Bartl, J.; Dvorska, L.; Svastova, P.; du Maine, R.; Machackova, M.; Yayo Ayele, W.; Horvathova, A. Epidemiology of paratuberculosis in wild ruminants studied by restriction fragment length polymorphism in the Czech Republic during the period 1995–1998. Vet. Microbiol. 2000, 77, 231–251. [Google Scholar] [CrossRef]
- Gerritsmann, H.; Stalder, G.L.; Spergser, J.; Hoelzl, F.; Deutz, A.; Kuebber-Heiss, A.; Walzer, C.; Smith, S. Multiple strain infections and high genotypic diversity among Mycobacterium avium subsp. paratuberculosis field isolates from diseased wild and domestic ruminant species in the eastern Alpine region of Austria. Infect. Genet. Evol. 2014, 21, 244–251. [Google Scholar] [CrossRef]
- Eamens, G.; Walker, D.; Porter, N.; Fell, S. Pooled faecal culture for the detection of Mycobacterium avium subsp paratuberculosis in goats. Aust. Vet. J. 2007, 85, 243–251. [Google Scholar] [CrossRef]
- de Juan, L.; Mateos, A.; Domínguez, L.; Sharp, J.M.; Stevenson, K. Genetic diversity of Mycobacterium avium subspecies paratuberculosis isolates from goats detected by pulsed-field gel electrophoresis. Vet. Microbiol. 2005, 106, 249–257. [Google Scholar] [CrossRef]
- Sergeant, E.S.G.; McAloon, C.G.; Tratalos, J.A.; Citer, L.R.; Graham, D.A.; More, S.J. Evaluation of national surveillance methods for detection of Irish dairy herds infected with Mycobacterium avium ssp. paratuberculosis. J. Dairy Sci. 2019, 102, 2525–2538. [Google Scholar] [CrossRef]
- Sodoma, E. Ergebnisse und Erfahrungen aus den labordiagnostischen Analysen im freiwilligen Paratuberkulose-Bekämpfungsprogramm in Tirol. Berl. Munch. Tierarztl. Wochenschr. 2021, 134, 1–10. [Google Scholar] [CrossRef]
- Stewart, D.J.; Vaughan, J.A.; Stiles, P.L.; Noske, P.J.; Tizard, M.L.V.; Prowse, S.J.; Michalski, W.P.; Butler, K.L.; Jones, S.L. A long-term bacteriological and immunological study in Holstein-Friesian cattle experimentally infected with Mycobacterium avium subsp. paratuberculosis and necropsy culture results for Holstein-Friesian cattle, Merino sheep and Angora goats. Vet. Microbiol. 2007, 122, 83–96. [Google Scholar] [CrossRef]
- Bastida, F.; Juste, R.A. Paratuberculosis control: A review with a focus on vaccination. J. Immune Based Ther. Vaccines 2011, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Schleicher, C.; Gonano, M.; Prodinger, W.M.; Pacciarini, M.; Glawischnig, W.; Ryser-Degiorgis, M.-P.; Walzer, C.; Stalder, G.L.; Lombardo, D.; et al. Red deer as maintenance host for bovine tuberculosis, Alpine region. Emerg. Infect. Dis. 2015, 21, 464–467. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrott, J.; Sodoma, E.; Dünser, M.; Tichy, A.; Khol, J.L. Mycobacterium avium subsp. paratuberculosis in Sheep and Goats in Austria: Seroprevalence, Risk Factors and Detection from Boot Swab Samples. Animals 2023, 13, 1517. https://doi.org/10.3390/ani13091517
Schrott J, Sodoma E, Dünser M, Tichy A, Khol JL. Mycobacterium avium subsp. paratuberculosis in Sheep and Goats in Austria: Seroprevalence, Risk Factors and Detection from Boot Swab Samples. Animals. 2023; 13(9):1517. https://doi.org/10.3390/ani13091517
Chicago/Turabian StyleSchrott, Juliane, Eva Sodoma, Michael Dünser, Alexander Tichy, and Johannes Lorenz Khol. 2023. "Mycobacterium avium subsp. paratuberculosis in Sheep and Goats in Austria: Seroprevalence, Risk Factors and Detection from Boot Swab Samples" Animals 13, no. 9: 1517. https://doi.org/10.3390/ani13091517
APA StyleSchrott, J., Sodoma, E., Dünser, M., Tichy, A., & Khol, J. L. (2023). Mycobacterium avium subsp. paratuberculosis in Sheep and Goats in Austria: Seroprevalence, Risk Factors and Detection from Boot Swab Samples. Animals, 13(9), 1517. https://doi.org/10.3390/ani13091517




